Abstract
Background
Hypertensive individuals on blood pressure (BP)–lowering treatment with BP in the normal or high‐normal range have higher cardiovascular risk than untreated persons with usual BP in the same range. This residual risk (relative and absolute) is not well quantified and may be attributable in part to the higher burden of subclinical disease in treated individuals.
Methods and Results
We assigned 3024 Framingham Offspring Cohort participants to 5 categories based on systolic BP (SBP) and diastolic BP (DBP) and use of BP‐lowering treatment: (1) untreated SBP/DBP <120/80 mm Hg; (2) untreated SBP/DB ≥120/80 to <140/90 mm Hg; (3) treated SBP/DBP <140/90 mm Hg; (4) untreated SBP/DBP ≥140/90 mm Hg; and (5) treated SBP/DBP ≥140/90 mm Hg. A composite subclinical disease score was constructed, including information on left ventricular hypertrophy, systolic dysfunction, carotid ultrasound abnormality, peripheral artery disease, and microalbuminuria. The prevalence of subclinical disease rose across BP groups, as did the event rates for incident cardiovascular disease (449 events, median follow‐up of 11 years; group 1, 0.65 event per 100 person‐years; group 5, 3.20 events per 100 person‐years; P<0.0001 for trend). On multivariable adjustment, treated hypertensives in groups 3 and 5 had 50% (95% CI 13% to 99%) and 28% (95% CI −6% to 73%) higher hazards, respectively, of developing cardiovascular disease compared with their untreated counterparts with similar levels of BP (groups 1 and 2 and group 4, respectively). The increased risk of cardiovascular disease in treated hypertensives was attributable in part to greater subclinical disease burden.
Conclusions
Treated hypertensives have higher subclinical cardiovascular disease burden, which partly explains their higher cardiovascular disease risk compared with untreated persons with similar BP levels.
Keywords: blood pressure, cardiovascular disease, subclinical disease
Introduction
High blood pressure (BP) is a major cardiovascular disease (CVD) risk factor.1 Observational studies established a graded positive relation between BP levels and CVD risk,1, 2 and randomized controlled trials demonstrated that reductions in BP levels through antihypertensive treatment were paralleled by reductions in CVD events.3, 4 Ample evidence indicates that antihypertensive treatment also reduces the burden of subclinical CVD, including regression of left ventricular (LV) mass, carotid intima‐media thickness, and urinary albumin excretion, during short‐term follow‐up.5, 6, 7, 8 Most of the latter analyses, however, focused on selected parameters of subclinical disease and were performed in clinical trials with limited durations of follow‐up6, 8 or in clinical samples of modest size.5, 7
In some population‐based and clinical samples, higher CVD risk has been reported in persons on antihypertensive treatment9, 10, 11; however, the residual cardiovascular risk and particularly the subclinical disease burden in people on antihypertensive treatment in the community has not been adequately quantified. Those on antihypertensive treatment in the community have both greater duration of hypertension and higher levels of BP, both of which may drive the higher burden of subclinical disease in these people.
It is unclear whether BP reduction to normal or high‐normal levels by BP‐lowering medications “normalizes” CVD risk and subclinical disease burden. We aimed to quantify the burden of subclinical CVD and the risk of incident CVD associated with different levels of BP in the community, focusing on comparing treated versus untreated persons in the community with similar BP levels. We hypothesized that at similar BP levels, treated hypertensives have higher subclinical CVD burden and greater risk of overt CVD than persons with similar BP who are not on treatment. In addition, we postulated that the greater residual CVD risk in treated hypertensives is attributable in part to the greater subclinical disease burden in these individuals.
Methods
Study Sample
The present investigation was performed using data from attendees of the sixth examination cycle (conducted between 1995 and 1998) of the Framingham Offspring Cohort.12 Participants were comprehensively characterized with respect to CVD risk factors and subclinical disease.13 Participants with prevalent CVD (n=412) and missing BP levels (n=3) were excluded from the analyses. An additional 8 participants with missing follow‐up information were excluded, yielding a sample size of 3024 for analyses of incident CVD. In a subsample of 1915 participants, several measures of subclinical CVD burden were available. All participants provided written informed consent, and the study protocol was approved by the institutional review board at Boston University Medical Center.
Measurement of BP and Subclinical CVD
A more detailed description of the subclinical disease assessment is provided in Data S1.
