Abstract
Background
We performed a nationwide population‐based cohort study to investigate the long‐term risk of stroke after coronary artery bypass grafting in patients with type 1 and type 2 diabetes.
Methods and Results
All patients who underwent primary coronary artery bypass grafting in Sweden from 2000 through 2011 were included from the SWEDEHEART register. We excluded patients with prior stroke, and patients who had a stroke or died within 30 days of surgery. The National Diabetes Register was used to identify patients with type 1 and type 2 diabetes. Incident stroke (ischemic and hemorrhagic), and all‐cause mortality was obtained by record linkage with the National Patient Register and the Cause of Death register. We used multivariable Cox regression to estimate the risk of stroke in relation to type of diabetes. A total of 53 820 patients (type 1 diabetes [n=714], type 2 diabetes [n=10 054], no diabetes [n=43 052]) were included. During a mean follow‐up of 7.4 years (398 337 person‐years), in total, 8.0% (n=4296) of the patients had a stroke: 7.3% (n=52) in patients with type 1 diabetes, 9.1% (n=915) in patients with type 2 diabetes, and 7.7% (n=3329) in patients with no diabetes. The multivariable adjusted hazard ratio (95% CI) for all stroke was 1.59 (1.20–2.11) in type 1 diabetes, and 1.32 (1.23–1.43) in type 2 diabetes.
Conclusions
The long‐term risk for stroke after coronary artery bypass grafting was increased in patients with type 1 and type 2 diabetes, compared to patients with no diabetes.
Keywords: coronary artery bypass graft surgery, coronary artery disease, diabetes mellitus, long‐term follow‐up, stroke
Introduction
Rates of cardiovascular disease (CVD) such as myocardial infarction and stroke among patients with diabetes have declined substantially the last 2 decades, where the magnitude of reduction is greatest for myocardial infarction.1 Prevention of CVD by a combination of lifestyle changes, and lowering blood glucose, blood pressure, and lipid levels, both in type 12, 3 and type 2 diabetes may explain a large part of this reduction.4, 5
The more rapid decline in myocardial infarction might partly be explained by improvements in interventional cardiology, but also because of improved pharmacological therapy.1 Percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are currently the available options for revascularization in patients with coronary artery disease. For diabetes patients, the results of most studies have favored CABG over PCI as the preferred method for revascularization, especially in multivessel disease.6, 7 In the Strategies For Multivessel Revascularization in Patients with Diabetes (FREEDOM) trial, the hypothesis of CABG being more favorable than PCI with drug‐eluting stents among diabetes patients for long‐term outcome was tested.8 The risk of all‐cause mortality and myocardial infarction was significantly lower in patients who underwent CABG compared to those who underwent PCI.8 However, the stroke rate, on the other hand, was higher in the CABG group.9 The reason for a higher stroke incidence after CABG is not known but may reflect higher prevalence of cerebral vascular disease in patients with diabetes.
To the best of our knowledge, no previous study has reported on the association between type 1 and type 2 diabetes and long‐term risk of stroke after CABG.
We performed a nationwide population‐based cohort study to investigate the long‐term risk of stroke in patients with type 1 and type 2 diabetes compared to patients without diabetes.
Methods
This was a nationwide population‐based observational cohort study, and it was approved by the regional Human Research Ethics Committee, Stockholm, Sweden. The need for informed consent was waived.
Study Population and Data Sources
We included all patients who underwent primary nonemergent CABG in Sweden from the Swedish Web‐system for Enhancement and Development of Evidence‐based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) register.10, 11, 12 We excluded patients with a history of stroke, patients with prior cardiac surgery, patients who underwent emergency surgery (within 24 hours of decision to operate), and patients who had a stroke or died within 30 days of surgery. The data from the SWEDEHEART register were combined with information from the National Diabetes Register13 using the unique Swedish personal identity numbers,14 as previously described.15 Record linkages were performed by the Swedish National Board of Health and Welfare and data were anonymized according to regulations. The National Patient Register16 was used to obtain prior diagnoses, and The Longitudinal integration database for health insurance and labor market studies17 was used to retrieve educational level, household disposable income, country of birth, and marital status.
Definition of Diabetes
In the Swedish National Diabetes Register, the epidemiological definition of type 1 diabetes was onset of diabetes before the age of 30 years and treatment with insulin only, and type 2 diabetes was defined as diabetes treated with diet or oral hypoglycemic agents alone, or age of ≥40 years at onset of diabetes and treatment with insulin alone or in combination with oral hypoglycemic agents.13
Outcomes
Follow‐up started on January 1, 2000 and ended on December 31, 2012. We only used the first hospital admission for stroke in patients with multiple stroke admissions. The following International Classification of Diseases (ICD) codes for a primary discharge diagnosis was used to identify incident stroke: all stroke (ICD codes I60 to 64), ischemic stroke (ICD codes I63 to 64), and hemorrhagic stroke (ICD codes I60 to 62). The validity of stroke diagnosis in the National Patient Register had been shown to be high.16 The Cause of Death Register was used to ascertain dates of death.
