Introduction
Arterial stiffness has re‐emerged over the past 20 years as an important predictor of cardiovascular outcomes.1 However, the evidence on the role of arterial stiffness in the development of heart failure (HF) is limited to 2 large studies with some conflicting resulting.2, 3 Furthermore, both studies lack the distinction between HF phenotypes, which could be associated differently with arterial stiffness. Thus, it is particularly pleasing to see new data on this topic from the Framingham Heart Study 4 in this issue of the Journal of the American Heart Association.
Arterial stiffness is the hallmark of arterial aging.5 Several measurements for quantifying arterial stiffness have been proposed, which are summarized in Figure. Carotid–femoral pulse‐wave velocity (PWV) is the reference measurement of arterial stiffness,6 and it is calculated by measuring the time taken for the pressure wave to travel the distance between the carotid and the femoral artery, which is generally measured over the body surface. Timing is obtained by measuring the interval between the foot of the carotid and the femoral waveforms taken with a tonometer. Parameters generally referred to as arterial wave reflection are instead estimated by placing a tonometer on the surface of a peripheral vessel (ideally the radial artery) and applying mathematical processes to predict the waveform in the more proximal aortic circulation. The derived aortic pressure waveform is the result of a forward‐traveling pressure wave (Pf), generated by left ventricular (LV) ejection, and a backward‐traveling reflected pressure wave (Pb), generated by peripheral vascular resistance, vessels tapering, and bifurcations (Figure). These 2 components can be separated by simultaneously measuring the aortic flow waveform with echocardiography, or assuming a “triangular” aortic flow waveform (ie, pseudoflow) through the identification of the inflection point (ie, the point visible on the upward part of the aortic pressure waveform that corresponds to the foot of the backward‐reflected pressure wave). Augmented pressure above the inflection point is assessed subtracting Pf from central pulse pressure, and augmentation index (AI) estimated as augmented pressure expressed as a percentage of the central pulse pressure. The reflection magnitude instead is the ratio of the magnitudes of the backward wave and the forward wave (Pb/Pf).
Figure 1.
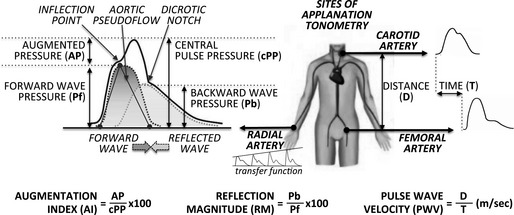
Estimation of major arterial stiffness and wave reflection parameters using applanation tonometry.
From a physiological standpoint, the stiffer the arterial system, the higher the PWV, and the faster the reflected pressure wave returns from the periphery to the proximal aorta, adding earlier to the forward wave and eventually reaching the heart at systole instead of diastole, thus causing (1) augmentation of central systolic blood pressure increasing cardiac loading, and (2) reduction of central diastolic blood pressure decreasing coronary perfusion.7 Several studies linked PWV and indexes of wave reflection with LV dysfunction,7, 8, 9, 10, 11 which constituted the background for investigating their potential contribution to the development of HF. In an earlier analysis of 2844 participants of the Health Aging and Body Composition study (mean age 74±3 years, 48% men, 26% prevalent cardiovascular disease [CVD], median follow‐up 4.6 years), Sutton‐Tyrrell and colleagues failed to demonstrate an association of PWV with incident HF (n=181, 6.4%).3 However, it is worth noting that in this study, PWV was measured using the foot of the Doppler flow wave instead of the pressure wave, whose identification may be more challenging.6 More recently, in 5960 participants of the Multi‐Ethnic Study of Atherosclerosis study (mean age 62 years, 48% men, no prevalent CVD, median follow‐up 7.6 years), Chirinos and colleagues demonstrated an independent association between reflection magnitude and incident HF (n=104, 1.7%). On the contrary, AI and pulse pressure amplification were not associated with incident HF after multivariate adjustments.2
In this issue of the Journal of the American Heart Association, Tsao and colleagues aimed at examining the association between arterial stiffness and incident HF with a specific focus on the 2 main phenotypes of HF. They used data from 2 study cohorts of the Framingham Heart Study population, with available tonometric measures of PWV and aortic pressure waveform.4 The analysis included 2539 participants (mean age 64±12 years, 44% men, about 30% prevalent CVD). At a median follow‐up of 10.1 years, there were 170 (6.7%) incident HF cases. In multivariate models, the authors found an independent relationship of incident HF with PWV, but not with central pulse pressure, Pf, and AI.4
A major limitation of current research in this field lies in methodological differences among studies. Whereas there is some agreement on the prognostic role of PWV, the real debate is generally on the contribution of wave reflection. Chirinos and colleagues argue that wave reflection has an independent value,2 while Tsao and colleagues do not.4 AI is considered to offer the best prognostic contribution when measured in younger individuals free from CVD.12 Thus this may partially explain the positive findings of the Multi‐Ethnic Study of Atherosclerosis study, which included a particularly healthy population with no prevalent CVD. The advocates of wave reflection, however, have been capitalizing on reflection magnitude rather than AI. Unfortunately, Tsao and colleagues did not calculate it.4 Similarly, a limitation of reflection magnitude tested in the Multi‐Ethnic Study of Atherosclerosis study is that it was calculated using a pseudoflow waveform, and not the individual's aortic flow waveform.2 The optimal study should utilize simultaneously measured aortic flow and pressure recordings.
