abstract
Mitophagy is a conserved, mitochondria-specific autophagic clearance process. We recently discovered an intricate regulatory network that balances mitophagy with mitochondrial biogenesis. Proper coordination of these opposing processes is important for stress resistance and longevity. Nodal regulatory factors that contribute to mitochondrial homeostasis have also been linked to carcinogenesis, highlighting mitophagy as a potential target for therapeutic interventions against cancer.
Keywords: Aging, autophagy, Caenorhabditis elegans, homeostasis, mitochondria, mitophagy, stress, tumorigenesis
Macroautophagy, hereafter referred to as autophagy, is a highly conserved lysosomal degradation process targeting large and possibly toxic structures, including protein aggregates, organelles, or pathogens. Mitochondria-selective autophagy (mitophagy) plays a pivotal role in the maintenance of mitochondrial homeostasis, regulating the size and quality of the mitochondrial population. In addition, mitophagy eliminates damaged mitochondria under diverse stress conditions. Healthy mitochondria are also removed when attenuation of mitochondrial function is required upon hypoxia, caloric restriction, or during certain developmental processes. Mitochondrial surveillance and quality control mechanisms, including mitophagy, decline with age and in several pathologies, causing progressive deterioration of mitochondrial function. Deregulation of mitophagy is closely linked to cancer development and progression. Thus, elucidation of the mechanisms governing mitophagy holds promise for novel anticancer interventions.1
Mitophagy is essential for mammalian erythrocyte maturation from hematopoietic stem cell (HSC)-derived early progenitors. Mice with impaired autophagy in HSCs develop atypical myeloproliferation and die within 12 weeks, recapitulating many symptoms of human myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Autophagy-deficient HSCs display a substantial increase in mitochondrial mass, accompanied by elevation of mitochondrial ROS production and DNA damage, in addition to higher proliferation and apoptosis rates.2 Mice lacking Parkin, a component of the E3 ubiquitin ligase complex and a key mitophagy regulator, spontaneously develop liver tumors and are susceptible to irradiation-induced lymphomagenesis.3 Notably, the human Parkin gene PARK2 maps to a site on chromosome 6 that is commonly deleted in several types of cancer.4 In C. elegans, inhibition of mitophagy increases mitochondrial mass, uncouples respiration from ATP production, enhances mitochondrial ROS production, and increases cytoplasmic calcium levels. These phenotypes are commonly observed in aged animals, and across large evolutionary distance.5 Increased ROS contribute to carcinogenesis by causing DNA damage and triggering aberrant alterations in gene expression. Therefore, in addition to the manifestation of pro-aging phenotypes, impairment of mitophagy potentially facilitates tumorigenesis.
Cancer cells within several types of solid tumors induce autophagy and mitophagy to adjust to their microenvironment of limited nutrient and oxygen availability. In the largely hypoxic solid tumor environment, energy production shifts from oxidative phosphorylation to glycolysis, leading to increased glucose uptake and reduced oxygen consumption, a phenomenon known as the Warburg effect. Hypoxia inducible factor 1α (HIF1α) mediates the adaptation of cancer cells to hypoxia. In solid tumors of breast and colon cancer, autophagy is induced upon hypoxia in a HIF1α-dependent manner. Furthermore, HIF1α upregulates mammalian mitophagy receptors BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3) and BCL2/adenovirus E1B 19 kDa interacting protein 3-like (BNIP3L/NIX) in response to hypoxia.6 Mitophagy induction has thus been proposed to be part of a hypoxia adaptation response that promotes cancer cell survival.7 Similarly, genetic upregulation of DCT-1, the C. elegans homolog of BNIP3 and BNIP3L/NIX, confers resistance against a variety of stressors at the organismal level.5
Notably, we found that DCT-1 upregulationn under mitophagy-inducing conditions is mediated by SKN-1, the nematode homolog of mammalian nuclear factor erythroid-derived 2-like 2 (Nrf2/NFE2L2), a transcription factor that becomes activated upon oxidative stress to preserve mitochondrial homeostasis. In sharp contrast to HIF1α, which is known to downregulate mitochondrial biogenesis,8 SKN-1 stimulates the expression of core mitochondrial components, promoting the assembly of fresh mitochondria.5 Our findings reveal a new layer of mitophagy regulation, which interfaces with mitochondrial biogenesis resulting in rejuvenation of the cell's mitochondrial pool. These observations highlight SKN-1/NRF2/NFE2L2 as a new anticancer target whose activation could induce both mitophagy and mitochondrial biogenesis. This dual coordinating role may shield mitochondrial metabolism from oncogenic transformation by opposing the Warburg effect to increase healthspan (Fig. 1).
Figure 1.
