Abstract
Background
Observational studies evaluating the possible interaction between proton pump inhibitors (PPIs) and clopidogrel have shown mixed results. We conducted a systematic review comparing the safety of individual PPIs in patients with coronary artery disease taking clopidogrel.
Methods and Results
Studies performed from January 1995 to December 2013 were screened for inclusion. Data were extracted, and study quality was graded for 34 potential studies. For those studies in which follow‐up period, outcomes, and multivariable adjustment were comparable, meta‐analysis was performed.
The adjusted odds or hazard ratios for the composite of cardiovascular or all‐cause death, myocardial infarction, and stroke at 1 year were reported in 6 observational studies with data on individual PPIs. Random‐effects meta‐analyses of the 6 studies revealed an increased risk for adverse cardiovascular events for those taking pantoprazole (hazard ratio 1.38; 95% CI 1.12–1.70), lansoprazole (hazard ratio 1.29; 95% CI 1.09–1.52), or esomeprazole (hazard ratio 1.27; 95% CI 1.02–1.58) compared with patients on no PPI. This association was not significant for omeprazole (hazard ratio 1.16; 95% CI 0.93–1.44). Sensitivity analyses for the coronary artery disease population (acute coronary syndrome versus mixed) and exclusion of a single study due to heterogeneity of reported results did not have significant influence on the effect estimates for any PPIs.
Conclusions
Several frequently used PPIs previously thought to be safe for concomitant use with clopidogrel were associated with greater risk of adverse cardiovascular events. Although the data are observational, they highlight the need for randomized controlled trials to evaluate the safety of concomitant PPI and clopidogrel use in patients with coronary artery disease.
Keywords: adverse cardiovascular outcomes, clopidogrel, coronary artery disease, medication interaction, proton pump inhibitor
Introduction
In patients with acute coronary syndromes (ACS), dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor improves cardiovascular (CV) outcomes and is recommended by national guidelines for 1 year after initial hospitalization.1, 2 Clopidogrel, now available in generic form, remains the most widely used P2Y12 inhibitor taken by post‐ACS outpatients. Clopidogrel is a prodrug that depends on cytochrome P450, or CYP, liver enzymes to metabolize it into active form. Due to the increased risk of gastrointestinal (GI) bleeding associated with clopidogrel and DAPT, many post‐ACS patients are also started on a proton pump inhibitor (PPI) or H2 receptor antagonist. PPI medications (the most frequently prescribed drugs to reduce the risk of GI bleeding) also interact with the CYP liver enzymes and thus may inhibit the conversion of clopidogrel to its active metabolite and potentially alter its efficacy. Several observational studies have been completed to evaluate possible medication interaction between PPI and DAPT and its association with CV outcomes.3, 4, 5, 6 These studies have had mixed results, with some showing a potential interaction between clopidogrel and PPIs that is associated with worse CV outcomes and others showing no association with outcomes. Very few randomized data are available to answer this question, and a recently meta‐analysis of this topic concluded that observational and randomized data on PPIs have divergent results.7
Despite conflicting data and possible equipoise in the field, the US Food and Drug Administration issued a black box warning about the use of clopidogrel concomitant with PPI agents, specifically, omeprazole and esomeprazole, for which there is the most experience; in the case of omeprazole, data are from both observational and randomized, controlled studies. Uncertainty remains about extending this association to other PPIs because there seems to be variation in efficacy among drugs in this class. Each PPI has a differential effect on CYP metabolism and thus may have incongruous effects on clopidogrel metabolism and subsequent CV outcomes. Recent meta‐analyses have grouped all PPIs together, likely for greater power, to evaluate the relationship among PPIs, clopidogrel, and CV outcomes8; however, based on pharmacology, there may be differential interactions between individual PPIs and clopidogrel. The aim of this study is to determine the relationship between individual PPI medications and adverse CV events in patients with both acute and stable coronary artery disease (CAD) taking clopidogrel.
