Abstract
Background
Flow‐mediated dilation (FMD) is used to assess endothelial function through changes in vascular diameter after hyperemia. High‐fat meal (HFM) has been shown to induce endothelial dysfunction; recent studies, however, reported conflicting results in obese African American women (AAW). Differences in the method used to analyze FMD may explain these discrepancies.
Methods and Results
In protocol 1, we assessed the time course of FMD and compared the repeatability of FMD using the individual maximum peak dilation (FMD peak) and the dilation at 60 seconds (FMD 60). Sixteen AAW (age, 42±10.4 years; body mass index [BMI], 39±5.8 kg/m2) were studied on 2 occasions, 4 weeks apart, under fasting conditions (study 1 and study 2). In protocol 2, we used the most repeatable measurement from protocol 1 to assess changes in endothelial function after an HFM in 17 AAW (agen 42±11.1 years; BMIn 38±5.6 kg/m2). We found that FMD peak was the most repeatable measurement (N=16; study 1, 5.31±3.12% and study 2, 5.80±2.91%; r=0.94). After an HFM, the baseline brachial artery diameter significantly increased at 2 hours (0.10 mm; 95% confidence interval [CI], 0.01–0.18; P=0.03) and at 4 hours (0.17 mm; 95% CI, 0.09–0.25; P<0.001). At 2 hours, the FMD peak decreased compared with pre‐HFM (−1.76; 95% CI, −3.55–0.02; P≤0.05).
Conclusions
The individual's maximum peak dilation after hyperemia is the most consistent measure to assess the effect of an HFM on endothelial function. Endothelial dysfunction occurred at 2 hours after an HFM in AAW.
Clinical Trial Registration
URL: https://clinicaltrials.gov/ Unique identifiers: NCT01334554 and NCT02126735.
Keywords: African American, endothelial dysfunction, high fat diet, obesity
Introduction
Vascular reactivity is an important determinant of cardiovascular health; its impairment, also known as endothelial dysfunction, has been recognized as a strong, independent risk factor for future cardiovascular disease and mortality.1
Since its introduction in 1992, brachial artery flow‐mediated dilation (FMD) has been used to evaluate vascular reactivity induced by hyperemia in conduit arteries2 and determine differences between healthy subjects and those at risk for cardiovascular disease. The FMD index is calculated by dividing the change in the arterial diameter in response to hyperemia by the initial baseline diameter, multiplying by 100.
Although the majority of human vascular reactivity studies have been performed in the fasting state, impairment in vascular reactivity has also been documented in the postprandial state, particularly after a high‐fat meal (HFM).3, 4, 5 These findings generated great interest because they added to the pathophysiological mechanisms by which fat intake contributes to cardiovascular disease. Not all studies, however, have been consistent with these findings, and some report no change,6 or even improvement in vascular reactivity after an HFM mostly in African Americans.7 Possible explanations for the discrepancy include differences in the analysis method and type of fat consumed.8 African American women (AAW) are particularly vulnerable to postprandial endothelial dysfunction because of the high‐fat consumption in their diet9 and the presence of already impaired endothelial function at baseline.10
In recent years, extensive work has been done to refine the FMD method. Among the new tools is the automatic edge detection software, which allows continuous recording of brachial artery diameter after hyperemic stimuli. These advances have greatly improved the reproducibility and repeatability of this technique.11, 12, 13 There is, however, significant heterogeneity in the methods used to analyze the FMD after a meal stimulus, which may potentially impact the results and conclusions. One of these inconsistencies is the use of different time points for calculation of FMD (60 seconds, 90 seconds, or maximum peak dilation posthyperemia),7, 14, 15 as well as absent reporting of baseline diameter and flow.5, 16 Hence, there remains a need for a standardized method to assess vascular reactivity in response to a meal using FMD.
The present study investigated different approaches to evaluate the effect of a high‐fat challenge on vascular reactivity measured using FMD in AAW. In protocol 1, we first determined the repeatability of FMD using dilation at a fixed time point (60 seconds; FMD60), maximum peak dilation (FMDpeak), and the time when peak vasodilation was reached. In protocol 2, we applied the most robust measurement found in protocol 1 to assess changes in vascular reactivity after a meal.
Methods
Subjects
Protocol 1. Repeatability studies
Sixteen obese AAW, participants in the clinical trial (NCT01334554), were included in this protocol. FMD was performed on 2 occasions, separated by 4 weeks (study 1: baseline and study 2: 4 weeks after). As such, all premenopausal patients were evaluated during the same menstrual phase each time. Estradiol levels were available for 13 patients; for study 1, the range was 40.56 to 487.53 pg/mL with a mean±SD of 204.6±135.4 pg/mL. For study 2, the range was 38.35 to 570.12 pg/mL with a mean±SD of 225.4±148.1 pg/mL (P=0.330). The correlation between measurements was 0.8 (P<0.001).
In addition, the subjects were requested to keep their usual physical activity and diet in between measurements.
Protocol 2. Differences in FMD induced by HFM
Seventeen obese AAW who participated in the clinical trial (NCT02126735) were included in this protocol.
