Abstract
Background
The effect of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation (PAF) remains unknown.
Methods and Results
We performed circumferential pulmonary vein isolation (CPVI) and voltage mapping of left atrium (LA) during sinus rhythm in 122 consecutive patients with symptomatic PAF (age, 55.4±9.4 years; 73.8% men). Low‐voltage zones (LVZs) were semiquantitatively estimated and presented as low‐voltage index (LVI). Each patient's daily alcohol consumption history was recorded at baseline and classified into alcohol abstainers, moderate drinkers, and heavy drinkers based on the National Institute on Alcohol Abuse and Alcoholism definition. Follow‐up was ≥12 months for AF recurrence. Alcohol abstainers and moderate and heavy drinkers were 70 (57.4%), 13 (10.6%), and 39 (32.0%), respectively. In total, LVZs were observed in 44 patients (36.1%). Daily alcohol consumption independently predicted presence of LVZs (odds ratio [OR], 1.097; 95% confidence interval [CI], 1.001–1.203; P=0.047). During mean follow‐up of 20.9±5.9 months, 40 patients (35.1%) experienced AF recurrence. Success rate was 81.3%, 69.2%, and 35.1% in alcohol abstainers, moderate drinkers, and heavy drinkers, respectively (overall log rank, P<0.001). Multivariate analysis showed that both alcohol consumption and LVI were independent predictors of AF recurrence (hazard ratio [HR], 1.579; 95% CI, 1.085–2.298; P=0.017; HR, 2.188; 95% CI, 1.582–3.026; P<0.001, respectively). Furthermore, mediation analysis revealed that LVZs acted as a partial mediator in effect of alcohol consumption on AF ablation outcomes.
Conclusions
Daily alcohol consumption was associated with atrial remodelling, and heavy drinkers have substantial risk for AF recurrence after CPVI.
Keywords: alcohol, atrial fibrillation, catheter ablation, electrophysiology mapping
Introduction
During the past decade, radiofrequency catheter ablation (RFCA) has been a curative treatment for drug‐refractory atrial fibrillation (AF), with pulmonary vein (PV) isolation (PVI) as its cornerstone.1 However, unfavorable ablation outcomes were observed in patients with various comorbidities, such as hypertension and enlarged left atrium (LA).2 Substrate remodeling that resulted from these adverse clinical factors was postulated to be one of the requisite underlying mechanisms for AF recurrence.
The 3‐dimensional (3D) navigation system‐based LA electroanatomical mapping (EAM) has been an important technique for not only guiding catheters, but also reflecting substrate remodeling. Previous studies employing EAM and delayed enhancement/magnetic resonance imaging (DE‐MRI) have shown that low‐voltage zones (LVZs) could serve as surrogates for atrial remodeling and are associated with poor ablation outcomes.3, 4
Although it has long been recognized that acute substantial alcohol consumption could increase the risk of new‐onset AF, which might be attributed to a hyperadrenergic state, impaired vagal tone, and intra‐ and interatrial conduction disturbances,5, 6 the impact of chronic alcohol consumption on the ablation outcome and the underlying substrate remodeling in patients with paroxysmal atrial fibrillation (PAF) remain unknown. Therefore, the present study aimed to evaluate whether alcohol consumption could affect the LVZ in LA or the ablation outcome after circumferential PVI (CPVI) in patients with PAF.
Methods
Study Population
One hundred forty consecutive patients with symptomatic PAF who were presented in sinus rhythm and underwent the initial CPVI procedure in our center between September 2012 and March 2014 were prospectively enrolled. Previously ablated patients, patients with significant valvular abnormalities, LA diameter (LAD) in parasternal long axis >60 mm, left ventricular ejection fraction (LVEF) <45%, and intracardiac thrombus were excluded from the study. AF duration was defined as the time from the first diagnosis of AF to the ablation procedure. The study was approved by the institutional review board, and all participants gave written informed consent. This study complies with the Declaration of Helsinki.
Alcohol Consumption Assessment
Alcohol consumption was assessed during a baseline clinical visit. Participants were inquired for information on whether they regularly consumed alcohol, average alcohol consumption per day, and the number of days per month that they consumed alcohol. Ethanol weight content differed among beverages: 5% for beer; 12.5% for red wine; 45% for hard liquor. One drink was defined as an average of 15 g of ethanol.7 We used the cut‐off values based on the definition by National Institute on Alcohol Abuse and Alcoholism8 on daily alcohol consumption to classify participants’ level of consumption as abstainers (no alcohol consumption history), moderate drinkers (up to 1 drink/day for women and up to 2 drinks/day for men), and heavy drinkers (>1 drink/day for women and >2 drinks/day for men).