Methods
A systematic review was performed to evaluate the current literature and to collect and synthesize available data. This analysis was part of a broader systematic review assessing the effectiveness and safety of antiplatelet and anticoagulant medications used to treat patients with unstable angina and non–ST‐segment elevation myocardial infarction (MI).9 The broader systemic review was funded from the Agency for Healthcare Research and Quality. The National Library of Medicine medical subject headings keyword nomenclature was used. The search areas for inclusion in this study were PubMed, Embase, and the Cochrane Database of Systematic Reviews from January 1, 1995, to December 30, 2013. The gray literature areas of research, including registries, conference abstracts, and completed studies, were also searched for relevant articles to include in our analysis (ClinicalTrials.gov, World Health Organization, International Clinical Trials Registry Platform Search Portal, ProQuest Conference Papers index).
Study Population
Study designs included were cohort (prospective and retrospective), case–control, and cross‐sectional. There were very few randomized controlled trials for data, and the inclusion of their data would not have been reasonable for statistical and conceptual reasons. Studies were included if they were English‐language studies comparing the concomitant use of clopidogrel and PPIs in the postdischarge treatment of patients with ACS or those undergoing percutaneous coronary intervention for CV outcomes and follow‐up for at least 1 year. The studies must also have analyzed individual PPIs separately, including but not limited to omeprazole, esomeprazole, pantoprazole, lansoprazole, and rabeprazole. Studies were excluded if they did not provide outcomes analyses for individual PPIs or if they had follow‐up for <12 months or if they did not provide multivariable modeling with adjusted odds ratios (ORs) or hazard ratios (HRs). Outcomes of interest included the composite CV outcomes of CV and/or all‐cause mortality, nonfatal MI, stroke, rehospitalization, and revascularization.
Study Selection
The studies were graded on the quality of data provided. Articles were independently reviewed by 2 investigators (C.M. and J.W.), and a third investigator served as an arbitrator in cases of disagreement (R.D.). Studies were included based on the aforementioned inclusion criteria and then were examined for data abstraction.
Statistical Methods
Data abstraction
Data abstraction forms and evidence tables were created to collect and summarize source data. The tables contained information on the study population, the individual PPI agents included in the study, outcomes measured, time of follow‐up, and unadjusted and adjusted point estimates. The data were initially abstracted by a single investigator, with review for completeness and errors performed by a second investigator.
Data synthesis
The primary literature was summarized by abstraction of ORs and HRs reported by the authors. We expected that the relationship between omeprazole and adverse CV events could be confounded by multiple factors, many of which were risk factors for both GI bleeding risk and CV risk. These included age, sex, weight, renal function, and other medical comorbidities including previous GI bleeding. These factors were typically incorporated into multivariable logistic regression or propensity modeling for the studies that we included in our analyses. If available, we evaluated each study for the inclusion of information on the use of appropriate concomitant secondary prevention medications for CAD.
Both unadjusted and adjusted ORs and HRs were initially considered for the analysis. Random‐effects modeling was used. Meta‐analyses of direct comparison were performed if outcomes and follow‐up periods were found to be comparable. An assessment of publication bias by constructing a funnel plot of samples, plotting study sample size versus reported ORs or HRs (log scale), was planned if the number of studies were sufficient (ie >10). Sensitivity analyses were performed including tests for possible heterogeneous effects (Q statistic) if only ACS patients were included versus a mixed (stable and ACS) population. If any 1 study was found to have disparate results, a “one‐out” sensitivity analysis was performed to determine whether there was any significant change in the results. All statistical analyses of the aggregate, deidentified data were performed by the Duke Clinical Research Institute using Comprehensive Meta‐Analysis version 2 (Biostat).
Results
On searching the literature, 36 potential studies were initially identified for further evaluation. After application of the aforementioned exclusion criteria (Figure 1), 2 studies were excluded as duplicates, and 4 randomized controlled trials were excluded. In addition, 24 studies were excluded after being found to lack data on 1‐year follow‐up and outcomes for individual PPIs. The final study sample included 6 observational studies, 5 of good quality and 1 of fair quality, that included >41 000 patients4, 5, 6, 10, 11, 12 (Table 1). In the specified studies within our sample, data on outcomes were available for omeprazole, esomeprazole, pantoprazole, lansoprazole, and rabeprazole. Due to data constraints, rabeprazole was excluded from further analyses because insufficient data were available to synthesize. We also found that the use of concomitant medications was excellent (>75%) including aspirin, beta blockers, and statins and more variable use of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers in studies that reported these data (all studies except that by Kreutz et al10).