The HFM consisted of a high‐fat shake formulated according to the protocol of Patsch et al.17 The meal, which participants were instructed to consume within 10 minutes, had 700 calories/m2 body surface area (2.93 MJ/m2 body surface area); 3% of calories were derived from protein, 14% from carbohydrate, and 83% from fat sources. Cholesterol content was 240 mg and the ratio of polyunsaturated fat to saturated fat was 0.06.
Antihypertensive, lipid‐lowering medications, vitamins, and antioxidants were discontinued 2 weeks before the study.
We analyzed FMD at 3 time points: baseline (before HFM) and 2 and 4 hours post‐HFM.
All studies adhered to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects. The studies were approved by the Vanderbilt Institutional Review Board, and they were conducted in accord with institutional guidelines. All subjects provided informed consent.
Procedures
Subjects were admitted to the Vanderbilt Clinical Research Center on study days. All assessments were performed early in the morning, in a quiet, temperature‐controlled room (22–23°C) and after a minimum 8‐hour fast. Subjects were kept in the supine position for 20 minutes before starting data acquisition. Blood pressure was measured using an automated sphygmomanometer (Dinamap; GE Medical Systems Information Technologies, Milwaukee, WI).
Vascular Assessment and FMD
The experimental subject's dominant arm was positioned with the palm facing forward and fixed with a positioning pillow that molds to the arm (Versa form; Patterson Medical, Warrenville, IL). Vascular images were obtained by a single ultrasound trained technician (J.G.) using a 9.3‐MHz linear array vascular probe attached to a high‐resolution ultrasound machine (iU22; Phillips, Bothell, WA). To minimize movement during data acquisition, the probe was fixed in place by the means of a mechanical arm (CIVCO Medical Solutions, Coralville, IA). FMD was performed following published guidelines.13, 18, 19 The right brachial artery was scanned over a longitudinal section of 3 to 5 cm above the elbow. Depth and gain settings were optimized to identify the interface between the lumen and the blood vessel wall. A pneumatic cuff was placed at the forearm 5 cm distal to the elbow. The brachial artery diameter was measured at baseline over 60 seconds. After, the cuff was inflated to a pressure of 50 mm Hg above the systolic blood pressure of the individual for 5 minutes to provide the ischemic stimulus. The cuff was deflated and images of the artery were obtained continuously for 180 seconds after cuff release.
The ultrasound technician (J.G.) documented anatomical landmarks when positioning the ultrasound probe in the brachial artery. These landmarks were used to reposition the probe during repeated studies.
Brachial Artery Diameter Analysis
The images were saved as a DICOM file and later analyzed using a continuous edge detection and wall tracking software (Brachial analyzer 5.0; Medical Imaging Applications LLC, Iowa City, IA). Regions of interest were identified by a trained operator on the software and kept consistent for repeated studies in the same subject. We obtained brachial artery diameters every 0.033 seconds. In protocol 1, the operator was blinded. In protocol 2, blinding to the intervention (HFM) was not possible because of the substantial increase in flow after a meal observed in all the recordings. To avoid analysis bias, the operator analyzed all studies pertaining to the same subjects simultaneously using the same region of interest.
The first author (A.M.) developed a program in Visual Basic for Applications (VBA; Microsoft Office 2010) that allowed us to implement the method previously validated by Green et al. to correct for changes in brachial artery during the cardiac cycle.12 Similar to the work of Green et al., a smoothing window routine was set to calculate the median of a prespecified number of consecutive samples, then shift to the next data bracket that overlaps with a fraction of the samples just analyzed. Our program allows for the parameters to be changed as desired. For our analysis, owing to the author's preference, we used a data bracket of 50 samples with a 20% overlap (Figure 1). The software facilitated the automatic detection of the maximum diameter posthyperemia (peak) decreasing the dependence on the operator to identify the peak diameter on a tracing with varying blood vessel diameter depending on the cardiac cycle.
Figure 1.
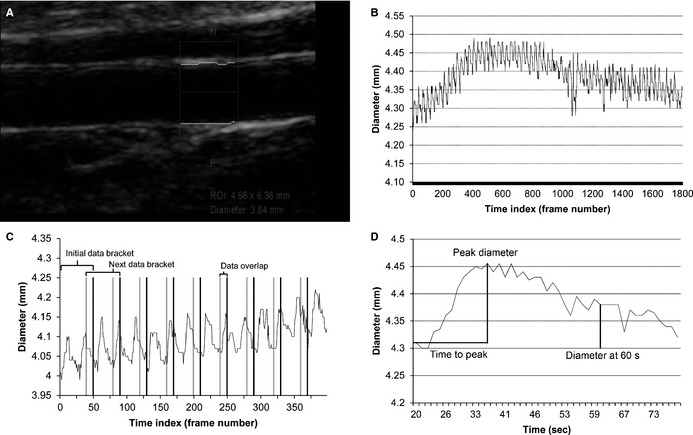
A, Ultrasound image of the brachial artery. The box is the region of interest used by the continuous edge detection and wall tracking software to determine brachial artery diameter and flow measurements. B, Vessel diameter vs frames curve (raw data) generated by the automatic edge detector software. C, The method we used to smooth the curve for peak detection. We calculated the median of a specific number of consecutive samples and then shifts to the next data bracket that overlap with the samples just analyzed. For all of our analysis, we used a data bracket of 50 samples with a 20% overlap. D, Final graphic (vessel diameter vs time) after our method is applied. Our custom design software calculated automatically the time to peak and peak dilation. ROI indicates region of interest.