Voltage Mapping and Catheter Ablation
After access to the LA using the Brockenborough needle and 8‐French Swartz sheath (SRO; St. Jude Medical, MN), detailed endocardial voltage mapping of the LA was performed with a circular decapolar catheter (PV 12, APT) before ablation and in sinus rhythm in all cases. Eighteen patients were excluded from the study because sinus rhythm could not be maintained during the mapping procedure. In case of presence of “false LVZ” resulting from inadequate contact between the circular catheter and LA tissue, any area showing abnormal voltage was reassessed with a 4‐mm irrigated ablation catheter (Therapy Cool Path Duo or Cool Flex; St. Jude Medical).
We use the semiquantitative method previously described to assess LA substrate remodeling.9 The LA was divided into 6 separate zones (Figure 1), which were the roof between the left superior pulmonary vein (LSPV) and right superior pulmonary vein (RSPV), anterior wall between roof and mitral valve annulus (MVA), posterior wall between roof and MVA, lateral wall between left atrial appendage (LAA), and MVA containing the ridge between LAA and LSPV, floor, and septum between right PV antrum and floor. Each zone involving LVZ was given 1 point. We examined every beat to exclude mechanically induced premature beats. As elsewhere reported, LVZ was defined as sites of >3 adjacent low‐voltage points (bipolar voltage amplitude <0.5 mV) and dense scar was defined as bipolar voltage amplitude <0.1 mV.3
Figure 1.
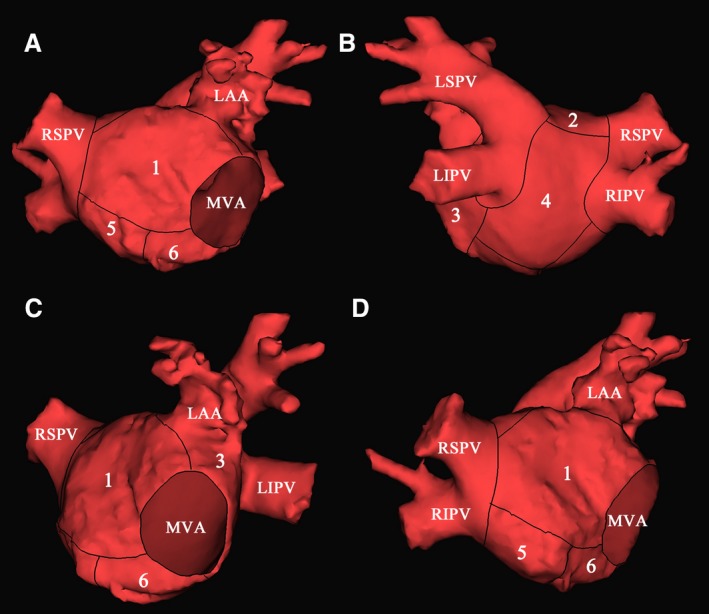
Segmentation of LA in AP (A), PA (B), LAO (C), and RAO (D) view. Numbers 1 to 6 indicate anterior wall, roof, lateral wall, posterior wall, septum, and floor, respectively. AP indicates anteroposterior; LA, left atrium; LAA, left atrial appendage; LAO, left anterior oblique; LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; MVA, mitral valve annulus; PA, posteroanterior; RAO, right anterior oblique; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein.
Intravenous heparin was administered after the trans‐septal puncture to maintain an activated clotting time of 300 to 350 seconds throughout the procedure. We used a 4‐mm irrigated ablation catheter with power settings ≤40 W at the posterior wall and ≤50 W elsewhere to perform CPVI, an upper temperature limit of 43°C, and a flow rate of 17 mL/min. 3D mapping navigation systems (EnSite Velocity, St. Paul, MN) were used to facilitate the ablation. CPVI was performed by sequential application of radiofrequency energy at the antrum of the pulmonary veins. The endpoint was isolation of the pulmonary veins with proof of both exit and entrance block. A pace‐and‐ablate approach previously described was also employed to produce durable PVI.10
Postablation Management and Follow‐up
All patients were monitored for 48 hours in the hospital after the procedure and treated with warfarin or dabigatran therapy for at least 3 months. Antiarrhythmic drugs were continued for 3 months after the procedure and was then stopped if no AF recurrence was found. All the patients were instructed to abstain from alcohol postablation.
After the 3‐month blanking period, subsequent follow‐up consisted of a clinical interview, electrocardiograms (ECGs), and 24‐hour Holter monitoring every 3 months for 1 year, and then every 6 months. In addition, the ECGs were recorded at the time of symptoms. AF recurrence was defined as symptomatic and/or asymptomatic episodes of AF/atrial flutter/atrial tachycardia lasting >30 seconds identified on the 12‐lead surface ECG or Holter monitoring.1
Statistical Analysis
Continuous variables were described as the mean±SD for normally distributed data and median (25% to 75% quartile) for non‐normally distributed data, and comparisons between groups were performed with Student t test (normally distributed data) or Kruskal–Wallis test (non‐normally distributed data). Categorical variables were described as counts and compared by chi‐square analysis. Survival curves were generated with the Kaplan–Meier analysis and compared by log rank tests. Binominal logistic regression was used to calculate the odds ratio (OR) and 95% confidence interval (CI) for the presence of LVZ, and Cox regression analysis was used to determine the independent predictors of AF recurrence, with a determination of hazard ratio (HR) and 95% CI for each variable in the model. Variables selected for testing in the multivariate analysis were those with P<0.05 in the univariate models. A receiver operating characteristic (ROC) analysis was used to determine the cut‐off value of daily alcohol consumption for predicting AF recurrence. Average alcohol consumption per day was treated as a continuous variable in logistic regression, and degree of alcohol consumption was treated as a categorical variable in Cox regression.