Statistical Analyses
Baseline characteristics were described as frequencies and percentages for categorical variables and means and standard deviations for continuous variables. Differences in baseline characteristics between patients with and without diabetes were compared using analysis of variance or χ2 test. Person‐time in days was counted from the date of surgery until the date of incident stroke, death, or the end of follow‐up (December 31, 2012). For the analysis of the stroke outcome, patients who died during follow‐up were censored on the date of death. We used Cox regression to estimate the risk of incident stroke, all‐cause mortality, and a combined end point (stroke or all‐cause mortality) in patients with type 1 diabetes or type 2 diabetes compared with patients without diabetes. The adjusted Cox models included all the variables listed in Table 1 as covariates and the models were stratified by calendar year of surgery and hospital. Patient age and body mass index was modeled using restricted cubic splines, and all other variables were included as categorical terms.
Table 1.
Baseline Characteristics in 53 820 Patients Who Underwent Coronary Artery Bypass Surgery in Sweden During 2000–2011 According to Diabetes Type
| All Patients | No Diabetes | Diabetes Type 1 | Diabetes Type 2 | P Value | |
|---|---|---|---|---|---|
| Number of patients, % | 53 820 | 43 052 (80%) | 714 (1%) | 10 054 (19%) | |
| Age, mean (SD) | 67.36 (9.43) | 67.52 (9.54) | 56.80 (9.64) | 67.42 (8.46) | <0.001 |
| Female sex | 11 874 (22%) | 9234 (21%) | 313 (44%) | 2327 (23%) | <0.001 |
| Birth region | |||||
| Nordic countries | 49 158 (91%) | 39 560 (92%) | 695 (97%) | 8903 (89%) | <0.001 |
| Other | 4657 (9%) | 3487 (8%) | 19 (3%) | 1151 (11%) | <0.001 |
| Education | |||||
| <10 years | 23 480 (46%) | 18 545 (45%) | 226 (32%) | 4709 (49%) | <0.001 |
| 10 to 12 years | 19 375 (38%) | 15 383 (38%) | 334 (47%) | 3658 (38%) | <0.001 |
| >12 years | 8447 (16%) | 6977 (17%) | 150 (21%) | 1320 (14%) | <0.001 |
| Marital status | |||||
| Married | 35 627 (66%) | 28 637 (67%) | 426 (60%) | 6564 (65%) | <0.001 |
| Other | 18 193 (34%) | 14 415 (33%) | 288 (40%) | 3490 (35%) | <0.001 |
| Household disposable income (tertiles) | |||||
| T1 (lowest) | 17 454 (32%) | 13 848 (32%) | 197 (28%) | 3409 (34%) | <0.001 |
| T2 | 17 783 (33%) | 14 147 (33%) | 201 (28%) | 3435 (34%) | <0.001 |
| T3 (highest) | 18 556 (34%) | 15 030 (35%) | 316 (44%) | 3210 (32%) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 27.14 (4.01) | 26.79 (3.86) | 25.95 (4.09) | 28.72 (4.25) | <0.001 |
| eGFR | |||||
| >60 mL/min per 1.73 m2 | 37 762 (76%) | 30 241 (77%) | 424 (63%) | 7097 (75%) | <0.001 |
| 45 to 60 mL/min per 1.73 m2 | 8211 (17%) | 6532 (17%) | 104 (15%) | 1575 (17%) | <0.001 |
| 30 to 45 mL/min per 1.73 m2 | 2712 (5%) | 2022 (5%) | 53 (8%) | 637 (7%) | <0.001 |
| 15 to 30 mL/min per 1.73 m2 | 528 (1%) | 373 (1%) | 29 (4%) | 126 (1%) | <0.001 |
| End‐stage renal disease | 420 (1%) | 303 (1%) | 65 (10%) | 52 (1%) | <0.001 |
| Hypertension | 17 343 (32%) | 12 596 (29%) | 292 (41%) | 4455 (44%) | <0.001 |
| Hyperlipidemia | 12 172 (23%) | 9296 (22%) | 180 (25%) | 2696 (27%) | <0.001 |
| Peripheral vascular disease | 3940 (7%) | 2914 (7%) | 132 (18%) | 894 (9%) | <0.001 |
| Prior PCI | 7886 (15%) | 6164 (14%) | 131 (18%) | 1591 (16%) | <0.001 |
| Chronic pulmonary disease | 3397 (6%) | 2599 (6%) | 27 (4%) | 771 (8%) | <0.001 |
| Prior myocardial infarction | 26 440 (49%) | 20 785 (48%) | 369 (52%) | 5286 (53%) | <0.001 |
| Left ventricular ejection fraction | |||||
| >50% | 25 887 (69%) | 20 648 (70%) | 347 (69%) | 4892 (64%) | <0.001 |
| 30 to 50% | 9929 (26%) | 7501 (25%) | 128 (26%) | 2300 (30%) | <0.001 |
| <30% | 1878 (5%) | 1420 (5%) | 25 (5%) | 433 (6%) | <0.001 |
| Heart failure | 7103 (13%) | 5366 (12%) | 124 (17%) | 1613 (16%) | <0.001 |
| Atrial fibrillation | 4727 (9%) | 3780 (9%) | 20 (3%) | 927 (9%) | <0.001 |
| History of cancer | 3039 (6%) | 2448 (6%) | 28 (4%) | 563 (6%) | 0.125 |
| Alcohol dependency | 1012 (2%) | 821 (2%) | 7 (1%) | 184 (2%) | 0.179 |
| Type of surgery | |||||