Laboratory Analysis
Blood samples were collected on ice and centrifuged immediately at 5°C for 20 minutes. Serum or plasma were separated and stored at −80°C until the time of assay. Blood samples were obtained pre‐HFM and 2 and 4 hours post‐HFM to assess for changes in triglyceride, glucose, insulin, free fatty acids, and F2‐isoprostane levels. For triglycerides, we used a commercial kit (R84098 Triglycerides GPO; Cliniqa, San Marcos, CA) adapted for use on microtiter plates. Plasma glucose was measured with a glucose analyzer (YSI Life Sciences, Yellow Springs, OH). Plasma insulin concentrations were determined by MILLIPLEX(r) MAP Human Metabolic Hormone Magnetic Bead Panel immunoassay (Millipore, Billerica, MA). Free fatty acids were measured using the NEFA‐HR(2) Kit (Wako, Sopachem BV, Ochten, the Netherlands). F2‐isoprostanes were measured in plasma using negative ion gas chromatography mass spectroscopy.20
Statistical Analysis
Data are presented as mean±SD or 95% confidence interval (CI), unless otherwise stated. Repeatability analysis was assessed through Pearson correlation coefficient. Mean and SD of the absolute differences between study 1 and study 2 for each of the subjects were also reported. Bland‐Altman plots were also generated for further analysis of FMD calculated using maximum peak diameter (FMDpeak), peak at 60 seconds posthyperemia (FMD60), and time to peak, seconds. The variation between time points and limits of agreement are included in the graphs.
To investigate the effect of a HFM on endothelial dysfunction, mixed‐effects models were fitted on discrete time points before and after the HFM. Random subject effect was used for endpoints baseline flow, peak flow velocity, baseline diameter, peak diameter, 60 seconds diameter, FMDpeak, and FMD60, respectively. Inferences were drawn based on t tests. Intraclass correlation coefficient (ICC) was calculated for the measurements based on the model.
Area under the curve (AUC) for changes in triglyceride levels (AUC trig), baseline flow velocity (AUC flow), and baseline brachial artery diameter (AUC artery) were calculated using the trapezoid method. Two‐sided P values ≤0.05 were considered statistically significant. The analysis was performed using R software (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria).
With regard to the sample size calculation for protocol 2, we determined that a sample size of 17 obese African Americans would give us 80% power to detect an absolute decrease of 2.3% in FMD with HFM based on paired t test with a type I error rate of 0.05, assuming an SD of 3.1 (FMDpeak, protocol 1) and a within‐subject correlation coefficient of 0.5. A decrease of 2.3% was considered a clinical meaningful difference. Previous studies demonstrated a significant effect of HFM on FMD with a similar number of subjects.3, 4
Results
Protocol 1. Repeatability Studies
Table 1 shows the patient's characteristics. Except for hypertension, all participants were free of cardiovascular disease. Table 2 shows the correlation coefficients for baseline flow, baseline brachial artery diameter (before cuff inflation), peak flow, maximum peak diameter, brachial artery diameter at 60 seconds, and velocity time integral (VTI; after cuff deflation). Absolute differences between measurements are also included in Table 2.
Table 1.
Patients’ Characteristics
| Protocol 1 | Protocol 2 | |
|---|---|---|
| N | 16 | 17 |
| Age, y | 42±10.4 | 42±11.1 |
| BMI | 39±5.8 | 38±5.6 |
| Hypertension (%) | 13 (81) | 9 (53) |
| Postmenopausal (%) | 5 (31) | 6 (35) |
| Systolic blood pressure, mm Hg | 128±10.4 | 131.6±12.7 |
| Diastolic blood pressure, mm Hg | 79±6.8 | 82.8±11.7 |
| Heart rate | 78±1 | 68.47±11.93 |
| Waist size, cm | 109.5±10.6 | 105.4±11.8 |
| BUN, mg/dL | 11.1±4.9 | 11.9±4.1 |
| Creatinine, mg/dL | 0.8±0.1 | 0.8±0.1 |
| Fasting glucose, mg/dL | 96.3±12.6 | 86±6.6 |
| Insulin, μU/mL | 13.1±7.7 | 23.4±18.3 |
| HOMA‐IR | 2.6±1.7 | 2.8±1.7 |
| Triglycerides, mg/dL | 70.4±28.5 | 61.6±25.8 |
Data presented as mean±SD or frequency. Blood pressures and heart rate were measured at the screening visit after sitting for 5 minutes. BMI indicates body mass index; BUN, blood urea nitrogen; HOMA‐IR, homeostatic model assessment of insulin resistance.
Table 2.