At the sixth examination cycle, BP levels were measured twice on the participant's left arm using a mercury column sphygmomanometer after the participant had been seated for ≥5 minutes. To determine the ankle brachial index, BP was measured twice on both the arms and at both the ankles using an 8‐MHz Doppler pen probe and an ultrasonic Doppler flow detector (Park Medical Electronics Inc), as described elsewhere.14 Participants also underwent transthoracic echocardiography, and M‐mode images were used to determine measurements of LV structure. LV mass was calculated as described by Devereux et al.15 The sex‐specific 80th percentile of LV mass was used as a cut point for echocardiographic evidence of LV hypertrophy, consistent with prior publications (Table S1).13, 16 LV fractional shortening and visually assessed ejection fraction served as measures of LV systolic function. In addition, a 12‐lead electrocardiogram was obtained with participants in supine position. Sex‐specific Cornell voltage criteria were used to assess electrocardiographic evidence of LV hypertrophy.13, 17 Ultrasound of the carotid arteries was performed, as described previously.18 Images of the common carotid artery and the internal carotid artery were obtained using a 7.5‐MHz transducer and a 5.0‐MHz transducer, respectively.18 These images were used to calculate near‐ and far‐wall intima‐media thickness. Stenoses of the carotid arteries were likewise assessed, with ≥25% stenoses indicating significant narrowing of the carotid artery. Intima‐media thickness values that met or exceeded the sex‐specific 80th percentile were defined as increased carotid artery intima‐media thickness (Table S1).13 Urinary albumin and creatinine were measured in a morning spot urine sample. Urine albumin was assayed using an immunoturbidimetric test (Tina‐quant albumin assay; Roche Diagnostics), and urinary creatinine was determined with a modified Jaffe method. The glomerular filtration rate was estimated using the Chronic Kidney Disease Epidemiology Collaboration formula.19
Subclinical Disease Score
We quantified the cumulative subclinical disease burden by generating a subclinical disease score, as reported previously.13 A detailed description of the score is provided in Table S1. In essence, this score is the sum of 5 dichotomized indices of subclinical atherosclerosis and target organ damage: LV hypertrophy (by electro‐ or echocardiography), LV systolic dysfunction (by echocardiography), abnormal ultrasound of the carotid arteries (increased intima‐media thickness or carotid artery stenosis), peripheral artery disease (abnormal ankle brachial index), and glomerular endothelial dysfunction (indicated by microalbuminuria).13 The score ranged from 0 (none of the above abnormal) to 5 (all abnormal). In addition to this score, we evaluated the 5 individual components of the score. We also modeled subclinical disease as a binary variable (yes/no). The complete score and its components were available for 1915 participants.
Assessment of Incident CVD
Participants were under surveillance for incident CVD events. Cardiovascular events were adjudicated by a panel of 3 physicians, and strokes were adjudicated by a panel of 3 neurologists, using standardized criteria. Our primary outcome of interest was a first CVD event over 11.1 years (median) of follow‐up; events were defined as fatal and nonfatal myocardial infarction, angina, coronary insufficiency (acute coronary syndrome), peripheral vascular disease (intermittent claudication), cerebrovascular events (stroke or transient ischemic attack), and heart failure.20
Statistical Methods
Based on BP levels and the intake of antihypertensive medication at examination cycle 6, participants were categorized into 5 BP groups: (1) usual (untreated) systolic BP (SBP)/diastolic BP (DBP) <120/80 mm Hg, (2) usual (untreated) SBP/DBP ≥120/80 to <140/90 mm Hg, (3) SBP/DBP <140/90 mm Hg on antihypertensive medication, (4) usual (untreated) SBP/DBP ≥140/90 mm Hg, and (5) SBP/DBP ≥140/90 mm Hg on antihypertensive medication.
If the SBP and DBP values of a participant belonged to different BP groups, the participant was assigned to the higher BP group using the higher of the SBP or DBP value; for example, an untreated participant with BP of 130/70 mm Hg would belong to group 2.
Association of BP Group With Measures of Subclinical Disease
We estimated the multivariable‐adjusted mean subclinical disease score for each BP group and tested for differences between BP groups using generalized linear models. We adjusted these models for age, sex, total and high‐density lipoprotein cholesterol, lipid‐lowering medication, smoking, diabetes, estimated glomerular filtration rate, and aspirin use. Furthermore, for each BP group, we estimated multivariable‐adjusted odd ratios for the presence of subclinical disease (defined as a score ≥1) and for each of the 5 components of the score, using multiple logistic regression analysis with the first BP group (SBP/DBP <120/80 mm Hg) as the referent. Because subclinical disease was relatively common in some BP groups (Table S2), we also estimated relative risks for the presence of subclinical disease using Poisson regression with robust error variance.21 In additional analyses, we specifically compared group 5 with group 4 and compared group 3 with groups 1 and 2 groups 1 and 2 combined with respect to the cumulative subclinical disease score using a general linear model to compare treated hypertensives with untreated participants with comparable levels of BP.
Association of BP Group With Incident CVD
We assessed the absolute risk of CVD (event rates per 100 person‐years) according to BP group (as defined) over a median follow‐up period of 11.1 years. Furthermore, we graphically displayed the association between BP group at examination cycle 6 and incident CVD after examination cycle 6 using Kaplan–Meier curves. To adjust for potential confounders, including age, sex, total and high‐density lipoprotein cholesterol, lipid‐lowering medication, smoking, diabetes, estimated glomerular filtration rate, and aspirin use, we estimated Cox proportional hazard models using the BP group as an exposure variable after verifying that the assumption of proportionality of hazards was met. We performed 2 main analyses. In the first analysis, we estimated hazard ratios (HRs) for incident CVD for each BP group using BP group 1 (untreated SBP/DBP <120/80 mm Hg) as the referent category. In additional analyses, we specifically compared treated hypertensives with participants with similar BP who were not on BP‐lowering treatment; to this end, multivariable‐adjusted HRs were calculated for incident CVD in group 5 (treated BP ≥140/90 mm Hg), with group 4 (usual BP ≥140/90 mm Hg) as the referent category, and for group 3 (treated BP <140/90 mm Hg), with groups 1 and 2 combined (usual BP <140/90 mm Hg) as the referent category, using the full sample and contrasting the respective subgroups. In secondary analyses, we calculated a propensity score using multivariable logistic regression analysis (outcome was BP group membership) including the following covariates: age, male sex, SBP and DBP, body mass index, smoking, diabetes, total and high‐density lipoprotein cholesterol, and lipid‐lowering treatment. We then adjusted the above‐mentioned analyses additionally for the propensity score. Finally, we adjusted the statistical models relating BP group to incident CVD for the duration of hypertension and the duration of BP‐lowering treatment.