| Isolated CABG | 46 432 (86%) | 36 886 (86%) | 674 (94%) | 8872 (88%) | <0.001 |
| CABG+valve | 6996 (13%) | 5809 (13%) | 40 (6%) | 1147 (11%) | <0.001 |
| CABG+other | 392 (1%) | 357 (1%) | 0 (0%) | 35 (0%) | <0.001 |
| Mechanical heart valve | 1348 (3%) | 1085 (3%) | 12 (2%) | 251 (2%) | 0.362 |
Data are shown as n (%) unless otherwise noted. End‐stage renal disease was defined as eGFR <15 mL/min per 1.73 m2 or on dialysis/renal transplant. CABG indicates coronary artery bypass grafting; eGFR, estimated glomerular filtration rate; PCI, percutaneous coronary intervention.
Missing data (educational level [4.7%], left ventricular ejection fraction [30%], renal function [7.8%], and body mass index [9.2%]) were handled by multiple imputation by chained equations.18 The imputation models included all variables in Table 1, and also the event indicator and the Nelson−Aalen estimator of the cumulative baseline hazard.19 Separate imputation models were used for the stroke and death outcomes. Ten data sets were imputed and estimates from these data sets were combined according to Rubin's rules. Missing data for country of birth (5 patients), household disposable income (27 patients), and marital status (27 patients) were imputed with the most common category.
Data management and statistical analyses were performed using Stata 14.0 (Stata Corp, College Station, TX) and R version 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Population and Patient Characteristics
A total of 53 820 patients (mean age 67 years, 22% women) who underwent isolated CABG (n=46 432), or CABG and heart valve surgery (n=6996), or CABG in combination with another cardiac procedure (n=392) from 2000 to 2011 were identified from the SWEDEHEART register. Patient characteristics according to type of diabetes are presented in Table 1. Patients with type 1 diabetes (714/53 820, 1%) were ≈10 years younger than patients with type 2 diabetes (10 054/53 820, 19%) and patients without diabetes (43 052/53 820, 80%). Patients with type 1 diabetes were more often female, more likely to have chronic kidney disease or end‐stage renal disease, and more likely to have peripheral vascular disease compared to patients with type 2 diabetes or no diabetes. Atrial fibrillation was less common in patients with type 1 diabetes (3%) compared to patients with type 2 diabetes (9%) or no diabetes (9%).
Long‐Term Risk of Stroke
During a mean follow‐up time of 7.4 (SD 3.5) (median 7.7) years (398 337 person‐years), in total, 8.0% (4296 of 53 820) of the patients had a stroke: 7.3% (52 of 714) of the patients with type 1 diabetes, 9.1% (915 of 10 054) of the patients with type 2 diabetes, and 7.7% (3329 of 43 052) of the patients with no diabetes (Table 2). The age‐adjusted stroke rate according to type of diabetes is shown in Figure.
Table 2.
Long‐Term Event Rates and Relative Risks for Stroke in Patients Who Underwent Coronary Artery Bypass Surgery According to Diabetes Type
| Outcome | Events/Person‐Years | Incidence Rate Per 1000 Person‐Years (95% CI) | Age‐Adjusted Hazard Ratio (95% CI) | P Value | Multivariable Adjusteda Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| All stroke | ||||||
| No diabetes | 3329/324 569 | 10.3 (9.9–10.6) | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Type 1 DM | 52/5099 | 10.2 (7.8–13.4) | 1.68 (1.27–2.21) | <0.001 | 1.59 (1.20–2.11) | 0.001 |
| Type 2 DM | 915/68 669 | 13.3 (12.5–14.2) | 1.33 (1.24–1.43) | <0.001 | 1.32 (1.23–1.43) | <0.001 |
| Ischemic stroke | ||||||
| No diabetes | 2848/326 902 | 8.7 (8.4–9.0) | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Type 1 DM | 42/5135 | 8.2 (6.0–11.1) | 1.59 (1.17–2.16) | 0.003 | 1.51 (1.10–2.06) | 0.010 |
| Type 2 DM | 814/69 050 | 11.8 (11.0–12.6) | 1.39 (1.28–1.50) | <0.001 | 1.37 (1.26–1.48) | <0.001 |
| Hemorrhagic stroke | ||||||
| No diabetes | 592/338 371 | 1.7 (1.6–1.9) | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Type 1 DM | 11/5293 | 2.1 (1.2–3.8) | 1.90 (1.04–3.48) | 0.038 | 1.82 (0.98–3.38) | 0.056 |
| Type 2 DM | 124/72 135 | 1.7 (1.4–2.0) | 1.01 (0.83–1.22) | 0.946 | 1.02 (0.84–1.25) | 0.825 |
DM indicates diabetes mellitus.