In addition, we used mediation analysis following the procedure described by Baron and Kenny,11 a method that uses 3 models to test for the statistical significance of a mediator effect. The first model was used to show that the independent variable was a significant predictor of the outcome variable; then, in the second model, we tested whether the independent variable was a significant predictor of the mediator. Finally, the third model, which contained both the independent and mediator variables entered simultaneously with the outcome variable, was used to verify the 2 conditions that must be met if a mediator effect is present: (1) The mediator is a significant predictor of the outcome variable and (2) the direct relationship of the independent variable to the outcome variable is less significant than it was in the first model (Figure 2).
Figure 2.
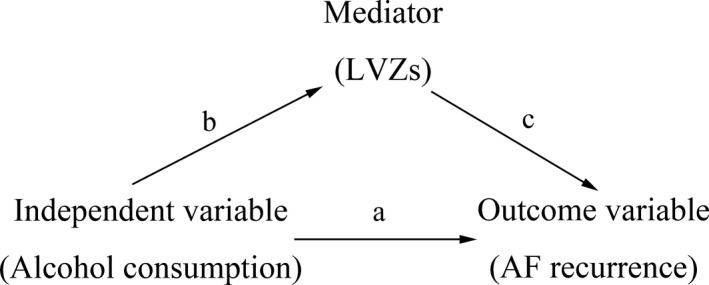
Statistical model of a mediator effect. AF indicates atrial fibrillation; LVZs, low‐voltage zones.
All tests were 2‐tailed, and a statistical significance was established at P<0.05. All analyses were performed using SPSS software (version 18.0; SPSS, Inc., Chicago, IL).
Results
Baseline Clinical and Procedural Characteristics of the Study Population
The studied population included 122 adults with a history of PAF, predominantly men (90 men; 73.8%) with a mean age of 55.4±9.4 years, among whom 70 patients (57.4%) were alcohol abstainers. In the remaining patients, 13 (10.6%) were moderate drinkers and 39 (32.0%) had a heavy drinking history (Table 1).
Table 1.
Baseline Characteristics of the Study Population
| Baseline Characteristics | Alcohol Abstainers (n=70) | Moderate Drinkers (n=13) | Heavy Drinkers (n=39) | P Value |
|---|---|---|---|---|
| Age, y | 57.0±10.1 | 52.5±9.3 | 53.6±8.0 | 0.117 |
| Male sex, n (%) | 40 (57.1) | 12 (92.3) | 38 (97.4) | <0.001 |
| AF duration, month | 42 (17.25–84) | 36 (24–90) | 60 (24–108) | 0.365 |
| Failed AADs | 1 (0, 2) | 1 (0, 1) | 1 (0, 2) | 0.520 |
| Propafenone, n (%) | 15 (21.4) | 2 (15.4) | 10 (25.6) | 0.718 |
| β blocker, n (%) | 26 (37.1) | 3 (23.1) | 10 (25.6) | 0.352 |
| Amiodarone, n (%) | 27 (38.6) | 3 (23.1) | 17 (43.6) | 0.421 |
| Sotalol, n (%) | 5 (7.1) | 2 (15.4) | 3 (7.7) | 0.655 |
| BMI, kg/m2 | 26.1±4.1 | 25.1±2.4 | 26.3±3.2 | 0.605 |
| Alcohol consumption, drink/day | 0 | 1.1 (0.7–1.3) | 5.6 (2.7–9.4) | <0.001 |
| CHADS2 score | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.546 |
| CHADS2–VASc score | 1 (0.75–2) | 0 (0–1) | 1 (0–1) | 0.006 |
| Echocardiography | ||||
| LAD, mm | 35.7±3.4 | 35.8±4.9 | 37.3±4.1 | 0.104 |
| LVED, mm | 46.7±3.6 | 48.0±4.3 | 48.4±4.5 | 0.095 |
| LVEF, % | 66.1±5.2 | 64.2±4.6 | 64.6±4.5 | 0.214 |
| Comorbidity | ||||
| Hypertension, n (%) | 29 (41.4) | 3 (23.1) | 17 (43.6) | 0.406 |
| Diabetes mellitus, n (%) | 6 (8.6) | 2 (15.4) | 4 (10.3) | 0.748 |
| CHD, n (%) | 6 (8.6) | 0 (0) | 2 (5.1) | 0.474 |
| SHD, n (%) | 2 (2.9) | 0 (0) | 1 (2.6) | 0.830 |
| LVI | 0 (0–1) | 1 (0–1.5) | 0 (0–2) | 0.036 |
| Roof low voltage, n (%) | 14 (20.0) | 6 (46.2) | 14 (35.9) | 0.063 |
| Lateral wall low voltage, n (%) | 6 (8.6) | 4 (30.8) | 10 (25.6) | 0.024 |
| Anterior wall low voltage, n (%) | 4 (5.7) | 0 (0) | 6 (15.4) | 0.112 |
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; BMI, body mass index; CHD, coronary heart disease; LAD, left atrial diameter; LVED, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVI, low‐voltage index; SHD, structural heart disease.