Flow‐Mediated Dilation Parameters
| Parameters | Study 1 | Study 2 | r | P Value | Absolute Difference |
|---|---|---|---|---|---|
| Baseline flow, m/s | 0.20±0.09 | 0.20±0.07 | 0.88 | <0.001 | 0.03±0.03 |
| Peak flow, m/s | 1.08±0.26 | 0.99±0.27 | 0.67 | 0.005 | 0.17±0.15 |
| Peak VTI, m | 0.91±0.29 | 0.84±0.24 | 0.64 | 0.011 | 0.19±0.12 |
| Baseline diameter, mm | 3.96±0.52 | 3.90±0.61 | 0.90 | <0.001 | 0.24±0.12 |
| Peak diameter, mm | 4.16±0.48 | 4.12±0.60 | 0.88 | <0.001 | 0.26±0.12 |
| Diameter at 60 seconds, mm | 4.08±0.55 | 4.10±0.59 | 0.83 | <0.001 | 0.30±0.14 |
Data presented as mean±SD, baseline (before cuff inflation). r indicates Pearson's correlation coefficient; VTI, velocity time integral, measurement of reactive hyperemia.
FMDpeak had the lowest bias for agreement (0.47; 95% CI, −2.55 to 1.16), and the highest correlation coefficient (r=0.940; 5.31±3.12% [study 1] and 5.80±2.91% [study 2]; Figure 2). The bias for agreement for FMD60 was −2.33 (95% CI, −8.84 to 4.18), and the correlation coefficient was r=0.383 (2.51±2.70% [study 1] and 4.84±3.22% [study 2]). The time to peak had the highest bias for agreement −3.59 (95% CI, −32.67 to 25.49) and the lowest correlation coefficient (r=0.353; 43.57±11.21 seconds [study 1] and 47.16±14.45 seconds [study 2]).
Figure 2.
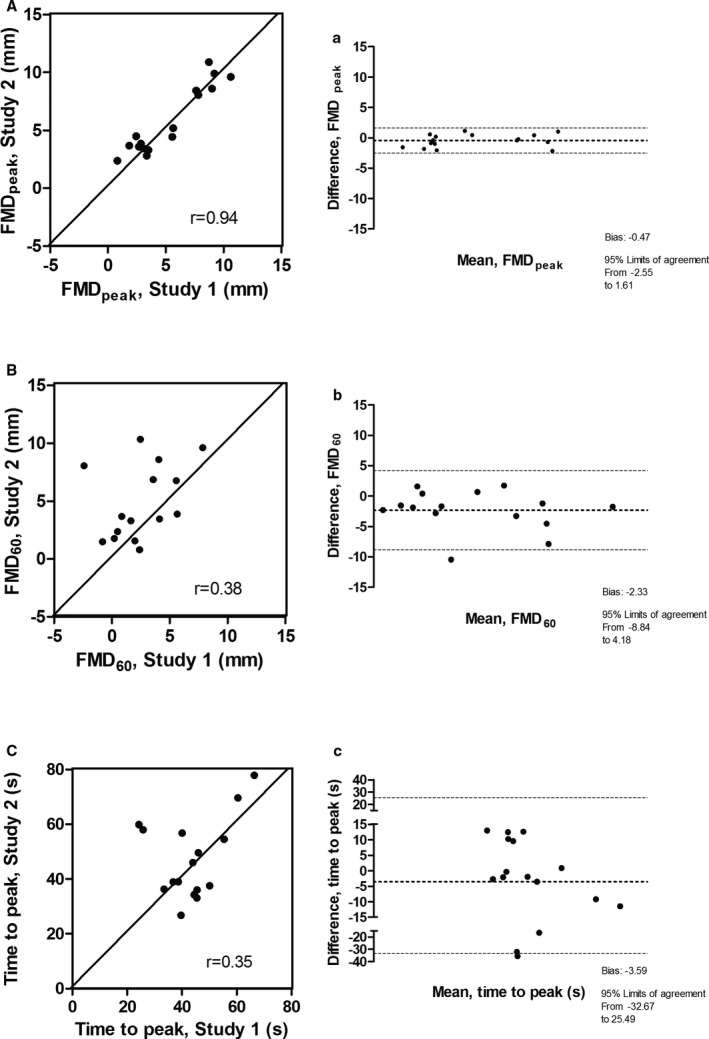
Left panel (A) showed the correlation between 2 measurements using flow‐mediated dilation (FMD)peak, separated by 4 weeks (study 1 and study 2); right panel (A) showed the respective Bland‐Altman plot. Left panel (B) showed the correlation between study 1 and study 2 with FMD 60; right panel (B) showed the respective Bland‐Altman plot. Left panel (C) showed the correlation between study 1 and study 2 with time to peak; right panel (C) showed the respective Bland‐Altman plot.
Our study showed that the FMDpeak was the most consistent and repeatable measurement in obese AAW. Time to peak in these subjects occurred, on average, ≈45 seconds posthyperemia. FMD60 and time to peak had poor repeatability.
Protocol 2. Effect of High‐Fat Intake on Vascular Reactivity
Table 1 shows patients’ characteristics. Except for hypertension, all participants were free of cardiovascular disease.
Triglyceride levels increased after the HFM; pre‐HFM and 2‐ and 4‐hour triglyceride levels were 62±25.82, 92±36 and 125±58.28 mg/dL, respectively.