Model included all variables reported in Table 1.
Figure 1.
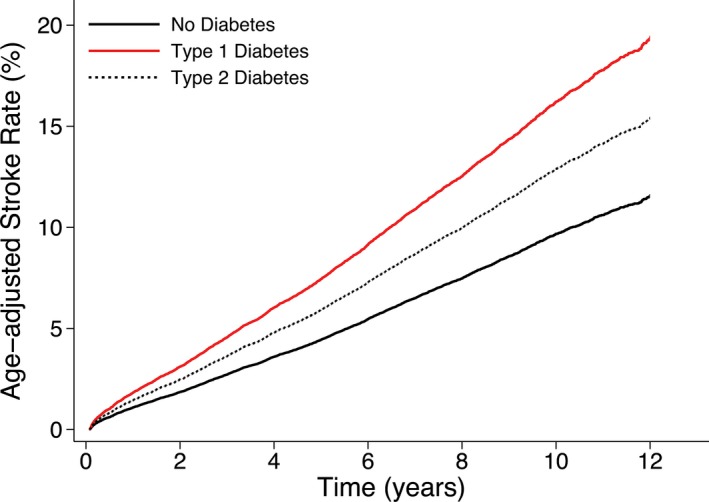
Age‐adjusted stroke rate in 53 820 patients who underwent coronary artery bypass grafting in Sweden between 2000 and 2011 according to type of diabetes. The age‐adjusted risk for stroke was significantly higher in patients with type 1 and type 2 diabetes compared with patients without diabetes.
The crude incidence rate of all stroke, ischemic stroke, and hemorrhagic stroke, respectively, are shown in Table 2.
The risk for stroke was significantly higher in patients with type 1 and type 2 diabetes compared with patients without diabetes in the age‐adjusted and multivariable adjusted analyses (Table 2). After multivariable adjustment for all variables reported in Table 1, the hazard ratio (95% CI) for all stroke was 1.59 (1.20–2.11) in type 1 diabetes, and 1.32 (1.23–1.43) in type 2 diabetes. The age‐adjusted and multivariable‐adjusted risks for the subtypes of stroke (ischemic and hemorrhagic) are shown in Table 2.
Long‐Term Risk of All‐Cause Mortality and the Combination of Stroke and All‐Cause Mortality
During a mean follow‐up time of 7.8 (SD 3.5) (median 7.7) years (421 576 person‐years), in total, 22% (11 737 of 53 820) patients died of any cause: 22% (155 of 714) in type 1 diabetes, 21% (2130 of 10 054) in type 2 diabetes, and 22% (9452 of 43 052) in patients with no diabetes (Table 3). The crude incidence rates, and also the age‐adjusted and multivariable‐adjusted risks of all‐cause mortality and the combination of stroke and all‐cause mortality, respectively, are shown in Table 3. The multivariable adjusted risk for all‐cause mortality, and also the combined outcome, was twice as high in patients with type 1 diabetes compared to patients without diabetes. The risk for these outcomes was only slightly higher in patients with type 2 diabetes compared to patients without diabetes.
Table 3.
Long‐Term Event Rates and Relative Risks for All‐Cause Mortality or a Combination of Stroke and All‐Cause Mortality in Patients Who Underwent Coronary Artery Bypass Surgery According to Diabetes Type
| Outcome | Events/Person‐Years | Incidence Rate Per 1000 Person‐Years (95% CI) | Age‐Adjusted Hazard Ratio (95% CI) | P Value | Multivariable Adjusteda Hazard Ratio (95% CI) | P Value |
|---|---|---|---|---|---|---|
| All‐cause mortality | ||||||
| No diabetes | 9452/341 303 | 27.7 (27.1–28.3) | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Type 1 DM | 155/5399 | 28.7 (24.5–33.6) | 2.34 (1.99–2.75) | <0.001 | 1.98 (1.68–2.33) | <0.001 |
| Type 2 DM | 2130/74 875 | 28.4 (27.3–29.7) | 1.10 (1.05–1.16) | <0.001 | 1.05 (1.00–1.10) | 0.078 |
| Stroke or death | ||||||
| No diabetes | 11 205/290 883 | 38.5 (37.8–39.2) | 1.00 (Ref.) | 1.00 (Ref.) | ||
| Type 1 DM | 184/4571 | 40.3 (34.8–46.5) | 2.19 (1.89–2.54) | <0.001 | 1.86 (1.60–2.16) | <0.001 |
| Type 2 DM | 2661/62 738 | 42.4 (40.8–44.1) | 1.18 (1.13–1.23) | <0.001 | 1.10 (1.06–1.15) | <0.001 |
DM indicates diabetes mellitus.