Figure 1.
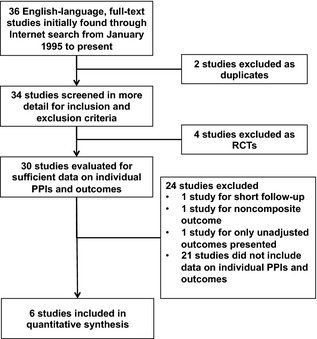
Study selection sample flow diagram depicting the results of our literature search and resulting exclusion of studies from quantitative analyses. PPIs indicates proton pump inhibitors; RCT, randomized controlled trial
Table 1.
Study Characteristics
| Study; Population; Quality | Total Sample Sizea PPI vs No PPI Samples | PPIs, n (%) | Outcomes/Time Period | Number of Outcomes | Covariates for Adjusted OR/HR |
|---|---|---|---|---|---|
| Kreutz, 201010; general PCI population; good |
Total: 16 690 PPI: 6828 No PPI: 9862 |
Omeprazole, 2307 (34%) Pantoprazole, 1653 (24%) Lansoprazole, 785 (11%) Esomeprazole, 3257 (48%) |
Composite 1‐year CV mortality, nonfatal MI, stroke, revascularization |
Total: 3476; No PPI: 1766; PPI: 1710 |
Age, sex, hospitalization, stent, DM, HTN, CHF, CRI, HL, Meds |
| Ray, 20105; mixed PCI population; good |
Total: 20 596 PPI: 7226 No PPI: 8995 |
Omeprazole, 683 (9%) Pantoprazole, 4708 (62%) Lansoprazole Esomeprazole Rabeprazole |
Composite 1‐year CV mortality, nonfatal MI, stroke, sudden cardiac death |
Total: 1041; No PPI: 580; PPI: 461 |
Age, sex, health insurance, CABG, PCI, meds, CHF, stroke, PAD |
| Simon, 201111; FAST‐MI, all MI population; good |
Total: 2744 PPI: 1449 No PPI: 900 |
Omeprazole, 993 (69%) Pantoprazole, 99 (7%) Lansoprazole, 46 (3%) Esomeprazole, 311 (22%) |
Composite 1‐year total mortality, nonfatal MI, stroke |
Total: 225; No PPI: 100; PPI: 125 |
GRACE score, sex, smoking, HL, HTN, DM |
| Van Boxel, 201012; mixed CAD population; fair |
Total: 18 139 PPI: 5734 No PPI: 12 405 |
Omeprazole, 1826 (32%) Pantoprazole, 2618 (46%) Esomeprazole, 1092 (19%) Rabeprazole, 133 (2%) |
Composite 1‐year total mortality, nonfatal MI, stroke, UA |
Total: 1584 No PPI: 830; PPI: 754 |
Age, Gender, Meds, CHF, MI, CVD, COPD, CRI, DM, PUD |
| Charlot, 20106; all MI population; good |
Total: 56 406 PPI: 6753 No PPI: 17 949 |
Omeprazole, 2717 (17%) Pantoprazole, 4698 (30%) Lansoprazole, 2798 (18%) Esomeprazole. 5316 (34%) |
Composite 1‐year CV mortality, nonfatal MI, stroke |
Total: 2564; No PPI: 1506; PPI: 1058 |
Age, gender, income, shock, DM, PUD, PCI, pulmonary edema, CVD, cancer, arrhythmia, CRI, ARF, meds |
| O'Donoghue 20094; TRITON‐TIMI 38, ACS population; good |
Total: 13 608 PPI: 4529 No PPI: 9079 |
Omeprazole, 1675 (37%) Pantoprazole, 1844 (41%) Lansoprazole, 441 (10%) Esomeprazole, 613 (14%) |
Composite 1‐year CV mortality, MI, stroke |
Total: 781; No PPI: 526; PPI: 255 |
Age, sex, ethnicity, HTN, HL, DM, Tob, index event, previous MI, CABG, CVD, PAD, CrCl, DES, Meds, BMI, Hgb, BP, heart rate |
ACS indicates acute coronary syndromes; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; CRI, chronic renal insufficiency; CV, cardiovascular; CVD, cerebrovascular disease; DES, drug eluting stent; DM, diabetes mellitus; Hgb, hemoglobin; HR, hazard ratio; HTN, hypertension; meds, concomitant medications; MI, myocardial infarction; OR, odds ratio; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention; PPI, proton pump inhibitor; PUD, peptic ulcer disease; UA, unstable angina.