Table 3 shows the parameter estimates obtained from the mixed‐effect model that evaluated the effect of HFM on vascular reactivity parameters, such as baseline flow, peak flow, baseline brachial diameter, peak brachial diameter and FMDpeak, and FMD60. Measurements at 2 and 4 hours post‐HFM were compared to pre‐HFM (before meal ingestion). There was a significant increase in baseline flow at 2 and 4 hours post‐HFM compared with pre‐HFM. The peak flow obtained after cuff release was similar at 2 and 4 hours post‐HFM.
Table 3.
Effect of High‐Fat Shake on FMD Parameters: Mixed‐Effect Model Parameter Estimates
| Parameters | 2 h Post‐HFM vs Pre‐HFM | 4 h Post‐HFM vs Pre‐HFM | ||
|---|---|---|---|---|
| Estimate [95% CI] | P Value | Estimate [95% CI] | P Value | |
| Baseline flow, m/s | 0.12 [0.04–0.19] | 0.004 | 0.12[0.05–0.19] | 0.002 |
| Peak flow, m/s | 0.04 [−0.06 to 0.14] | 0.478 | 0.05 [−0.04 to 0.15] | 0.288 |
| Baseline diameter, mm | 0.10 [0.01–0.18] | 0.030* | 0.17 [0.09–0.25] | <0.001** |
| Peak diameter, mm | 0.04 [−0.04 to 0.12] | 0.348 | 0.15 [0.07–0.23] | <0.001** |
| Diameter at 60 seconds, mm | 0.07 [−0.02 to 0.16] | 0.130 | 0.16 [0.07–0.24] | <0.001** |
| FMDpeak, % | −1.76 [−3.48 to −0.05] | 0.050 | −0.83 [−2.47 to 0.81] | 0.329 |
| FMD60, % | −0.95 [−2.88 to 0.98] | 0.342 | −0.63 [−2.48 to 1.22] | 0.508 |
CI indicates confidence interval; FMD, flow‐mediated dilation; HFM, high‐fat meal.
*P<0.05; **P<0.01; pre‐HFM (before meal ingestion); post‐HFM (after meal ingestion).
Brachial artery diameter significantly increased 2 and 4 hours after the HFM, suggesting that the HFM induced vasodilation. Hyperemia induced a similar increase in the maximum peak diameter pre‐HMF and 2 hours post‐HFM. At 4 hours post‐HFM, however, hyperemia significantly increased the maximum peak diameter compared to pre‐HFM measurements (Figure 3A and 3B).
Figure 3.
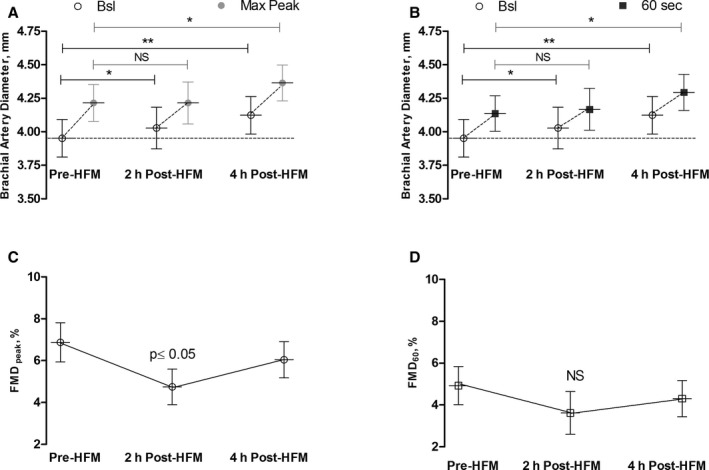
A, Baseline brachial artery diameter and maximum peak diameter postcuff deflation pre‐HFM and at 2 and 4 hours post‐HFM. B, Baseline brachial artery diameter and 60 seconds diameter postcuff deflation pre‐HFM and 2 and 4 hours post‐HFM. C, Changes in FMD peak post‐HFM. D, Changes in FMD 60 after HFM. *P<0.05; **P<0.001. FMD indicates flow‐mediated dilation; HFM, high‐fat meal; NS, not significant.
We also determined the changes in FMDpeak and FMD60 with the HFM. The HFM decreased FMDpeak at 2 hours postmeal (P≤0.05); this effect was transient, and FMDpeak was partially restored at 4 hours. The decrease in FMDpeak at 2 hours postmeal was, in part, explained by the increase in the baseline brachial artery diameter and a reduced increase in peak diameter after hyperemia. Similar trends were observed with FMD60; the effect of the meal at 2 hours, however, was attenuated and nonsignificant (P=0.342; (Figure 3B and 3D.
There were no associations between the changes in triglyceride levels (AUC trig) after an HFM and FMD parameters, such as baseline artery diameter (AUC artery, r=0.10; P=0.709) or baseline flow velocity (AUC flow, −0.39; P=0.12). We also examined the changes in glucose, free fatty acids, insulin, and isoprostane with a HFM (Figure 4). Changes in free fatty acids, insulin, and isoprostanes did not predict the changes in FMDpeak (P=0.16, 0.98, and 0.98, respectively).
Figure 4.