LVZ in Relationship With Alcohol Consumption
In total, LVZ was observed in 44 patients (36.1%), of whom the low‐voltage index (LVI) score was 1, 2, and 3 in 25 (56.8%), 14 (31.8%), and 5 (11.4%) patients, respectively. Roof, lateral wall, and anterior wall of LA were the most frequently affected areas, which were found in 34 (77.3%), 19 (43.2%), and 10 (22.7%) patients, respectively.
Compared with patients without LVZ, the daily alcohol consumption was significantly higher in patients with LVZ in LA (0.8 [0.0–4.9] drink/day vs 0 [0.0–2.7] drink/day; P=0.040). Subgroup analysis revealed that daily alcohol consumption was significantly higher in patients with LVZ in anterior wall (P=0.039), roof (P=0.030), lateral wall (P=0.002), and posterior wall (P=0.011) than that of patients without LVZ in the corresponding zone.
Significant difference was detected on LVI of abstainers and moderate and heavy drinkers (overall, P=0.020). Compared with alcohol abstainers, the percentage of patients with LVZ in lateral (P=0.033) and posterior wall (P=0.045) of LA was significantly higher in drinkers (the combination of moderate and heavy drinkers; Figure 3). In multivariate analysis, daily alcohol consumption and AF duration were independent predictors of the presence of LVZ, with an OR of 1.097 (95% CI, 1.001–1.203) and 1.009 (95% CI, 1.002–1.017), respectively (Table 2). For every drink of daily alcohol consumption, the probability of presence of LVZ increased by 9.7%.
Figure 3.
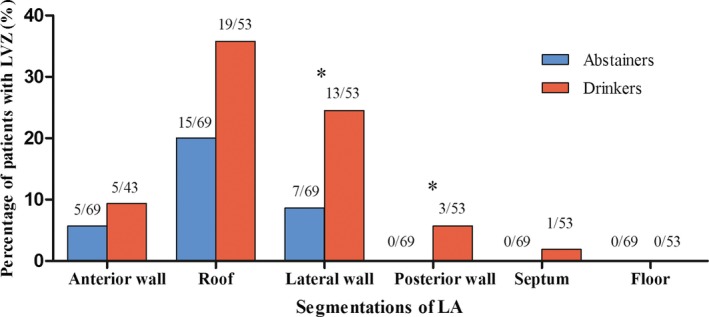
Distribution of LVZs in different segments of LA in alcohol abstainers and drinkers. Asterisk indicates comparison between groups with P<0.05. LA indicates left atrium; LVZs, low‐voltage zones.
Table 2.
Uni‐ and Multivariate Analyses for Predictors of LVZ
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| P Value | OR (95% CI) | P Value | OR (95% CI) | |
| Daily alcohol consumption | 0.046 | 1.096 (1.002–1.200) | 0.047 | 1.097 (1.001–1.203) |
| Age | 0.136 | 1.032 (0.990–1.075) | ||
| Sex | 0.844 | 1.087 (0.471–2.508) | ||
| AF duration | 0.007 | 1.010 (1.003–1.017) | 0.004 | 1.009 (1.002–1.017) |
| Failed AADs | 0.389 | 1.227 (0.771–1.953) | ||
| BMI, kg/m2 | 0.325 | 0.948 (0.853–1.054) | ||
| CHADS2 score | 0.519 | 0.871 (0.574–1.324) | ||
| CHADS2‐VASc score | 0.828 | 1.040 (0.729–1.483) | ||
| LAD | 0.883 | 0.993 (0.900–1.095) | ||
AADs indicates antiarrhythmic drugs; AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; LAD, left atrial diameter; LVZ, low‐voltage zone; OR, odds ratio.
Predictors for Long‐Term Outcome After CPVI
During a mean follow‐up of 20.9±5.9 months (12–30 months), 10 patients were lost to follow‐up. In the remaining 112 patients, 40 (35.7%) experienced AF recurrence after a single CPVI procedure.