For each study, the sample size shown is for patients also taking clopidogrel.
Omeprazole
Six observational studies including 10 201 patients assessed the effect of omeprazole when added to DAPT in both stable CAD and postdischarge ACS patients for the composite end point of CV or all‐cause mortality, nonfatal MI, and stroke at 1 year.4, 5, 6, 10, 11, 12 The result was an estimated HR of 1.16 (95% CI 0.93–1.44) (Figure 2A). There was evidence of extreme heterogeneity (Q value 28.70 for 5 df, P<0.001). The I2 value was 82.58.
Figure 2.
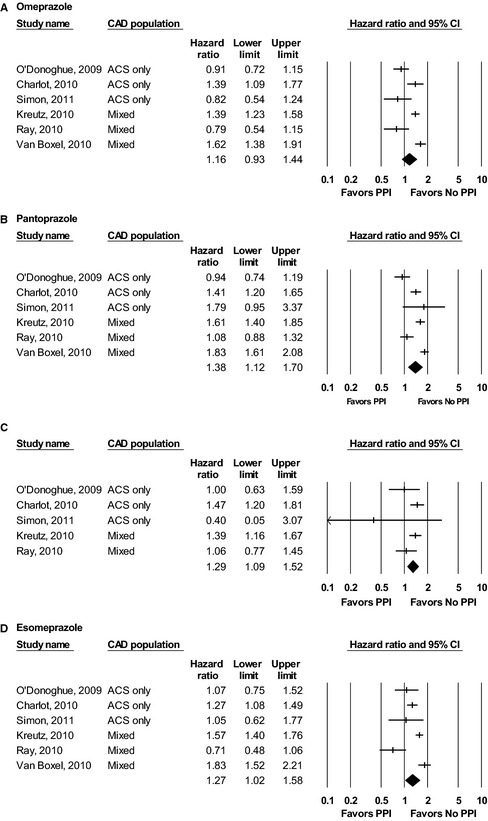
Results of individual PPI meta‐analyses across included studies: (A) omeprazole, (B) pantoprazole, (C) lansoprazole, (D) esomeprazole. ACS indicates acute coronary syndromes; CAD, coronary artery disease; PPI, proton pump inhibitor.
Pantoprazole
Six observational studies including 15 620 patients assessed the effect of pantoprazole when added to DAPT in both stable CAD and postdischarge ACS patients for the composite end point of CV or all‐cause mortality, nonfatal MI, and stroke at 1 year.4, 5, 6, 10, 11, 12 The result was an estimated HR of 1.38 (95% CI 1.12–1.70) (Figure 2B). There was evidence of extreme heterogeneity (Q value 36.50 for 5 df, P<0.001). The I2 value was 86.30.
Lansoprazole
Five observational studies assessed the effect of lansoprazole when added to DAPT in 4681 stable and post‐ACS CAD patients for the composite end point of CV or all‐cause mortality, nonfatal MI, and stroke at 1 year.4, 5, 6, 10, 11 The result was an estimated HR of 1.29 (95% CI 1.09–1.52) (Figure 2C). There was no evidence of significant heterogeneity (Q value 5.89 for 4 df, P=0.208). The I2 value was 32.07.