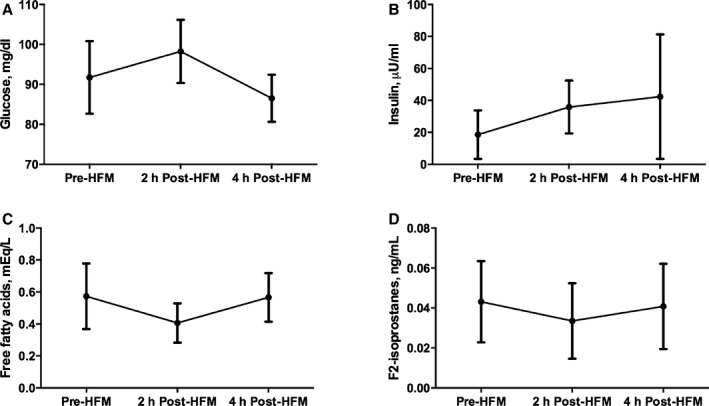
Changes in glucose (A), insulin (B), free fatty acids (C), and isoprostane (D) levels before and 2 and 4 hours after high‐fat meal (HFM).
We determined ideal sample sizes for studies that compared the effect of HFM on FMD in different populations using our proposed approach. Figure 5 shows power curves analyses for future studies.
Figure 5.
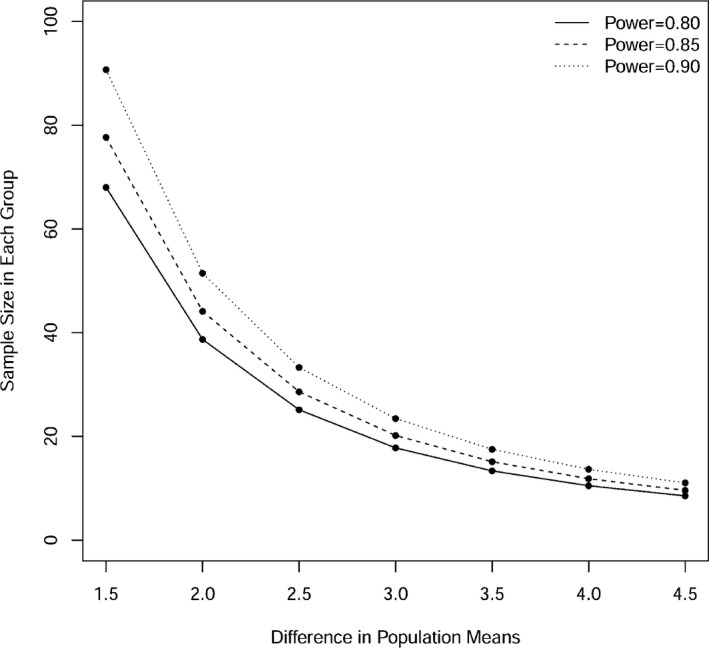
Power curves for future studies in order to detect indicated differences in the effect of HFM on FMD peak between African American and Caucasians, using 2‐sample t test with type I error rate of 0.05 and SD of 3.1. FMD indicates flow‐mediated dilation; HFM, high‐fat meal.
Discussion
The effect of an HFM on endothelial function remains controversial. Impaired endothelial function has been reported in most,3, 4, 21, 22 but not all,6, 23 previous studies. Recently, 2 different studies reported no effect of a high‐fat diet on endothelial function in obese African Americans, suggesting a racial disparity.7, 16 The primary goal of the present study was to determine whether these observed differences were explained by different methodological approaches to the analyses of FMD after a high‐fat challenge.
First, we found that FMD calculated using the maximum peak dilation after hyperemia was the most robust and repeatable method to assess vascular reactivity in obese AAW. The time to peak after hyperemia occurred at 45 seconds, earlier than the arbitrary traditional 60 seconds used to calculate FMD (Figure 1). The correlation coefficient between studies of the FMDpeak was 0.9 compared with 0.3 for FMD60. The FMD60 (2.51±2.70%) was significantly lower compared with FMDpeak (5.31±3.12%). These findings have important implications; if we were to test an intervention that aimed to improve endothelial function in this population, the sample size would be significantly greater when FMD60 is selected owing to the poor within‐subject correlation of this measurement.
These findings highlight the importance of following the time course of the brachial artery diameter after hyperemia, as previously suggested by other investigators.11, 12 Liuni et al.,11 using a similar methodology, reported that FMD60 has less repeatability that FMDpeak. In the same study, they showed that both measurements were blunted when nitric oxide synthase inhibitor (L‐NMMA) was infused, suggesting that these changes in vascular reactivity are nitric oxide–dependent and they do not reflect the action of other vasodilators also released during hyperemia.24 Black et al.12 reported that the time to peak varies among different populations; in the healthy young, it occurred at ~50 seconds, but in an older and sedentary population it occurred at ~80 seconds. Our study showed that in middle age obese AAW, the time to peak occurred early (~45 seconds), similar to the young and healthy. It is noteworthy that the time to peak after hyperemia is not influenced by nitric oxide,11 suggesting that other factors, such as differences in vascular compliance and transduction, may play a role in the early vasodilator response observed in African Americans.
Second, we found that a high‐fat challenge induced a significant reduction in FMD, as measured by maximum peak dilation in obese African Americans. The maximum decrease in FMDpeak was observed 2 hours after the meal and was the result of a concurrent increase in baseline brachial artery diameter and attenuation in the brachial artery dilation after hyperemia. At 4 hours, the baseline brachial artery diameter continued to increase; the FMD, however, was partially restored because of a greater increase in the brachial artery dilation after hyperemia. The increase in baseline brachial artery diameter observed may result, in part, in underestimation of endothelial dysfunction post‐HFM.