The success rate was 81.3%, 69.2%, and 35.1% in alcohol abstainers, moderate drinkers, and heavy drinkers, respectively (overall log rank, P<0.001). Further analysis showed that the success rate of alcohol abstainers was slightly higher than that of moderate drinkers (log rank, P=0.356), whereas the success rate of heavy drinkers was significantly lower than that of moderate drinkers (log rank, P=0.032), which indicated that the effect of alcohol consumption on the ablation outcome of PAF may be dose dependent (Figure 4A). Additionally, compared to patients with LVZ, the success rate was significantly higher in patients without LVZ (83.6% vs 31.7%; log rank, P<0.001; Figure 4B). As was shown in the ROC curve, the optimal cut‐off value for daily alcohol consumption displaying the best predictive value was 0.74 drink/day (sensitivity=71.1% and specificity=75.7%; area under the curve=0.753; Figure 5).
Figure 4.
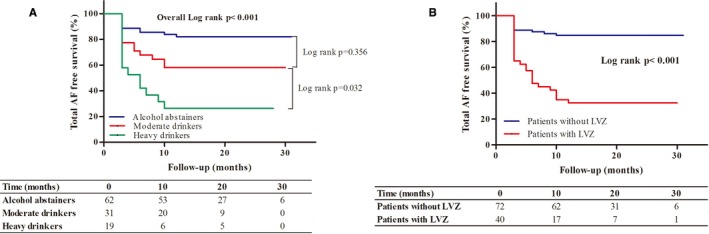
Kaplan–Meier curves for AF recurrence. A, AF recurrence in abstainers and moderate and heavy drinkers. B, AF recurrence in patients without and with LVZs. AF indicates atrial fibrillation; LVZ, low‐voltage zone.
Figure 5.
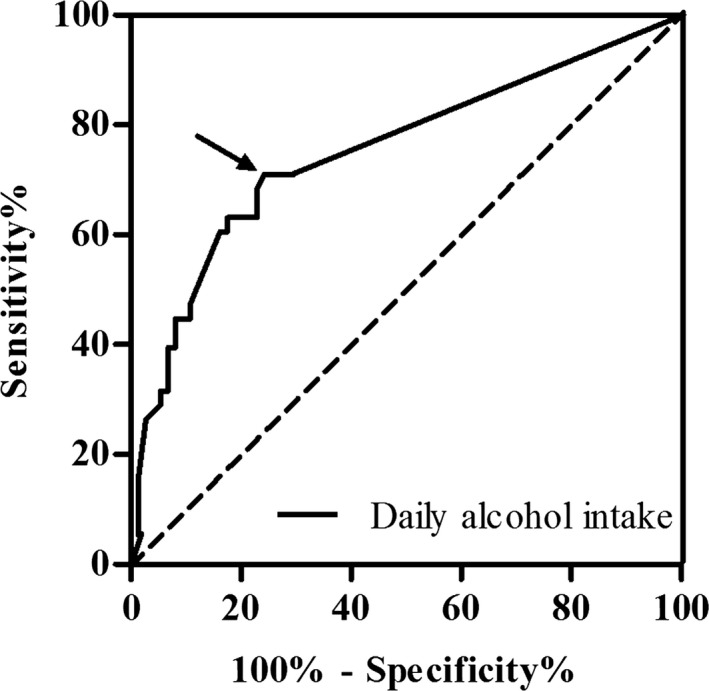
ROC curve of daily alcohol consumption for predicting 21‐month AF recurrence. Arrow shows the optimal cut‐off value for daily alcohol consumption. AF indicates atrial fibrillation; ROC, receiver operating characteristic.
In multivariate analysis, adjusted for LVI, LAD, and AF duration, alcohol consumption independently predicted long‐term outcomes after CPVI procedure in patients with PAF (HR, 1.579; 95% CI, 1.085–2.298; P=0.017). For every degree of alcohol consumption, the risk of AF recurrence increased with 58%. In addition, LVI and LAD were independent predictors of AF recurrence (HR, 2.188; 95% CI, 1.582–3.026; P<0.001; HR, 1.130; 95% CI, 1.036–1.233; P=0.006, respectively; Table 3).
Table 3.
Uni‐ and Multivariate Analyses for 21‐Month AF Recurrence
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| P Value | HR (95% CI) | P Value | HR (95% CI) | |
| Age | 0.139 | 1.026 (0.992–1.061) | ||
| Sex | 0.807 | 0.912 (0.434–1.915) | ||
| Alcohol consumption | <0.001 | 2.272 (1.556–3.318) | 0.017 | 1.579 (1.085–2.298) |
| LVI | <0.001 | 2.416 (1.797–3.248) | <0.001 | 2.188 (1.582–3.026) |
| BMI | 0.693 | 1.017 (0.936–1.104) | ||
| Failed AADs | 0.110 | 1.367 (0.931–2.008) | ||
| AF duration | 0.024 | 1.005 (1.001–1.009) | 0.189 | 1.003 (0.998–1.008) |
| CHADS2 score | 0.519 | 0.871 (0.574–1.324) | ||
| CHADS2‐VASc score | 0.913 | 0.984 (0.735–1.317) | ||
| LAD | 0.029 | 1.098 (1.010–1.195) | 0.006 | 1.130 (1.036–1.233) |
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; HR, hazard ratio; LAD, left atrial diameter; LVI, low‐voltage index.