Esomeprazole
Six observational studies including 11 200 patients assessed the effect of esomeprazole when added to DAPT in both stable CAD and postdischarge ACS patients for the composite end point of CV or all‐cause mortality, nonfatal MI, and stroke at 1 year.4, 5, 6, 10, 11, 12 The result was an estimated HR of 1.27 (95% CI 1.02–1.58) (Figure 2D). There was evidence of extreme heterogeneity (Q value 27.28 for 5 df, P<0.001). The I2 value was 81.67.
Sensitivity Analyses
Given the heterogeneity present in our results, we performed several sensitivity analyses. To explore potential heterogeneity within the study sample, we divided studies into groups: ACS‐only versus mixed or stable CAD populations. As seen in Table 2, there were no significant changes to the resultant summary HRs (with wider CIs) in either the ACS‐only or mixed population groups for any of the individual PPIs.
Table 2.
Sensitivity Analyses based upon CAD Population for Individual PPIs
| Drug | CAD Population | Studies (n) | Effect Size (95% CI) | P Value for Heterogeneity |
|---|---|---|---|---|
| Omeprazole | ACS only | 3 | 1.04 (0.76–1.41) | 0.31 |
| Mixed | 3 | 1.28 (0.97–1.71) | ||
| Pantoprazole | ACS only | 3 | 1.29 (0.89–1.77) | 0.45 |
| Mixed | 3 | 1.49 (1.14–1.95) | ||
| Lansoprazole | ACS only | 3 | 1.26 (0.91–1.74) | 0.96 |
| Mixed | 2 | 1.24 (0.92–1.68) | ||
| Esomeprazole | ACS only | 3 | 1.15 (0.83–1.60) | 0.57 |
| Mixed | 3 | 1.37 (1.01–1.85) |
ACS indicates acute coronary syndromes; CAD, coronary artery disease.
To further explore the effect of a possible outlier study, we repeated the entire analysis excluding the study by Simon et al due to the use of ORs and the small sample size (Figure 3A and 3B). As seen in Figure 3, there were no significant changes for the summary HR estimates for any of the individual PPIs or for the overall PPI effect estimate. Finally, given that the number of studies included in the quantitative analyses was substantially <10, publication bias analyses were not pursued because the power of those tests is not great enough to provide accurate estimates of bias with small sample sizes.13, 14
Figure 3.
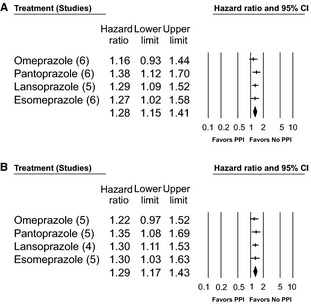
Sensitivity analyses of overall PPI effect (A) with and (B) without the study by Simon et al.11 PPI indicates proton pump inhibitor.
Discussion
In a systematic review of observational data available for the association of individual PPIs with adverse cardiac outcomes in CAD patients on clopidogrel, several PPIs previously assumed to be safe were found to have an association with harm. Omeprazole did not have a statistically significant association with adverse CV events, independent of CAD status (ACS versus stable CAD), whereas pantoprazole, lansoprazole, and esomeprazole were all significantly associated with adverse CV outcomes. There remains a need for randomized controlled trials or patient‐level meta‐analyses to evaluate the safety of individual PPIs for concomitant use with clopidogrel in patients with CAD.