To the best of our knowledge, only 2 studies7, 16 have examined the effect of an HFM on endothelial function in obese AAW. Swift et al.16 failed to demonstrate a significant reduction in FMD as measured by peak diameter. The study, however, had a small sample size; only 8 obese AAW were studied. Measurements of FMD were obtained only at 1 time point, 4 hours after the meal. In our study, the maximum changes in FMD occurred at 2 hours with a partially restoration of FMD at 4 hours; therefore, it could be possible that the investigators did not detect important early changes in vascular reactivity.
In a separate study, Muniyappa et al.7 determined the effect of an HFM on endothelial function in 18 AAW with insulin resistance and 18 Caucasian women. They concluded that there were no racial differences in the effect of the HFM on endothelial function. Of note, endothelial function was not impaired with the HFM, and on the contrary, it improved in both groups. The meal used in this study was rich in fat (40%), but also in carbohydrates (40%), raising the possibility that the improvement in endothelial function was the results of insulin‐dependent vasodilation. Also, FMD was calculated using brachial artery diameter measured at fixed intervals (60 and 90 seconds) after hyperemia. As previously discussed, we argue that these measurements have poor repeatability and do not reflect the true peak dilation after hyperemia in AAW.
Third, we observed that resting baseline brachial artery diameter increased significantly after an HFM. Previous studies have reported similar observations in young healthy volunteers.25, 26 Gokce et al.25 showed that an HFM (30% fat) increased brachial diameter by 6%. Similar to our study, the increase in brachial artery was observed as early as 2 hours post‐HFM. In our study, however, the maximum increase in brachial artery was observed at 4 hours post‐HFM. The changes in brachial artery diameter could not be attributed to an error in brachial artery measurements; we used the same methodology described in protocol 1 when we assessed the repeatability of our method. The ICC for repeated measurements of brachial artery diameters was optimal (0.96). Nonetheless, these observations highlight the importance of reporting baseline artery diameter when assessing FMD.
In conclusion, the present study showed that FMD calculated using the maximum peak dilation after hyperemia is the most robust method to assess the effect of a HFM on vascular reactivity in AAW. An HFM impairs endothelial dysfunction in AAW, and this effect occurred 2 hours postmeal.
Limitations
In our study, we decided not to correct FMD for changes in flow velocity. Ideally, flow velocity should be calculated by determining the AUC of the shear stress up to point of maximum dilation.27 However, the simultaneous recording of flow velocity and brachial artery diameter affects the accuracy of the edge‐detector software to measure changes in vessel diameter. We measured flow velocity 30 seconds after cuff deflation; however, the correction of FMD using this fixed arbitrary period of assessment produces spurious results.12 We did not include a Caucasian control group because the main objective of this study was to optimize the methodology of measuring FMD and use it to define the effect of a HFM in AAW. We did not obtain data on nonendothelial‐mediated vasodilation in African Americans.
Clinical Perspectives
Our results showed that FMD calculated using the maximum peak dilation after hyperemia is the most robust and repeatable method to assess vascular reactivity in obese AAW. Using this methodology, we assessed the effect of an HFM on endothelial function and found that endothelial function is impaired 2 hours after a meal. Our results contrast with previous studies that reported no effect of an HFM on endothelial function in this population and provide a potential explanation for this difference.
Sources of Funding
This work was supported, in part, by grant P01 HL056693, R01 HL102387, DRTC grant DK20593, Autonomic Rare Diseases Clinical Research Consortium Grant U54 NS065736, the Vanderbilt Clinical and Translational Science Award grant UL1 RR024975 from the National Center for Research Resources, and the National Institutes of Health (NIH). Shibao is supported by grant K23 HL103976 from the NIH, PhRMA Foundation Career Development Award, and the Doris Duke Clinical Scientist Career Development Award.