Impact of Subclinical Disease Burden on CVD Risk
To assess the extent to which the increased CVD risk in treated hypertensives was explained by their greater subclinical disease burden, we performed 2 sets of analyses. First, we calculated multivariable‐adjusted HRs for incident CVD in a subsample restricted to those participants on whom information regarding subclinical disease was available (n=1915). We then additionally adjusted our statistical model for the subclinical disease score (modeled as a continuous trait) and evaluated how much the HR for incident CVD was attenuated on such adjustment. In an alternative model, we adjusted for the presence of subclinical disease (defined as a subclinical disease score ≥1). Second, we calculated the absolute disease risk for incident CVD in each BP group, stratifying by presence versus absence of subclinical disease within each BP group; thus, at a given level of BP, we compared the absolute risks for incident CVD in those with versus without subclinical disease.
Results
Baseline characteristics stratified by BP group are displayed in Table 1. Mean SBP and DBP were lower in group 3 (treated hypertensives) compared with group 2 (untreated persons) (Table 1). In group 5 (treated hypertensives), participants had slightly higher levels of SBP but slightly lower levels of DBP, as in group 4 (untreated persons) (Table 1). Baseline characteristics of the subclinical disease samples are provided in Table S2. The average duration of hypertension in groups 4 and 5 were 6.9 years (SD 6.7) and 15.5 years (SD 7.8), respectively. The average duration of treatment for hypertension was 6.3 years (SD 6.3) and 8.4 years (SD 7.4) in groups 3 and 5, respectively.
Table 1.
Baseline Characteristics in the Overall Sample (n=3024), Stratified by Blood Pressure Group
| Characteristics | Overall Sample (N=3024) | |||||
|---|---|---|---|---|---|---|
| Group 1, <120/80 mm Hg, No Treatment (n=909) | Group 2, ≥120/80 to <140/90 mm Hg, No Treatment (n=970) | Group 3, <140/90 mm Hg, On Treatment (n=393) | Group 4, ≥140/90 mm Hg, No Treatment (n=413) | Group 5, ≥140/90 mm Hg, On Treatment (n=339) | P trend | |
| Clinical and biochemical features | ||||||
| Age, y, mean±SD | 53.7±8.8 | 57.9±9.1 | 61.0±8.5 | 61.4±9.1 | 65.7±8.1 | <0.0001 |
| Women, n (%) | 582 (64.0) | 503 (51.9) | 201 (51.2) | 211 (51.1) | 178 (52.5) | <0.0001 |
| SBP, mm Hg, mean±SD | 109±7 | 127±7 | 125±10 | 152±13 | 154±14 | <0.0001 |
| DBP, mm Hg; mean±SD | 68±6 | 77±7 | 75±8 | 84±10 | 82±10 | <0.0001 |
| Hypertension treatment, n (%) | 0 (0.0) | 0 (0.0) | 393 (100.0) | 0 (0) | 339 (100.0) | <0.0001 |
| BMI, kg/m² | 26.1±4.4 | 27.9±5.0 | 29.8±5.8 | 28.4±5.4 | 29.3±5.3 | <0.0001 |
| Smoking, n (%) | 165 (18.2) | 139 (14.3) | 39 (9.9) | 65 (15.8) | 32 (9.4) | 0.0004 |
| Diabetes, n (%) | 21 (2.3) | 52 (5.4) | 62 (15.8) | 32 (7.8) | 61 (18.0) | <0.0001 |
| Total cholesterol, mg/dL, mean±SD | 200±37 | 209±38 | 203±34 | 214±39 | 207±36 | <0.0001 |
| HDL cholesterol, mg/dL, mean±SD | 54±16 | 52±16 | 49±16 | 52±17 | 50±16 | <0.0001 |
DBP indicates diastolic blood pressure; HDL, high‐density lipoprotein; SBP, systolic blood pressure.
Association of BP Group With Subclinical Disease Burden
The subclinical disease score and the prevalence of subclinical disease (subclinical disease score ≥1) were higher in persons with increasing BP level (Table S2), a trend that persisted on multivariable adjustment (Figure 1A and 1B). At comparable levels of BP, treated hypertensives (groups 5 and 3) had significantly higher subclinical disease scores (Figure 1A) and greater odds (Figure 1B) and relative risks (Figure S1) of subclinical disease compared with untreated participants with similar (usual) BP levels (group 4 and groups 1 and 2 combined). Similar trends were observed for the individual components of the score, including LV hypertrophy, LV systolic dysfunction, carotid ultrasound abnormality, peripheral artery disease, and microalbuminuria (Figure S2), with generally higher odd ratios by the higher BP groups and with greater subclinical disease burden in treated hypertensives (groups 3 and 5) compared with their untreated counterparts with comparable levels of BP (groups 1 and 2 combined and group 4) (Figure S2).
Figure 1.
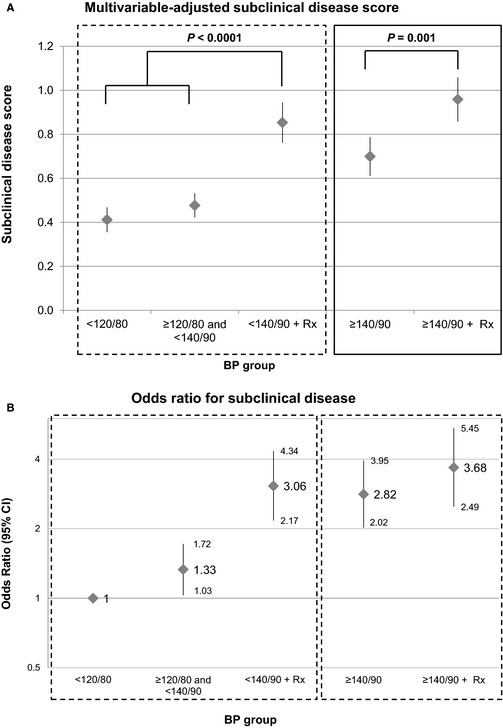
Multivariable‐adjusted subclinical disease score (A) and odds ratio for presence of subclinical disease (score ≥1) (B) by BP group. BP indicates blood pressure; hrx, blood‐pressure lowering medication.