Mediation Analysis
Table 4 shows the 3 models used to test the possible mediation effect of the presence of LVZs on the association between the alcohol consumption and the ablation outcomes after the CPVI procedure. Potential confounders, including age, sex, BMI, LAD, AF duration, and CHADS2 score, have been adjusted for in all 3 models. The first model demonstrates that alcohol consumption is significantly associated with AF recurrence. Moreover, the second model demonstrates that alcohol consumption is significantly associated with the presence of LVZs. Finally, the third model shows that when the variables “alcohol consumption” and “presence of LVZs” are entered simultaneously in the model to predict the outcome “AF recurrence,” the 2 variables are both independent predictors for AF recurrence, which confirms the partial mediating effect of the presence of LVZs.
Table 4.
Statistical Tests for a Mediator Effect of “LVZs” in the Association Between the Main Exposure “Alcohol Consumption” and the Outcome “AF Recurrence”
| Variable | Presence of LVZs | AF Recurrence | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | HR (95% CI) | P Value | |
| The first model | ||||
| Alcohol consumption | — | — | 1.105 (1.044–1.169) | 0.001 |
| The second model | ||||
| Alcohol consumption | 1.114 (1.007–1.232) | 0.036 | — | — |
| The third model | ||||
| Alcohol consumption | — | — | 1.074 (1.013–1.138) | 0.016 |
| The presence of LVZs | — | — | 4.858 (2.306–10.236) | <0.001 |
In the first model, the main exposure variable is a significant predictor of the outcome variable; in the second model, the main exposure variable is a significant predictor of mediator; in the third model, when the main exposure and the mediator are entered simultaneously in the model to predict the outcome, the association between the main exposure and the outcome is less significant than it was in the first model. All of the regression models have been adjusted for potential confounders including age, sex, BMI, LAD, AF duration, and CHADS2 score. AF indicates atrial fibrillation; HR, hazard ratio; LVZs, low voltage zones; OR, odds ratio.
Discussion
This study has shown: (1) LVZs were identified in 36.1% of patients with paroxysmal atrial fibrillation; (2) alcohol consumption was correlated with the presence as well as the extent of adverse atrial remodeling; and (3) alcohol consumption was associated with unfavorable ablation outcomes after CPVI procedure, especially in heavy drinkers. To the best of our knowledge, this is the first report focusing on the impact of alcohol consumption on atrial substrate remodeling and ablation outcomes.
Alcohol Consumption and Substrate Remodeling
It has long been recognized that atrial remodeling, which is characterized by fibrosis and altered electrophysiological characteristics, plays a fundamental role in the pathogenesis of AF.12 Combining the DE‐MRI with EAM, Oakes et al. elegantly demonstrated the close relationship between LVZ and fibrotic tissue.3 Furthermore, several clinical factors, including age, gender, AF type, and metabolic syndrome, have been demonstrated to independently predict the presence of LVZ in EAM.9, 13 However, whether alcohol consumption could influence the presence of LVZ remains unknown. In this study, we have demonstrated that daily alcohol consumption was an independent predictor of the presence of LVZ with an OR of 1.097 (95% CI, 1.001–1.203). Moreover, the LVI was significantly higher in heavy drinkers. Therefore, chronic alcohol consumption may prompt adverse atrial remodeling, and there may also be a dose‐response relationship.
Although numerous studies have already shown that acute alcohol consumption, especially binge drinking, predisposes patients to AF, which has been attributed to a hyperadrenergic state, impaired vagal tone, direct toxic effects of metabolite acetaldehyde, or free radicals from ethanol enzyme reaction,5, 6, 14 limited data exist on the how chronic alcohol consumption promotes atrial remodeling. Cardiac electrophysiological studies indicated a deleterious effect of alcohol consumption, whether acute or chronic, on atrial electrophysiological characteristics, including the prolongation of intra‐ and interatrial conduction and reduction in the atrial effective refractory period.15, 16, 17 Nevertheless, the relationship between long‐term alcohol consumption and atrial fibrosis have not been systematically studied. It has long been recognized that ethanol‐induced cell injury and fibrosis have played a central role in the pathogenesis of alcohol liver disease,18 prompting further investigation into whether alcohol toxicity may serve as the underlying mechanism for atrial fibrosis.
Alcohol Consumption and Long‐Term Outcomes After CPVI
Since the fundamental study by Haïssaguerre et al.,19 PV‐targeted strategy has been the cornerstone in AF ablation, especially for PAF.1 However, the long‐term success rate remains unsatisfactory. Recently, several clinical factors and biomarkers have been proposed for predicting AF recurrence, including CHADS2, CHADS2‐VASc scores, metabolic syndrome, high‐sensitivity C‐reactive protein, and big endothelin‐1.9, 20, 21, 22 However, the impact of alcohol consumption, a highly prevalent phenomenon, on ablation outcomes has not yet been investigated.