Although an abundance of observational data from individual studies shows a relationship between PPIs (as a group) and adverse CV outcomes, there are several plausible explanations for those findings. The most compelling argument remains that PPI use is a marker for high risk rather than a cause of poor CV outcomes. This is well illustrated by several studies of both clopidogrel and newer generation P2Y12 antagonists. Goodman et al evaluated the effect of PPIs on adverse CV events in post‐ACS patients taking either ticagrelor or clopidogrel in the PLATO trial.15 An important distinction is that although ticagrelor blocks the P2Y12 receptor, it is an active compound and thus, unlike clopidogrel, does not require metabolism by the CYP 2C19 system for activation. As such, there is no pharmacokinetic mechanism for interaction between PPIs and ticagrelor. The authors showed that patients taking PPIs or other non‐PPI GI drugs had significantly higher rates of adverse CV events in both the clopidogrel and ticagrelor treatment groups. Using landmark analyses for the start of PPIs either at the time of randomization or subsequently during the trial (day 2, 4, 9, 30, 60, 90, or 180), PPIs were only independently associated with adverse cardiac events if patients started them prior to or at randomization. These authors concluded that the most reasonable explanation for these findings was that PPI use served as a marker of patients at high risk for CV events and that the association of events with PPIs for patients on clopidogrel and ticagrelor was heavily confounded. Dunn et al came to a similar conclusion in analyzing the results of the CREDO trial for patients with and without the use of PPIs.16
Alternative mechanisms have been proposed to explain the association of PPIs with adverse CV events. In a large‐scale pharmacovigilance study, Shah et al examined the use of PPIs in an unselected group of patients to evaluate the association of PPIs with CV events and mortality. In their study, regardless of clopidogrel use, patients taking PPIs were at increased hazard for MI (HR 1.16; 95% CI 1.09–1.24) and a 2‐fold high hazard for all‐cause mortality (HR 2.00; 95% CI 1.07–3.78; P=0.031) compared with patients not taking PPIs.17 They did not find a similar association in patients on H2 receptor blocking agents. Although the exact mechanism by which PPIs would have this effect on a general population is unknown, the authors point to previous animal model studies indicating that PPI administration may be associated with decreased nitric oxide synthase activity, which is a known marker for vascular health and thrombosis. Although these findings are intriguing, they do not help elucidate the possible interaction between clopidogrel and PPIs. The most valid method to evaluate the relationship of PPIs and clopidogrel remains a randomized controlled trial.
At present, very little randomized controlled trial evidence is available to answer this question. Smaller randomized controlled trials have been performed with pantoprazole18 and omeprazole,19 evaluating platelet reactivity and function as end points. These studies showed that platelet reactivity is higher in patients on concomitant clopidogrel and omeprazole therapy compared with those on clopidogrel alone or those on clopidogrel and concomitant pantoprazole; however, none of these studies were large enough or adequately powered to study clinical outcomes. The COGENT trial was designed to evaluate the effect of omeprazole versus placebo on thrombotic and bleeding outcomes in patients prescribed DAPT with clopidogrel for at least 12 months.20 The trial enrolled only patients who were expected to receive at least 12 months of DAPT (with clopidogrel) and were not currently taking PPIs, H2 receptor antagonists, or other antacid GI agents. Unfortunately, the trial was stopped prematurely due to slow enrollment and lack of funding, but with the limited sample size and follow‐up available, patients taking omeprazole and DAPT versus those on DAPT alone had a similar risk of thrombotic events and a significantly decreased risk of GI bleeding. To date, these data represent the best available on the relationship between an individual PPI and adverse CV outcomes in patients with CAD. Despite these findings, the US Food and Drug Administration still issued a warning about the combination of omeprazole or esomeprazole with clopidogrel based on observational data.
When few randomized controlled trial data are available, researchers will often try to combine the best sources of data available to reach conclusions. Systematic reviews with meta‐analysis enable researchers and clinicians to use the findings across multiple studies and to increase power to answer important clinical questions. The results of our systematic review are consistent with a previous randomized controlled study20 indicating that omeprazole is not associated with adverse CV events in patients with CAD on concomitant clopidogrel. For the remaining individual PPIs, including pantoprazole, lansoprazole, and esomeprazole, our observational results add to previous literature but are strictly hypothesis generating and point to the need for more randomized controlled trial evidence evaluating the efficacy and safety of these PPIs in patients with CAD on concomitant clopidogrel. There is currently no solid evidence to prompt the change of an individual PPI therapy to another to avoid interaction with clopidogrel. At this time, due to inconsistencies in the available data, consideration should be given to changing the guidelines to reflect this uncertainty.