Association of BP Group With Incident CVD
During a median follow‐up of 11.1 years (range 0.005 to 14.4 years), 449 (201 women) of 3024 participants developed an incident CVD event. The Kaplan–Meier curves by BP group are displayed in Figure 2 and show unadjusted survival free of CVD. As expected, treated hypertensives (group 5, yellow line, and group 3, red line) had shorter cumulative survival free of CVD compared with their untreated counterparts with similar BP levels (group 4, blue line, and group 2, green line). The crude event rate was lowest (0.65 event per 100 person‐years) (Table 2) in group 1 (usual BP <120/80 mm Hg), rose with increasing BP, and was highest in group 5 (treated BP ≥140/90 mm Hg) (Table 2). At comparable levels of BP, treated hypertensives had higher event rates compared with their untreated counterparts (comparison of group 5 versus group 4 and of group 3 versus groups 1 and 2) (Table 2). Even after multivariable adjustment, participants in group 3 (on BP‐lowering treatment) had 50% (95% CI 13% to 99%; P=0.005) higher hazard of developing incident CVD compared with the untreated participants in groups 1 and 2 with similar BP levels (Table S3). On a similar note, participants in group 5 (on BP‐lowering treatment) tended to have greater hazards for incident CVD compared with (untreated) participants in group 4 (HR 1.28; 95% CI 0.94 to 1.73; P=0.12). Additional adjustment for a propensity score did not alter these results (Table S3); however, adjustment for the duration of hypertension and the duration of hypertension treatment attenuated the HR for incident CVD in groups 3 and 5 (Table 2, right column) and rendered the differences between treated and untreated participants with similar levels of BP statistically nonsignificant (Table S3, right column).
Figure 2.
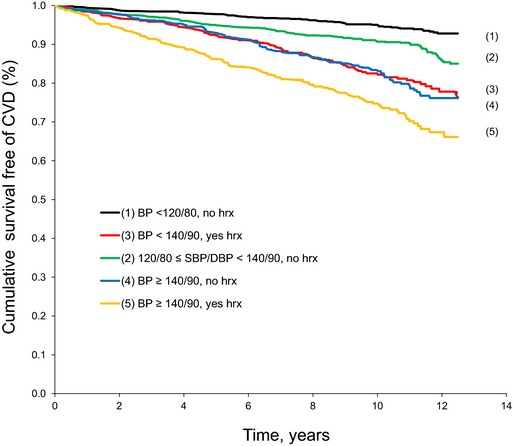
Unadjusted survival free of CVD by BP group (log‐rank P<0.0001). BP indicates blood pressure; CVD, cardiovascular disease; hrx, blood pressure–lowering medication.
Table 2.
Association of BP Group With Incident Cardiovascular Disease in Different Multivariable‐Adjusted Models in the Overall Sample (n=3024)
| BP Group, mm Hg | BP Treatment | No. Events/No. at Risk | Person‐Years | Crude Event Rates (95% CI)a | HR (95% CI)b | P Value | HR (95% CI)c | P Value | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | <120/80 | No | 65/909 | 9997 | 0.65 (0.51–0.83) | 1 | 1 | ||
| 2 | ≥120/80 to <140/90 | No | 116/970 | 10 170 | 1.14 (0.95–1.37) | 1.35 (1.00–1.84) | 0.054 | 1.36 (1.00–1.85) | 0.053 |
| 3 | <140/90 | Yes | 80/393 | 3842 | 2.08 (1.67–2.59) | 1.92 (1.36–2.71) | 0.0002 | 1.60 (1.08–2.38) | 0.020 |
| 4 | ≥140/90 | No | 88/413 | 4091 | 2.15 (1.75–2.64) | 1.94 (1.39–2.71) | 0.0001 | 1.97 (1.39–2.79) | 0.0001 |
| 5 | ≥140/90 | Yes | 100/339 | 3125 | 3.20 (2.64–3.88) | 2.44 (1.74–3.43) | <0.0001 | 1.96 (1.30–2.98) | 0.0015 |
| Total | 449/3024 | 31 225 | 1.44 (1.31–1.58) |
BP indicates blood pressure; HDL, high‐density lipoprotein; HR, hazard ratio.
Events per 100 person‐years.
HRs are adjusted for age, sex, total and HDL cholesterol, intake of lipid‐lowering medication, smoking, diabetes, aspirin use, and estimated glomerular filtration rate.
HRs are adjusted for age, sex, total and HDL cholesterol, intake of lipid‐lowering medication, smoking, diabetes, aspirin use, estimated glomerular filtration rate, duration of hypertension, and duration of hypertension treatment.
Impact of Subclinical Disease Burden on CVD Risk
Table 3 displays the multivariable‐adjusted HRs for incident CVD in those participants in whom all subclinical disease measures were available (n=1915). In essence, the association pattern in this subgroup (n=1915) was comparable to the results in the overall sample (Table 2). On additional adjustment for subclinical disease, the HRs for incident CVD were attenuated by 3.4% to 20.5% (Table 3). The attenuation was stronger with increasing BP levels and was greater in treated hypertensives compared with untreated participants with similar BP (Table 3).