Model included all variables reported in Table 1.
Importance of Duration, Treatment, and Glycemic Control
The univariate associations between duration of diabetes, treatment, and HbA1c, respectively, and all stroke is shown in Table 4. There was no significant association between HbA1c level and stroke in type 1 diabetes. However, it should be noted that the number of patients and strokes was low. There was no statistically significant association between duration of diabetes, or HbA1c levels, and stroke in type 2 diabetes. The risk of stroke was increased in patients treated with insulin compared to diet alone; the unadjusted hazard ratio was 1.23 (95% CI 1.02–1.48).
Table 4.
Duration and Treatment of Diabetes, and Glycemic Control in Patients With or Without Stroke
| Type 1 Diabetes | Type 2 Diabetes | |||||
|---|---|---|---|---|---|---|
| No Stroke | Stroke | HRa (95% CI) | No Stroke | Stroke | HRa (95% CI) | |
| DM duration, y, mean (SD) | 41.8 (11.0) | 41.5 (13.3) | 1.01 (0.98–1.03) | 8.47 (7.37) | 9.10 (7.62) | 1.01 (1.00–1.02) |
| Duration ≤10 years (Ref.) | 0 (0%) | 0 (0%) | — | 6125 (67%) | 596 (65%) | 1.00 |
| Duration >10 years | 662 (100%) | 52 (100%) | — | 3014 (33%) | 319 (35%) | 1.11 (0.96–1.27) |
| Treatment | ||||||
| Diet only (Ref.) | 0 (0%) | 0 (0%) | — | 2189 (24%) | 171 (19%) | 1.00 |
| Oral hypoglycemic agents | 0 (0%) | 0 (0%) | — | 3928 (43%) | 374 (41%) | 1.13 (0.95–1.36) |
| Insulin with or without oral hypoglycemic agents | 662 (100%) | 52 (100%) | — | 3022 (33%) | 370 (40%) | 1.23 (1.02–1.48) |
| HbA1c, mean (SD), % | 8.44 (1.09) | 8.46 (1.10) | 0.93 (0.68–1.28) | 7.41 (1.16) | 7.61 (1.23) | 1.07 (0.99–1.17) |
| HbA1c | ||||||
| ≤7.0% (Ref.) | 55 (11%) | 3 (8%) | 1.00 | 1993 (44%) | 142 (38%) | 1.00 |
| 7.1% to 8.0% | 134 (26%) | 12 (32%) | 1.63 (0.42–6.26) | 1378 (30%) | 117 (31%) | 1.10 (0.86–1.42) |
| 8.1% to 9.0% | 200 (38%) | 12 (32%) | 1.12 (0.30–4.27) | 757 (17%) | 68 (18%) | 1.04 (0.78–1.40) |
| 9.1% to 10.0% | 95 (18%) | 6 (16%) | 0.95 (0.22–4.15) | 278 (6%) | 35 (9%) | 1.46 (0.99–2.13) |
| >10.0% | 38 (7%) | 5 (13%) | 1.76 (0.33–9.26) | 113 (3%) | 13 (3%) | 1.26 (0.71–2.26) |
Data are shown as n (%) unless otherwise noted. DM indicates diabetes mellitus; HbA1c, glycosylated hemoglobin; HR, hazard ratio; Ref., reference category.
Univariate hazard ratio.
Discussion
In this long‐term nationwide follow‐up study of all patients who underwent CABG in Sweden during 12 years, we found that diabetes was associated with an increased risk of stroke compared with no diabetes. The increase in the risk of stroke was statistically significant in patients with both type 1 and type 2 diabetes after adjustment for clinically relevant baseline characteristics. Moreover, the risk of death in combination with stroke was doubled in patients with type 1 diabetes compared to those with no diabetes, and it was only slightly increased in patients with type 2 diabetes, compared to those with no diabetes.