Limitations
Our study had several limitations. Detailed data on drug regimen and duration and GI bleeding events were not available in all studies. There was variation in composite end point definition and covariates used in adjusted OR or HR, such as the use of all‐cause versus CV mortality, which may have introduced some imprecision into our effect estimates. In addition, we performed meta‐analysis combining HRs and ORs, which can be of questionable statistical validity if event rates are high. Event rates were uniformly ≤25%, and sensitivity analyses were performed excluding the study by Simon et al—the only study to use ORs—and found that results were very similar with or without those data. Moreover, individual end point results were not available, limiting our ability to use metaregression or indirect comparison methods. Finally, all data were based on observational studies that may be confounded by selection bias, in which sicker patients with more comorbidities are treated with a PPI and thus are at higher risk for adverse clinical outcomes.
Conclusions
Several frequently used PPIs previously thought to be safe for concomitant use with clopidogrel were associated with greater risk of adverse CV events. Although the data are observational, they highlight the need for randomized controlled trials or patient‐level meta‐analyses to evaluate the safety of concomitant PPI and clopidogrel use in patients with CAD.
Sources of Funding
Dr Sherwood was funded by NIH T‐32 training grant #5 T32 HL 7101‐37. Jones: Research grants—American Heart Association, AstraZeneca, Boston Scientific Corporation, Daiichi Sankyo.
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002245 doi: 10.1161/JAHA.115.002245)
References
- 1. Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL. 2012 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Unstable Angina/Non‐ST‐Elevation Myocardial Infarction (Updating the 2007 Guideline and Replacing the 2011 Focused Update): A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2012;126:875–910. [DOI] [PubMed] [Google Scholar]
- 2. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz CB, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso JE, Tracy CM, Woo YJ, Zhao DX, Force CAT. 2013 ACCF/AHA Guideline for the Management of ST‐elevation Myocardial Infarction: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–555. [DOI] [PubMed] [Google Scholar]
- 3. Ho PM, Maddox TM, Wang L, Fihn SD, Jesse RL, Peterson ED, Rumsfeld JS. Risk of adverse outcomes associated with concomitant use of clopidogrel and proton pump inhibitors following acute coronary syndrome. JAMA. 2009;301:937–944. [DOI] [PubMed] [Google Scholar]
- 4. O'Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, Michelson AD, Hautvast RW, Ver Lee PN, Close SL, Shen L, Mega JL, Sabatine MS, Wiviott SD. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton‐pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989–997. [DOI] [PubMed] [Google Scholar]
- 5. Ray WA, Murray KT, Griffin MR, Chung CP, Smalley WE, Hall K, Daugherty JR, Kaltenbach LA, Stein CM. Outcomes with concurrent use of clopidogrel and proton‐pump inhibitors: a cohort study. Ann Intern Med. 2010;152:337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Charlot M, Ahlehoff O, Norgaard ML, Jorgensen CH, Sorensen R, Abildstrom SZ, Hansen PR, Madsen JK, Kober L, Torp‐Pedersen C, Gislason G. Proton‐pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: a nationwide cohort study. Ann Intern Med. 2010;153:378–386. [DOI] [PubMed] [Google Scholar]
- 7. Melloni C, Washam JB, Jones WS, Halim SA, Hasselblad V, Mayer SB, Heidenfelder BL, Dolor RJ. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy: systematic review. Circ Cardiovasc Qual Outcomes. 2015;8:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siller‐Matula JM, Jilma B, Schror K, Christ G, Huber K. Effect of proton pump inhibitors on clinical outcome in patients treated with clopidogrel: a systematic review and meta‐analysis. J Thromb Haemost. 2010;8:2624–2641. [DOI] [PubMed] [Google Scholar]