This study has shown that alcohol consumption was associated with unfavorable long‐term outcomes after CPVI, especially in heavy drinkers (success rate of 35.1% in 21‐month follow‐up). Moreover, alcohol consumption was an independent predictor for AF recurrence with an HR of 1.579 (95% CI, 1.085–2.298). We highly suspect that it is the underlying substrate remodeling caused by alcohol toxicity that plays this role. Indeed, by mediation analysis, we confirmed that the presence of LVZs act as a mediator in the effect of alcohol consumption to ablation outcomes. Thus, exclusive CPVI may not be sufficient to maintain sinus rhythm in the long‐term and further substrate modification may have to be considered in heavy drinkers.
Recently, Rolf et al. proposed a novel LVZ‐guided individualized ablation strategy.13 In accord with our observation, patients with LVZ undergoing pure PVI had a higher recurrence rate, regardless of AF type. Moreover, further substrate modification improved the ablation outcomes to a level comparable with those without LVZ undergoing pure PVI.
Prevalence and Distribution of LVZs in PAF
Although focal triggering was believed to be the predominant mechanism for PAF, substrate remodeling, surrogated by LVZs, was also demonstrated to be present in many patients and associated with poor ablation outcome.3 Limited studies have characterized the prevalence and spatial distribution of LVZs in PAF, with high variability.3, 13, 23, 24 With computed tomography scanning and EAM, Hori et al. revealed LVZs in almost 89% of patients. Moreover, these LVZs were often located in areas such as anterior wall, posterior wall, and left PV antrum where aorta or vertebrae were adjacent.23 In a study by Rolf et al., LVZs were found in 9.7% of patients, with the most affected areas being anterior, septal, and posterior walls and roof. In this study, LVZs were found in 36.1% of patients with PAF, which is in accord with the observation by Watanabe et al.,24 who identified LVZs in 23 PAF patients (44.2%). Furthermore, we found the most frequently affected zones were roof and anterior and lateral walls. We believe that the discrepancies in LVZs’ prevalence and distribution lie in different sample sizes and different approaches to segmentation of LA. The ridge between LAA and LSPV was assigned to the anterior wall by Rolf et al., whereas the same region was included in the lateral wall in our study. Likewise, the area adjacent to the ascending aorta was assigned to the septum by Rolf et al., but to the anterior wall by Hori et al.23 and by us.
In addition, with the EnSite Array mapping system, our previous study showed that the ridge between LSPV and LAA and the roof between LSPV and RSPV were the most frequent sites where the fibrillatory wave front propagated slowly in patients with PAF and had the highest priority in an individualized ablation strategy.25 These sites were exactly where the LVZs were often distributed, which suggests that substrate remodeling starts in these specific areas.
Limitations
First, this is a single‐center observational study. Second, most female patients (93.8%) in our study are abstainers, which implies that our conclusion is not tenable for female patients. This is related both to traditional Chinese culture and our limited sample size; thus, a further large‐scale study to confirm the influence of alcohol consumption on atrial remodeling and ablation outcomes in Chinese women is necessary. Third, all patients undergo 24‐hours Holter monitoring, rather than implanted loop recorder during follow‐up, which may underestimate the recurrence rates.
Conclusions
Alcohol consumption is prevalent among candidates for PAF ablation, which is correlated with the presence as well as extent of adverse atrial remodeling. Furthermore, alcohol consumption is associated with poor ablation outcome after CPVI, especially in heavy drinkers. These observations may help electrophysiologists to identify patients at high risk for recurrence and plan appropriate ablation strategies for better rhythm control in these patients.
Disclosures
None.
Acknowledgments
The authors gratefully thank Professor Richard Sutton of the National Heart & Lung Institute, Imperial College, London, UK for help in preparation of this article.