Several previous studies have demonstrated that diabetes is an important risk factor for stroke.20 Both type 1 and type 2 diabetes are associated with an increased absolute risk of stroke, although expected to be higher in type 2 diabetes, which mostly is explained by the fact that patients with type 2 diabetes are older. Not only age, but also sex can influence the risk of stroke in diabetes. Diabetes is associated with a higher relative risk of stroke in women than in men,21 particularly in type 1 diabetes.22 In recent studies where patients with diabetes have been divided into type 1 and type 2 diabetes, a rather high incidence rate of stroke have been demonstrated both in type 1 and type 2 diabetes.23, 24 After separation of the diabetes types, an even further increased risk has been reported in type 1 diabetes.23, 24 In our study, the association between type 1 and type 2 diabetes and risk of stroke was rather similar even if point estimates suggested a slightly stronger association between type 1 diabetes and risk of stroke. Also, it was recently demonstrated that type 1 diabetes individuals had a considerably higher risk for premature stroke compared with nondiabetic individuals.25 Usually type 1 diabetes patients are at risk of stroke 10 to 15 years earlier than nondiabetes individuals; this risk is even higher in women.21, 26 The excess risk, not only for stroke, but also all‐cause mortality, and fatal and nonfatal CVD is greater in women than in men with type 1 diabetes.22 Besides differences in age and sex, chronic kidney disease and peripheral vascular disease were also more common in patients with type 1 diabetes compared to patients with type 2 diabetes in the present study. This may have contributed to the trend of a higher relative risk of stroke in the type 1 diabetes patients as compared with the relative risk in patients with type 2 diabetes. In contrast, the occurrence of atrial fibrillation was lower in type 1 diabetes patients.
We reported recently that patients with type 1 diabetes had more than a doubled long‐term risk of death after CABG compared with patients without diabetes, whereas patients with type 2 diabetes had almost the same risk as patients without diabetes.15 Also in the current study, all‐cause mortality was twice as high in type 1 diabetes patients compared to nondiabetes patients after multivariable adjustments for a number of potential risk factors. Interestingly, all‐cause mortality, and the combined outcome (stroke and death), were only slightly increased in type 2 diabetes patients compared to patients without diabetes. Others have demonstrated an increased risk of death in type 1 diabetes patients.27, 28 In a nationwide study, based on data from the Swedish National Diabetes Register, it was found that type 1 diabetes individuals, even at optimal glycemic control, had twice as high a risk of death, predominantly from CVD, compared to matched controls from the general population.27 When myocardial infarction, stroke, and heart failure are reported as separate outcomes in individuals with diabetes, usually coronary heart disease is predominant.29 In the present study, diabetes patients were more likely than nondiabetes patients to have a prior diagnosis of myocardial infarction or heart failure. In addition to the difference in age and sex, diabetes duration, chronic kidney disease, and peripheral artery disease were all more common in type 1 diabetes patients than in patients with type 2 diabetes. After multivariable adjustment, the hazard ratio for death only changed slightly, lending support to the idea that the pathophysiology underlying the excess risk of premature death in type 1 diabetes is still not well understood.29
Diabetes causes earlier onset of atherosclerosis. Involvement of hyperglycemia, obesity, hyperlipidemia, hypertension, and insulin resistance are all well‐known factors that all can induce and maintain atherosclerosis. Since we were not able to control for all these factors, we cannot exclude that there were differences between diabetes groups, or even between the nondiabetes group in terms of these risk factors. There were substantially more patients in the diabetes group compared to the nondiabetes group who were diagnosed with hypertension. This may, to some extent, explain the increased risk of stroke observed in the diabetes group. In diabetes, hypertension has a stronger association with stroke than hyperglycemia has.30, 31, 32 Nevertheless, the association between hyperglycemia and increased risk of stroke has been demonstrated in some,33 but not all studies2 in diabetes individuals. Long‐term studies in type 2 diabetes individuals comparing intensive versus standard glycemic control treatment have all failed to demonstrate a reduced risk of death and CVD (including fatal or nonfatal stroke).34, 35, 36 In one of these studies, the rate of death was even higher in the intensively treated group.34 The reason for this remains unclear.37 Moreover, there are differences in risk factors that are associated with an increased risk of ischemic stroke as compared to hemorrhagic stroke in diabetes. Hyperglycemia has a stronger association with ischemic than with hemorrhagic stroke.33 When we categorized glucose control (by means of HbA1c), we found no association between glucose control and stroke. Even though the numbers of all stroke were large, it should be noted that the numbers after this categorization, especially in the type 1 diabetes group, were low. Moreover, after we subgrouped stroke (there were much fewer hemorrhagic strokes in all patient groups), the hazard ratio for hemorrhagic stroke was increased in the type 1 diabetes patients, although the total numbers were too few to make any firm interpretations of that observation. Some studies have shown an association between hyperglycemia and hemorrhagic stroke,38 and other studies have not.33 Our finding is consistent with other reports demonstrating a weak or even a lack of association between HbA1c levels and stroke in diabetes. Finally, we also found that the risk of stroke was increased in type 2 diabetes patients treated with insulin compared to diet alone. It has been debated whether insulin per se induces atherosclerosis. However, in the ORIGIN study, therapy with basal insulin glargine for more than 6 years had a neutral effect on CVD outcomes.39 Therefore, it seems more likely that factors other than insulin may have confounded our observation (eg, type 2 diabetes patients treated with insulin are often older with longer diabetes duration and increased insulin resistance).