Table 3.
Impact of Subclinical Disease Burden on the HR for Incident Cardiovascular Disease in the Subsample With Available Subclinical Disease Measures (n=1915)
| BP Group, mm Hg | BP Treatment | HR (95% CI)a | HR (95% CI) b | Change in HR (%)c | HR (95% CI)d | Change in HR (%)c | |
|---|---|---|---|---|---|---|---|
| 1 | <120/80 | No | 1 | 1 | 1 | ||
| 2 | ≥120/80 to <140/90 | No | 1.48 (0.99–2.20) | 1.43 (0.96–2.13) | 3.37% decrease | 1.43 (0.96–2.13) | 3.37% decrease |
| 3 | <140/90 | Yes | 2.46 (1.57–3.85) | 2.07 (1.31–3.27) | 15.85% decrease | 2.14 (1.36–3.37) | 13.01% decrease |
| 4 | ≥140/90 | No | 2.15 (1.39–3.35) | 1.93 (1.24–3.01) | 10.23% decrease | 1.87 (1.20–2.93) | 13.02% decrease |
| 5 | ≥140/90 | Yes | 2.39 (1.52–3.78) | 1.90 (1.18–3.03) | 20.50% decrease | 2.05 (1.29–3.25) | 14.23% decrease |
BP indicates blood pressure; HDL, high‐density lipoprotein; HR, hazard ratio.
Multivariable‐adjusted model with adjustment for age, sex, total and HDL cholesterol, intake of lipid‐lowering medication, smoking, diabetes, aspirin use, and estimated glomerular filtration rate.
Multivariable model additionally adjusted for subclinical disease score, modeled as a continuous trait.
Change in HR on additional adjustment for the subclinical disease score or for the presence of subclinical disease, respectively, compared with the HR adjusted for age, sex, total and HDL cholesterol, intake of lipid‐lowering medication, smoking, diabetes, aspirin use, and estimated glomerular filtration rate only.
Multivariable model additionally adjusted for the presence vs absence of subclinical disease (defined as a subclinical disease score ≥1).
The absolute disease risk in each BP group, stratified by the presence versus absence of subclinical disease, is shown in Table 4. Across all levels of BP, participants with subclinical disease had a greater absolute risk of incident CVD compared with participants without subclinical disease.
Table 4.
Absolute Disease Risk by BP Group and Within Each BP Group Further Stratified by the Presence or Absence of Subclinical Disease (Defined as a Subclinical Disease Score ≥1)
| BP Group, mm Hg | BP Treatment | Subclinical Disease (Score ≥1) | No. Events/No. at Risk | No. Events/No. at Risk | Person‐Years | Crude Event Rates (95% CI)a | |
|---|---|---|---|---|---|---|---|
| 1. A. | <120/80 | No | Absent | 22/474 | 41/640 | 5294 | 0.42 (0.27–0.63) |
| 1. B. | <120/80 | No | Present | 19/166 | 1799 | 1.06 (0.67–1.65) | |
| 2. A. | ≥120/80 to <140/90 | No | Absent | 31/386 | 66/608 | 4192 | 0.74 (0.52–1.05) |
| 2. B. | ≥120/80 to <140/90 | No | Present | 35/222 | 2216 | 1.58 (1.13–2.19) | |
| 3. A. | <140/90 | Yes | Absent | 6/87 | 44/223 | 915 | 0.66 (0.30–1.46) |
| 3. B. | <140/90 | Yes | Present | 38/136 | 1255 | 3.03 (2.21–4.14) | |
| 4. A. | ≥140/90 | No | Absent | 16/98 | 48/243 | 994 | 1.61 (0.99–2.62) |
| 4. B. | ≥140/90 | No | Present | 32/145 | 1440 | 2.22 (1.58–3.13) | |
| 5. A. | ≥140/90 | Yes | Absent | 7/56 | 53/201 | 569 | 1.23 (0.59–2.57) |
| 5. B. | ≥140/90 | Yes | Present | 46/145 | 1335 | 3.44 (2.59–4.58) | |
| Total | 252/1915 | 20 008 | 1.26 (1.11–1.42) |
BP indicates blood pressure.
Events per 100 person‐years.
Discussion
Hypertension is a silent killer because people are often not aware of having high BP levels; even if they are aware of their high BP, often they are not treated. Furthermore, many treated patients are not well controlled.22
In a large sample from the general population, we compared the absolute and relative disease risks for incident CVD and the subclinical CVD burden according to different BP levels, with a specific focus on comparing treated and untreated participants with similar levels of BP. By doing so, we wanted to explore whether reduction of BP to normal or high‐normal levels through antihypertensive medication was paralleled by normalization of CVD risk to a level comparable to the risk experienced by untreated persons with similar levels of BP. Our main findings are summarized as follows. First, higher levels of BP were associated with higher burden of subclinical disease and with greater hazards for incident CVD prospectively. Second, at similar levels of BP, treated hypertensives had a more substantial burden of subclinical CVD and were at higher risk of incident CVD compared with untreated participants. Third, this increased CVD risk was partly related to the greater burden of subclinical disease in treated hypertensives and to the duration of hypertension and hypertension treatment.