Finally, the heterogeneity of the HF phenotype has never been addressed in previous studies, and the study by Tsao and colleagues appears underpowered to this goal.4 Indeed, a major paradigm shift in our understanding of HF occurred after the recognition that a clinical syndrome of HF was possible also in patients with normal LV ejection fraction, subsequently referred to as HF with preserved ejection fraction (HFpEF), as opposed to HF with reduced ejection fraction (HFrEF).13 HFpEF is currently diagnosed in at least half of patients with new‐onset HF, and it is associated with a high mortality rate, comparable to that of HFrEF.13 Arterial stiffness has been called into question, particularly in the pathogenesis of HFpEF.9, 10 Tsao and colleagues aimed to tackle the problem by performing subgroup analysis of participants who developed HFpEF (n=77, 45%) versus HFrEF (n=61, 36%). Thirty‐two of the 170 incident cases of HF (19%) were correctly excluded from the analysis because of a myocardial infarction between LV imaging and incident HF. PWV displayed no significant association with either HFpEF or HFrEF after accounting for clinical covariates.4 The authors should be complimented for reporting this valuable information, despite the very low number of events, which might have significantly hampered their efforts. In addition, LV ejection fraction was defined by either echocardiography or radionuclide angiography, with a threshold of 45%, which is lower than the currently recommended 50%.13 Thus, to date we remain without effective evidences of a differential contribution of arterial stiffness to the pathogenesis and development of HFrEF versus HFpEF. Remarkably, because women have a higher incidence of LV diastolic dysfunction and indexes of wave reflection than men,14 the latter have been increasingly suggested as responsible for the higher incidence of HFpEF in women,9, 10 but data in support of this concept are lacking. Unfortunately, both the Multi‐Ethnic Study of Atherosclerosis and the current Framingham Heart Study analyses were underpowered in terms of incident HF events to test this hypothesis. Tsao and colleagues reported a non‐significant interaction between PWV and sex for incident HF, which, however, is of no use.4 Future studies will need to be large and with long‐term follow‐up, in order to include a higher number of incident HF events that will have to be dichotomized in HFrEF versus HFpEF according to the definition of the most recent guidelines.13 Because survival is similarly poor in both forms of HF, yet to date there is no treatment of proven benefit for HFpEF,13 demonstration of a contribution of arterial stiffness and wave reflection to this syndrome would allow focus on new therapeutic approaches that primarily target the arterial rather than the cardiac walls.
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002807 doi: 10.1161/JAHA.115.002807)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
References
- 1. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 2. Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sutton‐Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well‐functioning older adults. Circulation. 2005;111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 4. Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, Benjamin EJ, Mitchell GF, Vasan RS. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4:e002189 doi: 10.1161/JAHA.115.002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alghatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, Scuteri A, Najjar SS, Ferrucci L, Lakatta EG. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on H . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. [DOI] [PubMed] [Google Scholar]
- 9. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2012;6:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shim CY, Park S, Choi D, Yang WI, Cho IJ, Choi EY, Chung N, Ha JW. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57:1226–1233. [DOI] [PubMed] [Google Scholar]
- 11. Canepa M, Alghatrif M, Strait JB, Cheng HM, Chuang SY, Chen CH, Brunelli C, Ferrucci L, Lakatta EG. Early contribution of arterial wave reflection to left ventricular relaxation abnormalities in a community‐dwelling population of normotensive and untreated hypertensive men and women. J Hum Hypertens. 2014;28:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo‐Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753–1760. [DOI] [PubMed] [Google Scholar]
- 13. Writing Committee M , Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation/American Heart Association Task Force on Practice G . 2013 ACCG/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 14. Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol. 2011;26:562–568. [DOI] [PubMed] [Google Scholar]