(J Am Heart Assoc. 2015;4:e002349 doi: 10.1161/JAHA.115.002349)
References
- 1. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9:632–696.e621. [DOI] [PubMed] [Google Scholar]
- 2. Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, Vidal B, Arriagada G, Mendez F, Matiello M, Molina I, Brugada J. Pre‐procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–841. [DOI] [PubMed] [Google Scholar]
- 3. Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJ, Rao SN, DiBella EV, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed‐enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R, Burgon NS, Greene T, Kim D, Dibella EV, Parker D, Macleod RS, Marrouche NF. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, Anasako Y, Nishigaki Y, Yachi Y, Iida KT, Ohashi Y, Yamada N, Sone H. Alcohol consumption and risk of atrial fibrillation: a meta‐analysis. J Am Coll Cardiol. 2011;57:427–436. [DOI] [PubMed] [Google Scholar]
- 6. Djousse L, Levy D, Benjamin EJ, Blease SJ, Russ A, Larson MG, Massaro JM, D'Agostino RB, Wolf PA, Ellison RC. Long‐term alcohol consumption and the risk of atrial fibrillation in the Framingham Study. Am J Cardiol. 2004;93:710–713. [DOI] [PubMed] [Google Scholar]
- 7. Conen D, Tedrow UB, Cook NR, Moorthy MV, Buring JE, Albert CM. Alcohol consumption and risk of incident atrial fibrillation in women. JAMA. 2008;300:2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. National Institute of Alcohol Abuse and Alcoholism . The Physicians’ Guide to Helping Patients With Alcohol Problems. Washington, DC: U.S. Department of Health and Human Services, National Institutesof Health; 1995. [Google Scholar]
- 9. Dinov B, Kosiuk J, Kircher S, Bollmann A, Acou WJ, Arya A, Hindricks G, Rolf S. Impact of metabolic syndrome on left atrial electroanatomical remodeling and outcomes after radiofrequency ablation of nonvalvular atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:483–489. [DOI] [PubMed] [Google Scholar]
- 10. Eitel C, Hindricks G, Sommer P, Gaspar T, Kircher S, Wetzel U, Dagres N, Esato M, Bollmann A, Husser D, Hilbert S, Zaker‐Shahrak R, Arya A, Piorkowski C. Circumferential pulmonary vein isolation and linear left atrial ablation as a single‐catheter technique to achieve bidirectional conduction block: the pace‐and‐ablate approach. Heart Rhythm. 2010;7:157–164. [DOI] [PubMed] [Google Scholar]
- 11. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 12. Verma A, Wazni OM, Marrouche NF, Martin DO, Kilicaslan F, Minor S, Schweikert RA, Saliba W, Cummings J, Burkhardt JD, Bhargava M, Belden WA, Abdul‐Karim A, Natale A. Pre‐existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: an independent predictor of procedural failure. J Am Coll Cardiol. 2005;45:285–292. [DOI] [PubMed] [Google Scholar]
- 13. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, Hindricks G, Piorkowski C. Tailored atrial substrate modification based on low‐voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;7:825–833. [DOI] [PubMed] [Google Scholar]
- 14. Balbao CE, de Paola AA, Fenelon G. Effects of alcohol on atrial fibrillation: myths and truths. Ther Adv Cardiovasc Dis. 2009;3:53–63. [DOI] [PubMed] [Google Scholar]
- 15. Steinbigler P, Haberl R, Konig B, Steinbeck G. P‐wave signal averaging identifies patients prone to alcohol‐induced paroxysmal atrial fibrillation. Am J Cardiol. 2003;91:491–494. [DOI] [PubMed] [Google Scholar]
- 16. Greenspon AJ, Schaal SF. The “holiday heart”: electrophysiologic studies of alcohol effects in alcoholics. Ann Intern Med. 1983;98:135–139. [DOI] [PubMed] [Google Scholar]
- 17. Gould L, Reddy CV, Becker W, Oh KC, Kim SG. Electrophysiologic properties of alcohol in man. J Electrocardiol. 1978;11:219–226. [DOI] [PubMed] [Google Scholar]
- 18. Louvet A, Mathurin P. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol. 2015;12:231–242. [DOI] [PubMed] [Google Scholar]
- 19. Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. [DOI] [PubMed] [Google Scholar]
- 20. Chao TF, Ambrose K, Tsao HM, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Hartono B, Chang HY, Wu TJ, Chen SA. Relationship between the CHADS(2) score and risk of very late recurrences after catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2012;9:1185–1191. [DOI] [PubMed] [Google Scholar]
- 21. Liu J, Fang PH, Dibs S, Hou Y, Li XF, Zhang S. High‐sensitivity C‐reactive protein as a predictor of atrial fibrillation recurrence after primary circumferential pulmonary vein isolation. Pacing Clin Electrophysiol. 2011;34:398–406. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Liu J, Fang P, Lei S, Li X, Hou Y, Zhang S. Big endothelin‐1 as a predictor of atrial fibrillation recurrence after primary ablation only in patients with paroxysmal atrial fibrillation. Herz. 2012;37:919–925. [DOI] [PubMed] [Google Scholar]
- 23. Hori Y, Nakahara S, Tsukada N, Nakagawa A, Hayashi A, Komatsu T, Kobayashi S, Sakai Y, Taguchi I. The influence of the external structures in atrial fibrillation patients: relationship to focal low voltage areas in the left atrium. Int J Cardiol. 2015;181:225–231. [DOI] [PubMed] [Google Scholar]
- 24. Watanabe Y, Nakano Y, Hidaka T, Oda N, Kajihara K, Tokuyama T, Uchimura Y, Sairaku A, Motoda C, Fujiwara M, Kawazoe H, Matsumura H, Kihara Y. Mechanical and substrate abnormalities of the left atrium assessed by 3‐dimensional speckle‐tracking echocardiography and electroanatomic mapping system in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2015;12:490–497. [DOI] [PubMed] [Google Scholar]
- 25. Yao Y, Zheng L, Zhang S, He DS, Zhang K, Tang M, Chen K, Pu J, Wang F, Chen X. Stepwise linear approach to catheter ablation of atrial fibrillation. Heart Rhythm. 2007;4:1497–1504. [DOI] [PubMed] [Google Scholar]