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002388 doi: 10.1161/JAHA.115.002388)
References
- 1. Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. [DOI] [PubMed] [Google Scholar]
- 2. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 3. Marchesi S, Lupattelli G, Schillaci G, Pirro M, Siepi D, Roscini AR, Pasqualini L, Mannarino E. Impaired flow‐mediated vasoactivity during post‐prandial phase in young healthy men. Atherosclerosis. 2000;153:397–402. [DOI] [PubMed] [Google Scholar]
- 4. Vogel RAC, Mary C, Plotnik Gary D. Effect of a single high‐fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. [DOI] [PubMed] [Google Scholar]
- 5. Ceriello A. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: effects of short‐ and long‐term simvastatin treatment. Circulation. 2002;106:1211–1218. [DOI] [PubMed] [Google Scholar]
- 6. Ayer JG, Harmer JA, Steinbeck K, Celermajer DS. Postprandial vascular reactivity in obese and normal weight young adults. Obesity. 2010;18:945–951. [DOI] [PubMed] [Google Scholar]
- 7. Muniyappa R, Sachdev V, Sidenko S, Ricks M, Castillo DC, Courville AB, Sumner AE. Postprandial endothelial function does not differ in women by race: an insulin resistance paradox? Am J Physiol Endocrinol Metab. 2012;302:E218–E225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nicholls SJ, Lundman P, Harmer JA, Cutri B, Griffiths KA, Rye KA, Barter PJ, Celermajer DS. Consumption of saturated fat impairs the anti‐inflammatory properties of high‐density lipoproteins and endothelial function. J Am Coll Cardiol. 2006;48:715–720. [DOI] [PubMed] [Google Scholar]
- 9. Gans KM, Burkholder GJ, Risica PM, Lasater TM. Baseline fat‐related dietary behaviors of white, Hispanic, and black participants in a cholesterol screening and education project in New England. J Am Diet Assoc. 2003;103:699–706; discussion 706. [DOI] [PubMed] [Google Scholar]
- 10. Loehr LR, Espeland MA, Sutton‐Tyrrell K, Burke GL, Crouse JR III, Herrington DM. Racial differences in endothelial function in postmenopausal women. Am Heart J. 2004;148:606–611. [DOI] [PubMed] [Google Scholar]
- 11. Liuni A, Luca MC, Lisi M, Dragoni S, di Stolfo G, Mariani JA, Uxa A, Gori T, Parker JD. Observations of time‐based measures of flow‐mediated dilation of forearm conduit arteries: implications for the accurate assessment of endothelial function. Am J Physiol Heart Circ Physiol. 2010;299:H939–H945. [DOI] [PubMed] [Google Scholar]
- 12. Black MA, Cable NT, Thijssen DH, Green DJ. Importance of measuring the time course of flow‐mediated dilatation in humans. Hypertension. 2008;51:203–210. [DOI] [PubMed] [Google Scholar]
- 13. Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, Friberg P, Deanfield JE. Methodological approaches to optimize reproducibility and power in clinical studies of flow‐mediated dilation. J Am Coll Cardiol. 2008;51:1959–1964. [DOI] [PubMed] [Google Scholar]
- 14. Padilla J, Harris RA, Fly AD, Rink LD, Wallace JP. The effect of acute exercise on endothelial function following a high‐fat meal. Eur J Appl Physiol. 2006;98:256–262. [DOI] [PubMed] [Google Scholar]
- 15. Tyldum GA, Schjerve IE, Tjonna AE, Kirkeby‐Garstad I, Stolen TO, Richardson RS, Wisloff U. Endothelial dysfunction induced by post‐prandial lipemia: complete protection afforded by high‐intensity aerobic interval exercise. J Am Coll Cardiol. 2009;53:200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swift DL, Weltman JY, Patrie JT, Barrett EJ, Gaesser GA, Weltman A. Evaluation of racial differences in resting and postprandial endothelial function in postmenopausal women matched for age, fitness and body composition. Ethn Dis. 2013;23:43–48. [PMC free article] [PubMed] [Google Scholar]
- 17. Patsch JR, Miesenbock G, Hopferwieser T, Muhlberger V, Knapp E, Dunn JK, Gotto AM Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12:1336–1345. [DOI] [PubMed] [Google Scholar]
- 18. Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow‐mediated dilation. Hypertension. 2010;55:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thijssen DH, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, Green DJ. Assessment of flow‐mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of f2‐isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. [DOI] [PubMed] [Google Scholar]
- 21. Bae JH, Bassenge E, Kim KB, Kim YN, Kim KS, Lee HJ, Moon KC, Lee MS, Park KY, Schwemmer M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis. 2001;155:517–523. [DOI] [PubMed] [Google Scholar]
- 22. Cortes B, Nunez I, Cofan M, Gilabert R, Perez‐Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high‐fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–1671. [DOI] [PubMed] [Google Scholar]
- 23. Djousse L, Ellison RC, McLennan CE, Cupples LA, Lipinska I, Tofler GH, Gokce N, Vita JA. Acute effects of a high‐fat meal with and without red wine on endothelial function in healthy subjects. Am J Cardiol. 1999;84:660–664. [DOI] [PubMed] [Google Scholar]
- 24. Doshi SN, Naka KK, Payne N, Jones CJ, Ashton M, Lewis MJ, Goodfellow J. Flow‐mediated dilatation following wrist and upper arm occlusion in humans: the contribution of nitric oxide. Clin Sci. 2001;101:629–635. [PubMed] [Google Scholar]
- 25. Gokce N, Duffy SJ, Hunter LM, Keaney JF, Vita JA. Acute hypertriglyceridemia is associated with peripheral vasodilation and increased basal flow in healthy young adults. Am J Cardiol. 2001;88:153–159. [DOI] [PubMed] [Google Scholar]
- 26. Raitakari OT, Lai N, Griffiths K, McCredie R, Sullivan D, Celermajer DS. Enhanced peripheral vasodilation in humans after a fatty meal. J Am Coll Cardiol. 2000;36:417–422. [DOI] [PubMed] [Google Scholar]
- 27. Tschakovsky ME, Pyke KE. Counterpoint: flow‐mediated dilation does not reflect nitric oxide‐mediated endothelial function. J Appl Physiol. 2005;99:1235–1237; discussion 1237–1238. [DOI] [PubMed] [Google Scholar]