In the Context of the Published Literature
Association of BP and BP treatment with clinical and subclinical CVD
Observational studies have established a positive and graded relation between BP levels and CVD risk.1, 2 Even slightly elevated BP levels (>120/80 mm Hg) are associated with higher risk of BP progression toward hypertension23 and with greater hazards for incident CVD events prospectively compared with participants with BP levels <120/80 mm Hg.1 Furthermore, BP is a key correlate of cardiovascular remodeling traits, including LV mass,24, 25 intima‐media thickness,26 and albuminuria.27 Randomized controlled trials consistently demonstrated that lowering high BP through pharmacological interventions was associated with reduced risks for CVD events.4 Consistent with the latter observations, measures of subclinical cardiac and vascular disease also improved in parallel to the reduction in BP on antihypertensive medication. In the LIFE study, the prevalence of LV hypertrophy decreased from 70% at baseline to 23% over 5 years of antihypertensive treatment, and such regression in LV mass was associated with reduced relative risks of CVD events.28 In other clinical trials, antihypertensive treatment was associated with benign structural remodeling of the common carotid artery.29, 30 Higher BP values were also associated with indices of early kidney damage.27, 31 Nevertheless, data regarding the residual CVD risk of treated hypertensives in the general population are limited.
Consistent with the above‐mentioned reports, in our large community‐based sample, we observed that higher BP values were associated with greater cumulative subclinical disease burden, including higher prevalence of LV hypertrophy and systolic dysfunction, carotid artery abnormalities, peripheral artery disease, and microalbuminuria. Furthermore, we estimated the absolute disease risks for incident CVD associated with different levels of BP in the community and demonstrated that treated hypertensives had a greater risk of incident CVD compared with untreated participants with similar BP. These observations are in line with the results from population‐based analyses from Japan and Sweden reporting greater risks of cardiovascular mortality and stroke in treated versus untreated persons.9, 10, 11 Finally, we report that the increased hazard for CVD is attenuated by ≈3% to 20% on adjustment for the greater subclinical disease burden in treated hypertensives, consistent with the concept that part of the increased CVD risk is related to the greater subclinical disease burden. Adjustment for the duration of hypertension and hypertension treatment also attenuated the increased CVD risk in persons with hypertension, underscoring that the cumulative exposure to high BP throughout the life course affects CVD risk. Alternative reasons for residual cardiovascular risk in treated hypertensives include noncompliance with medication intake and dietary recommendations.
Our results agree well with risk prediction models in the primary prevention setting. Multivariable‐adjusted statistical models that predict absolute disease risks for a first cardiovascular event in initially healthy persons revealed that the intake of antihypertensive medication is an independent adverse predictor in such models for various forms of CVD, even after accounting for measured BP levels.32, 33 In large clinical trial cohorts, prediction models have estimated the impact of antihypertensive treatment on the 5‐year risk for CVD.34 On a similar note, antihypertensive treatment was independently associated with the progression of important subclinical disease traits, including LV mass and mean aortic root diameter over the short term (4 years) and over the life course, above and beyond measured BP levels.24, 35
Clinical Implications
Our observations of increased CVD risk and subclinical disease burden in treated hypertensives (compared with untreated participants with similar BP) support the concept that the intake of antihypertensive medication is a marker for the chronicity and severity of elevated BP and for greater subclinical disease burden. It is conceivable that physicians treat patients with antihypertensive medication if patients have higher BP levels more frequently and over a longer period of time. Consequently, even though antihypertensive medications significantly lower BP and improve clinical outcome, treated individuals are still at higher CVD risk compared with untreated persons with similar usual BP levels. These observations suggest that those on BP‐lowering medication should be monitored closely for target organ damage and potential symptoms of overt CVD, even if their BP levels reach normal or high‐normal values. Given the well‐documented burden of hypertension with significant deficits in the awareness, treatment, and control of hypertension in the Unites States,22 these data underscore the need for better screening strategies for hypertension in the community to improve the rates of early detection and subsequent treatment and control of hypertension. Finally, our analyses emphasize the importance of primary prevention of hypertension because the CVD risk cannot be entirely eliminated by medications once hypertension has developed.
Strengths and Limitations
The large sample size, the community‐based design, the careful assessment of BP and clinical covariates, the broad panel of subclinical disease traits, and the prospective design with up to 14 years of follow‐up for incident CVD strengthen our investigation. Some limitations merit consideration. We focused our BP group classification on the mean of 2 BP measurements at 1 examination cycle (examination cycle 6). This examination cycle was chosen because it offered the broadest and most comprehensive spectrum of subclinical disease measures and because using it as the baseline examination ensured a sufficiently long follow‐up period for incident CVD analyses (follow‐up of up to 14 years, 449 events). Nevertheless, classification based on BP measurement and treatment information from a single examination cycle might have led to some nondifferential misclassification, biasing us toward the null hypothesis of no association between higher BP group and disease outcome. Our sample was middle‐aged and of European American descent. It is unknown whether our results are applicable to nonwhite ethnicities and other age groups.
It is well established that, compared with white persons, black women and men have a greater burden of hypertension36 (eg, age‐adjusted prevalences of 44.9% and 46.1% for men and women, respectively),36 earlier onset of hypertension,36 greater burden of subclinical disease (eg, higher prevalence of LV hypertrophy37, 38 and peripheral artery disease36, 39), and greater relative risk increases for CVD events (eg, stroke) for similar increments in SBP.36, 40 Consequently, similar analyses of the residual cardiovascular risk associated with BP‐lowering medication in black persons and other ethnic groups are warranted.
We cannot entirely rule out that the observed differences in CVD risk and subclinical disease burden between treated and untreated participants with similar BP values could be partially due to the fact that clinicians identified individuals at higher risk for CVD and prescribed them antihypertensive drugs (confounding by indication).