Strengths and Limitations
The main strength of the present study was the utilization of several high‐quality national Swedish healthcare registers. We were able to classify all patients according to the type of diabetes, because we used the National Diabetes Register where all diabetes patients in Sweden are registered with virtually complete coverage. This allowed us to investigate the importance of type 1 and type 2 diabetes, respectively, for the risk of stroke. In addition we used the National Patient Register to retrieve information on strokes during follow‐up. This register has complete coverage of Sweden since 1987, and has been validated for a number of diagnoses including stroke.16 Furthermore, since the registers we used are virtually complete and nationwide, the risk of misclassification of disease or outcome was small, and there was no loss to follow‐up. In addition, the level of health care provided to patients who undergo cardiac surgery in Sweden is comparable to the health care provided in the United States and other countries in Europe, which is why we believe that our results have a high external validity. To the best of our knowledge, this was the first study where the relation between subtypes of diabetes and risk of stroke was investigated in patients who underwent CABG.
As in every observational study, there may have been unmeasured risk factors that we could not adjust for in our statistical models. Even though our study population was large, there were few events when stroke was divided into subcategories (ischemic and hemorrhagic). Conclusions regarding the association between type of diabetes and subcategories of stroke must be drawn with caution. In addition, the point estimate suggested that the association between type 1 diabetes and stroke was stronger than the association between type 2 diabetes and stroke. However, even in this large study population, there were few events among patients with type 1 diabetes that led to confidence intervals being wide and overlapping. Our results cannot be generalized to the group of diabetes patients who undergo PCI. However, there is a general consensus that CABG is superior to PCI in patients with diabetes who are in need of multivessel revascularization, particularly in patients with more complex coronary artery disease.7 We had no information on medication with anticoagulants, which may ameliorate the risk of stroke, and may have influenced our results. Some of the patients without diabetes may in fact have had type 2 diabetes if tested, as shown in earlier studies.40 However, it is likely that these patients had a shorter duration of diabetes and thus may have had a lower risk of stroke. Another limitation was that our findings were not generalizable to patients with prior cardiac surgery or a prior stroke because our study population included only patients who underwent primary CABG without a prior stroke. Due to study design, we were not able to estimate the stroke risk associated with diabetes and CABG separately. In order to do that, we would have needed access to a control group of patients who did not undergo CABG, which we did not have.
Conclusions
Diabetes patients, compared to nondiabetes patients, had an increased long‐term risk of stroke after CABG that was independent of glycemic control and other cardiovascular risk factors. The risk of stroke was significantly higher in both type 1 and type 2 diabetes, compared to patients without diabetes. Moreover, the risk of death in combination with stroke was doubled in type 1 diabetes, whereas it was only slightly increased in patients with type 2 diabetes compared to patients without diabetes.
Sources of Funding
This study was supported by grants from the Swedish Society of Medicine (Nyström, Holzmann, Sartipy), Karolinska Institutet Foundations and Funds (Holzmann and Sartipy), the Mats Kleberg Foundation (Sartipy), and the Swedish Heart and Lung Foundation (Nyström).
Disclosures
None.
Acknowledgments
The authors are grateful to the steering committees of SWEDEHEART and NDR for providing data for this study.
(J Am Heart Assoc. 2015;4:e002411 doi: 10.1161/JAHA.115.002411)
Presented as an abstract at the Scientific Sessions of the American Heart Association, Nov 7‐11, 2015 in Orlando, FL.
References
- 1. Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes‐related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Writing Group for the DCCT/EDIC Research Group , Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, Lachin JM. Association between 7 years of intensive treatment of type 1 diabetes and long‐term mortality. JAMA. 2015;313:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 5. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 6. Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, Carrie D, Clayton TC, Danchin N, Flather M, Hamm CW, Hueb WA, Kahler J, Kelsey SF, King SB, Kosinski AS, Lopes N, McDonald KM, Rodriguez A, Serruys P, Sigwart U, Stables RH, Owens DK, Pocock SJ. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong EJ, Rutledge JC, Rogers JH. Coronary artery revascularization in patients with diabetes mellitus. Circulation. 2013;128:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S III, Bertrand M, Fuster V; Freedom Trial Investigators . Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. [DOI] [PubMed] [Google Scholar]
- 9. Palmerini T, Biondi‐Zoccai G, Reggiani LB, Sangiorgi D, Alessi L, De Servi S, Branzi A, Stone GW. Risk of stroke with coronary artery bypass graft surgery compared with percutaneous coronary intervention. J Am Coll Cardiol. 2012;60:798–805. [DOI] [PubMed] [Google Scholar]
- 10. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart. 2010;96:1617–1621. [DOI] [PubMed] [Google Scholar]
- 11. Harnek J, Nilsson J, Friberg O, James S, Lagerqvist B, Hambraeus K, Cider A, Svennberg L, Attebring MF, Held C, Johansson P, Jernberg T. The 2011 outcome from the Swedish health care registry on heart disease (SWEDEHEART). Scand Cardiovasc J. 2013;47(suppl 62):1–10. [DOI] [PubMed] [Google Scholar]
- 12. Emilsson L, Lindahl B, Koster M, Lambe M, Ludvigsson JF. Review of 103 Swedish healthcare quality registries. J Intern Med. 2015;277:94–136. [DOI] [PubMed] [Google Scholar]
- 13. Swedish National Diabetes Register . Annual report 2013. Available at: https://www.Ndr.Nu/pdf/annual_report_ndr_2013.Pdf. Accessed June 10, 2015.