- 9. Melloni C, Jones WS, Washam JB, Hasselblad V, Mayer SB, Halim S, Subherwal S, Alexander K, Kong DF, Heidenfelder BL, Irvine RJ, Wing L, Dolor RJ. Antiplatelet and Anticoagulant Treatments for Unstable Angina/Non‐ST Elevation Myocardial Infarction. Rockville, MD: Agency for Healthcare Research and Quality (US); 2013. Nov. Report No.: 13(14)‐EHC125‐EF. AHRQ Comparative Effectiveness Reviews. AHRQ Comparative Effectiveness Reviews. [PubMed] [Google Scholar]
- 10. Kreutz RP, Stanek EJ, Aubert R, Yao J, Breall JA, Desta Z, Skaar TC, Teagarden JR, Frueh FW, Epstein RS, Flockhart DA. Impact of proton pump inhibitors on the effectiveness of clopidogrel after coronary stent placement: the clopidogrel medco outcomes study. Pharmacotherapy. 2010;30:787–796. [DOI] [PubMed] [Google Scholar]
- 11. Simon T, Steg PG, Gilard M, Blanchard D, Bonello L, Hanssen M, Lardoux H, Coste P, Lefevre T, Drouet E, Mulak G, Bataille V, Ferrieres J, Verstuyft C, Danchin N. Clinical events as a function of proton pump inhibitor use, clopidogrel use, and cytochrome P450 2C19 genotype in a large nationwide cohort of acute myocardial infarction: results from the French Registry of Acute ST‐Elevation and Non‐ST‐Elevation Myocardial Infarction (FAST‐MI) registry. Circulation. 2011;123:474–482. [DOI] [PubMed] [Google Scholar]
- 12. van Boxel OS, van Oijen MG, Hagenaars MP, Smout AJ, Siersema PD. Cardiovascular and gastrointestinal outcomes in clopidogrel users on proton pump inhibitors: results of a large Dutch cohort study. Am J Gastroenterol. 2010;105:2430–2436; quiz 2437. [DOI] [PubMed] [Google Scholar]
- 13. Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta‐analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53:1119–1129. [DOI] [PubMed] [Google Scholar]
- 14. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodman SG, Clare R, Pieper KS, Nicolau JC, Storey RF, Cantor WJ, Mahaffey KW, Angiolillo DJ, Husted S, Cannon CP, James SK, Kilhamn J, Steg PG, Harrington RA, Wallentin L; Platelet Inhibition and Patient Outcomes Trial Investigators . Association of proton pump inhibitor use on cardiovascular outcomes with clopidogrel and ticagrelor: insights from the platelet inhibition and patient outcomes trial. Circulation. 2012;125:978–986. [DOI] [PubMed] [Google Scholar]
- 16. Dunn SP, Steinhubl SR, Bauer D, Charnigo RJ, Berger PB, Topol EJ. Impact of proton pump inhibitor therapy on the efficacy of clopidogrel in the CAPRIE and CREDO trials. J Am Heart Assoc. 2013;2:e004564 doi: 10.1161/JAHA.112.004564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shah NH, LePendu P, Bauer‐Mehren A, Ghebremariam YT, Iyer SV, Marcus J, Nead KT, Cooke JP, Leeper NJ. Proton Pump Inhibitor Usage and the Risk of Myocardial Infarction in the General Population. PLoS One. 2015;10:e0124653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cuisset T, Frere C, Quilici J, Poyet R, Gaborit B, Bali L, Brissy O, Morange PE, Alessi MC, Bonnet JL. Comparison of omeprazole and pantoprazole influence on a high 150‐mg clopidogrel maintenance dose the PACA (Proton Pump Inhibitors and Clopidogrel Association) prospective randomized study. J Am Coll Cardiol. 2009;54:1149–1153. [DOI] [PubMed] [Google Scholar]
- 19. Gilard M, Arnaud B, Cornily JC, Le Gal G, Lacut K, Le Calvez G, Mansourati J, Mottier D, Abgrall JF, Boschat J. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double‐blind OCLA (Omeprazole Clopidogrel Aspirin) study. J Am Coll Cardiol. 2008;51:256–260. [DOI] [PubMed] [Google Scholar]
- 20. Bhatt DL, Cryer BL, Contant CF, Cohen M, Lanas A, Schnitzer TJ, Shook TL, Lapuerta P, Goldsmith MA, Laine L, Scirica BM, Murphy SA, Cannon CP. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010;363:1909–1917. [DOI] [PubMed] [Google Scholar]