In conclusion, we determined subclinical disease burden and risk for incident CVD according to different levels of BP in the community, with a specific focus on comparing treated and untreated persons with similar BP levels. At comparable levels of BP, treated hypertensives had higher subclinical disease burden and greater risk of incident CVD compared with their untreated counterparts. This risk was partly attributable to the greater burden of subclinical disease. These observations support the concept that treated hypertensives should be monitored closely for target organ damage and potential symptoms of CVD, even if their BP levels reach normal or high‐normal levels. Furthermore, these data highlight the need for early detection, treatment, and appropriate follow‐up of patients with hypertension.
Sources of Funding
This work was supported by the Framingham Core Contracts NO1‐HC‐25195 and HHSN268201500001I (to Dr. Vasan).
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
Table S1. Definitions of Subclinical Vascular Disease
Table S2. Baseline Characteristics of the Subclinical Disease Sample by Blood Pressure Group
Table S3. Hazard Ratio for Incident Cardiovascular Disease in Different Statistical Models, With and Without Adjustment for a Propensity Score
Figure S1. Multivariable‐adjusted relative risks for presence of subclinical disease (score ≥1) by blood pressure group.
Figure S2. Odds ratio for selected subclinical disease abnormalities, stratified by blood pressure group.
(J Am Heart Assoc. 2015;4:e002155 doi: 10.1161/JAHA.115.002155)
Accompanying Data S1, Tables S1 through S3 and Figures S1 and S2 are available at http://jaha.ahajournals.org/content/4/11/e002155/suppl/DC1
References
- 1. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. [DOI] [PubMed] [Google Scholar]
- 2. Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153:598–615. [DOI] [PubMed] [Google Scholar]
- 3. Staessen JA, Gasowski J, Wang JG, Thijs L, Den Hond E, Boissel JP, Coope J, Ekbom T, Gueyffier F, Liu L, Kerlikowske K, Pocock S, Fagard RH. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet. 2000;355:865–872. [DOI] [PubMed] [Google Scholar]
- 4. Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, Godwin J, Qizilbash N, Taylor JO, Hennekens CH. Blood pressure, stroke, and coronary heart disease. Part 2, short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. [DOI] [PubMed] [Google Scholar]
- 5. Stumpe KO, Agabiti‐Rosei E, Zielinski T, Schremmer D, Scholze J, Laeis P, Schwandt P, Ludwig M. Carotid intima‐media thickness and plaque volume changes following 2‐year angiotensin II‐receptor blockade. The multicentre olmesartan atherosclerosis regression evaluation (MORE) study. Ther Adv Cardiovasc Dis. 2007;1:97–106. [DOI] [PubMed] [Google Scholar]
- 6. Zanchetti A, Bond MG, Hennig M, Neiss A, Mancia G, Dal Palu C, Hansson L, Magnani B, Rahn KH, Reid JL, Rodicio J, Safar M, Eckes L, Rizzini P. Calcium antagonist lacidipine slows down progression of asymptomatic carotid atherosclerosis: principal results of the European Lacidipine Study on Atherosclerosis (ELSA), a randomized, double‐blind, long‐term trial. Circulation. 2002;106:2422–2427. [DOI] [PubMed] [Google Scholar]
- 7. Hartford M, Wendelhag I, Berglund G, Wallentin I, Ljungman S, Wikstrand J. Cardiovascular and renal effects of long‐term antihypertensive treatment. JAMA. 1988;259:2553–2557. [PubMed] [Google Scholar]
- 8. Wachtell K, Dahlof B, Rokkedal J, Papademetriou V, Nieminen MS, Smith G, Gerdts E, Boman K, Bella JN, Devereux RB. Change of left ventricular geometric pattern after 1 year of antihypertensive treatment: the losartan intervention for endpoint reduction in hypertension (LIFE) study. Am Heart J. 2002;144:1057–1064. [DOI] [PubMed] [Google Scholar]
- 9. Asayama K, Ohkubo T, Yoshida S, Suzuki K, Metoki H, Harada A, Murakami Y, Ohashi Y, Ueshima H, Imai Y. Stroke risk and antihypertensive drug treatment in the general population: the Japan arteriosclerosis longitudinal study. J Hypertens. 2009;27:357–364. [DOI] [PubMed] [Google Scholar]
- 10. Asayama K, Satoh M, Murakami Y, Ohkubo T, Nagasawa SY, Tsuji I, Nakayama T, Okayama A, Miura K, Imai Y, Ueshima H, Okamura T. Cardiovascular risk with and without antihypertensive drug treatment in the Japanese general population: participant‐level meta‐analysis. Hypertension. 2014;63:1189–1197. [DOI] [PubMed] [Google Scholar]
- 11. Andersson OK, Almgren T, Persson B, Samuelsson O, Hedner T, Wilhelmsen L. Survival in treated hypertension: follow up study after two decades. BMJ. 1998;317:167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 13. Ingelsson E, Sullivan LM, Murabito JM, Fox CS, Benjamin EJ, Polak JF, Meigs JB, Keyes MJ, O'Donnell CJ, Wang TJ, D'Agostino RB Sr, Wolf PA, Vasan RS. Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes. 2007;56:1718–1726. [DOI] [PubMed] [Google Scholar]
- 14. Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PW. The ankle‐brachial index in the elderly and risk of stroke, coronary disease, and death: the Framingham Study. Arch Intern Med. 2003;163:1939–1942. [DOI] [PubMed] [Google Scholar]