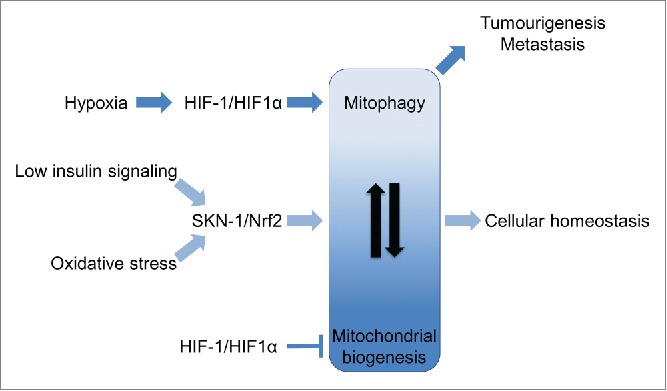
Coordination of mitochondrial biogenesis and mitophagy during aging and tumorigenesis. SKN-1/Nrf2/NFE2L2 (nuclear factor erythroid-derived 2-like 2) becomes activated under conditions of oxidative stress and low insulin/IGF-1 signaling. SKN-1 coordinates mitophagy and mitochondrial biogenesis, enhancing cellular homeostasis, longevity, and healthspan. Hypoxia-induced activation of HIF-1/HIF1α (hypoxia inducible factor 1α) stimulates mitophagy and quenches mitochondrial biogenesis. HIF1α-driven mitophagy leads to hypoxia adaptation and tumor cell survival, promoting tumorigenesis.
An additional interface between mitophagy and cancer lies in the context of insulin/insulin-like growth factor (IGF) signaling. Insulin/IGF signaling plays a key role in the development and progression of many human cancers. Although its role in the regulation of tumor growth is well established, its contribution to other phenotypes associated with malignancy, such as metastasis, is now also emerging.9 Decreased insulin signaling is an evolutionarily conserved molecular pathway that promotes longevity. We have shown that mitophagy is a significant contributor to lifespan extension under low insulin conditions. Indeed, inhibition of mitophagy shortens the lifespan of long-lived animals carrying lesions in daf-2, the gene encoding the sole insulin/IGF-1 receptor homolog in C. elegans. SKN-1 and the forkhead box O (FOXO) transcription factor DAF-16 underlie mitophagy induction under low insulin signaling conditions (Fig. 1). Targeting both insulin and IGF receptors specifically in cancer cells is a potentially effective strategy for anticancer treatment. Since mitophagy confers some of the beneficial effects of low insulin signaling, induction of mitophagy in specific types of cancer with increased insulin/IGF signaling could ameliorate tumor progression.
Mitophagy is emerging as a nexus of cellular and organismal physiology. Several mitophagy promoting conditions engage distinct transcription factors that impinge on cancer-associated processes.10 The extent of mitophagy induction is critical for the onset and progression of carcinogenesis. Impairment of mitophagy in healthy tissues can promote tumor formation and mobility of cancer cells, whereas mitophagy induction in hypoxic solid tumors promotes adaptation and tumor cell survival. Coordination of mitochondrial biogenesis and removal could provide a new pathway to circumvent the adverse effects of mitophagy in this context. Further dissection of this pathway could unravel new potential anticancer interventions targeting tumorigenesis by promoting mitochondrial rejuvenation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was funded by grants from the European Research Council, the European Commission 7th Framework Program and the Greek General Secretariat for Research and Technology.
References
- 1.Chourasia AH, Boland ML, Macleod KF. Mitophagy and cancer. Cancer Metab 2015; 3:4; PMID:25810907; http://dx.doi.org/ 10.1186/s40170-015-0130-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson AS, Mortensen M, Simon AK. Autophagy in the pathogenesis of myelodysplastic syndrome and acute myeloid leukemia. Cell Cycle 2011; 10:1719-25; PMID:21512311; http://dx.doi.org/ 10.4161/cc.10.11.15673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, et al.. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci U S A 2011; 108:16259-64; PMID:21930938; http://dx.doi.org/ 10.1073/pnas.1113884108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong Y,et al. Pan-cancer genetic analysis identifies PARK2 as a master regulator of G1/S cyclins. Nat Genet 2014; 46:588-94; PMID:24793136; http://dx.doi.org/ 10.1038/ng.2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 2015; 521:525-8; PMID:25896323; http://dx.doi.org/ 10.1038/nature14300 [DOI] [PubMed] [Google Scholar]
- 6.Sowter HM, Ratcliffe PJ, Watson P, Greenberg AH, Harris AL. HIF-1-dependent regulation of hypoxic induction of the cell death factors BNIP3 and NIX in human tumors. Cancer Res 2001; 61:6669-73; PMID:11559532 [PubMed] [Google Scholar]
- 7.Zhang H, et al.. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 2008; 283:10892-903; PMID:18281291; http://dx.doi.org/ 10.1074/jbc.M800102200 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Masson N, Ratcliffe PJ. Hypoxia signaling pathways in cancer metabolism: the importance of co-selecting interconnected physiological pathways. Cancer Metab 2014; 2:3; PMID:24491179; http://dx.doi.org/ 10.1186/2049-3002-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology 2011; 152:2546-51; PMID:21540285; http://dx.doi.org/ 10.1210/en.2011-0231 [DOI] [PubMed] [Google Scholar]
- 10.Lu H, et al.. Regulation and function of mitophagy in development and cancer. Autophagy 2013; 9:1720-36; PMID:24091872; http://dx.doi.org/ 10.4161/auto.26550 [DOI] [PubMed] [Google Scholar]