- 15. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 16. Lieb W, Gona P, Larson MG, Aragam J, Zile MR, Cheng S, Benjamin EJ, Vasan RS. The natural history of left ventricular geometry in the community: clinical correlates and prognostic significance of change in LV geometric pattern. JACC Cardiovasc Imaging. 2014;7:870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casale PN, Devereux RB, Alonso DR, Campo E, Kligfield P. Improved sex‐specific criteria of left ventricular hypertrophy for clinical and computer interpretation of electrocardiograms: validation with autopsy findings. Circulation. 1987;75:565–572. [DOI] [PubMed] [Google Scholar]
- 18. Maas R, Xanthakis V, Polak JF, Schwedhelm E, Sullivan LM, Benndorf R, Schulze F, Vasan RS, Wolf PA, Boger RH, Seshadri S. Association of the endogenous nitric oxide synthase inhibitor ADMA with carotid artery intimal media thickness in the Framingham Heart Study offspring cohort. Stroke. 2009;40:2715–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kannel WB, Wolf PA, Garrison RJ. Section 34. Some risk factors related to the annual incidence of cardiovascular disease and death using pooled repeated biennial measurements In: The Framingham Heart Study, 30‐Year Follow‐Up: An Epidemiological Investigation. Bethesda, MD: National Heart, Lung and Blood Institute; 1987. NIH Publication No. 87x20102703. [Google Scholar]
- 21. Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. [DOI] [PubMed] [Google Scholar]
- 22. Olives C, Myerson R, Mokdad AH, Murray CJ, Lim SS. Prevalence, awareness, treatment, and control of hypertension in United States counties, 2001–2009. PLoS One. 2013;8:e60308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non‐hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–1686. [DOI] [PubMed] [Google Scholar]
- 24. Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: clinical correlates of short‐ and long‐term change in the Framingham offspring study. Circulation. 2009;119:3085–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch‐Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oren A, Vos LE, Uiterwaal CS, Grobbee DE, Bots ML. Cardiovascular risk factors and increased carotid intima‐media thickness in healthy young adults: the Atherosclerosis Risk in Young Adults (ARYA) study. Arch Intern Med. 2003;163:1787–1792. [DOI] [PubMed] [Google Scholar]
- 27. Horner D, Fliser D, Klimm HP, Ritz E. Albuminuria in normotensive and hypertensive individuals attending offices of general practitioners. J Hypertens. 1996;14:655–660. [DOI] [PubMed] [Google Scholar]
- 28. Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris K, Aurup P, Dahlof B. Prognostic significance of left ventricular mass change during treatment of hypertension. JAMA. 2004;292:2350–2356. [DOI] [PubMed] [Google Scholar]
- 29. Stanton AV, Chapman JN, Mayet J, Sever PS, Poulter NR, Hughes AD, Thom SA. Effects of blood pressure lowering with amlodipine or lisinopril on vascular structure of the common carotid artery. Clin Sci (Lond). 2001;101:455–464. [PubMed] [Google Scholar]
- 30. Simon A, Gariepy J, Moyse D, Levenson J. Differential effects of nifedipine and co‐amilozide on the progression of early carotid wall changes. Circulation. 2001;103:2949–2954. [DOI] [PubMed] [Google Scholar]
- 31. Pontremoli R, Nicolella C, Viazzi F, Ravera M, Sofia A, Berruti V, Bezante GP, Del Sette M, Martinoli C, Sacchi G, Deferrari G. Microalbuminuria is an early marker of target organ damage in essential hypertension. Am J Hypertens. 1998;11:430–438. [DOI] [PubMed] [Google Scholar]
- 32. Pencina MJ, D'Agostino RB Sr, Larson MG, Massaro JM, Vasan RS. Predicting the 30‐year risk of cardiovascular disease: the Framingham Heart Study. Circulation. 2009;119:3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blacher J, Evans A, Arveiler D, Amouyel P, Ferrieres J, Bingham A, Yarnell J, Haas B, Montaye M, Ruidavets JB, Ducimetiere P. Residual cardiovascular risk in treated hypertension and hyperlipidaemia: the PRIME Study. J Hum Hypertens. 2010;24:19–26. [DOI] [PubMed] [Google Scholar]
- 34. Glynn RJ, L'Italien GJ, Sesso HD, Jackson EA, Buring JE. Development of predictive models for long‐term cardiovascular risk associated with systolic and diastolic blood pressure. Hypertension. 2002;39:105–110. [DOI] [PubMed] [Google Scholar]
- 35. Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation. 2010;122:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 37. Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, Oberman A, Kitzman DW, Hopkins PN, Liu JE, Devereux RB. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network Study. Hypertension. 2004;43:1182–1188. [DOI] [PubMed] [Google Scholar]
- 38. Drazner MH. Left ventricular hypertrophy is more common in black than white hypertensives: is this news? Hypertension. 2004;43:1160–1161. [DOI] [PubMed] [Google Scholar]
- 39. Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, Criqui MH. Ethnic‐specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. 2007;32:328–333. [DOI] [PubMed] [Google Scholar]
- 40. Howard G, Lackland DT, Kleindorfer DO, Kissela BM, Moy CS, Judd SE, Safford MM, Cushman M, Glasser SP, Howard VJ. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Table S1. Definitions of Subclinical Vascular Disease
Table S2. Baseline Characteristics of the Subclinical Disease Sample by Blood Pressure Group
Table S3. Hazard Ratio for Incident Cardiovascular Disease in Different Statistical Models, With and Without Adjustment for a Propensity Score
Figure S1. Multivariable‐adjusted relative risks for presence of subclinical disease (score ≥1) by blood pressure group.
Figure S2. Odds ratio for selected subclinical disease abnormalities, stratified by blood pressure group.