- 14. Ludvigsson JF, Otterblad‐Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holzmann MJ, Rathsman B, Eliasson B, Kuhl J, Svensson AM, Nyström T, Sartipy U. Long‐term prognosis in patients with type 1 and 2 diabetes mellitus after coronary artery bypass grafting. J Am Coll Cardiol. 2015;65:1644–1652. [DOI] [PubMed] [Google Scholar]
- 16. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Longitudinal integration database for health insurance and labour market studies (LISA by Swedish acronym). Available at: http://www.Scb.Se/en_/services/guidance-for-researchers-and-universities/scb-data/longitudinal-integration-database-for-health-insurance-and-labour-market-studies-lisa-by-swedish-acronym. Accessed June 10, 2015.
- 18. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 19. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012;11:261–271. [DOI] [PubMed] [Google Scholar]
- 21. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta‐analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–1980. [DOI] [PubMed] [Google Scholar]
- 22. Huxley RR, Peters SA, Mishra GD, Woodward M. Risk of all‐cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2015;3:198–206. [DOI] [PubMed] [Google Scholar]
- 23. Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO mutinational study of vascular disease in diabetes. Diabetologia. 2001;44(suppl 2):S54–S64. [DOI] [PubMed] [Google Scholar]
- 24. Janghorbani M, Hu FB, Willett WC, Li TY, Manson JE, Logroscino G, Rexrode KM. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses’ Health Study. Diabetes Care. 2007;30:1730–1735. [DOI] [PubMed] [Google Scholar]
- 25. Sundquist K, Li X. Type 1 diabetes as a risk factor for stroke in men and women aged 15–49: a nationwide study from Sweden. Diabet Med. 2006;23:1261–1267. [DOI] [PubMed] [Google Scholar]
- 26. Soedamah‐Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM. High risk of cardiovascular disease in patients with type 1 diabetes in the U.K.: a cohort study using the general practice research database. Diabetes Care. 2006;29:798–804. [DOI] [PubMed] [Google Scholar]
- 27. Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H, Dahlqvist S, Clements M, Rosengren A. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982. [DOI] [PubMed] [Google Scholar]
- 28. Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, Leese G, Leslie P, McCrimmon RJ, Metcalfe W, McKnight JA, Morris AD, Pearson DW, Petrie JR, Philip S, Sattar NA, Traynor JP, Colhoun HM; Scottish Diabetes Research Network Epidemiology Group, Scottish Renal Registry . Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA. 2015;313:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Ferranti SD, de Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, Magge SN, Marx N, McGuire DK, Orchard TJ, Zinman B, Eckel RH. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130:1110–1130. [DOI] [PubMed] [Google Scholar]
- 30. Ramirez‐Valiente JA, Lorenzo Z, Soto A, Valladares F, Gil L, Aranda I. Elucidating the role of genetic drift and natural selection in cork oak differentiation regarding drought tolerance. Mol Ecol. 2009;18:3803–3815. [DOI] [PubMed] [Google Scholar]
- 31. Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K, Lederballe‐Pedersen O, Nieminen MS, Omvik P, Oparil S, Wedel H, Aurup P, Edelman J, Snapinn S; Group LS . Cardiovascular morbidity and mortality in patients with diabetes in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:1004–1010. [DOI] [PubMed] [Google Scholar]
- 32. Accord Study Group , Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons‐Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail‐Beigi F. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hägg S, Thorn LM, Forsblom CM, Gordin D, Saraheimo M, Tolonen N, Waden J, Liebkind R, Putaala J, Tatlisumak T, Groop PH; FinnDiane Study G . Different risk factor profiles for ischemic and hemorrhagic stroke in type 1 diabetes mellitus. Stroke. 2014;45:2558–2562. [DOI] [PubMed] [Google Scholar]
- 34. Action to Control Cardiovascular Risk in Diabetes Study Group , Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail‐Beigi F, Grimm RH Jr, Probstfield JL, Simons‐Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Advance Collaborative Group , Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. [DOI] [PubMed] [Google Scholar]
- 36. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. [DOI] [PubMed] [Google Scholar]
- 37. Accord Study Group , Gerstein HC, Miller ME, Genuth S, Ismail‐Beigi F, Buse JB, Goff DC Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH Jr, Byington RP, Rosenberg YD, Friedewald WT. Long‐term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kruyt ND, Biessels GJ, DeVries JH, Luitse MJ, Vermeulen M, Rinkel GJ, Vandertop WP, Roos YB. Hyperglycemia in aneurysmal subarachnoid hemorrhage: a potentially modifiable risk factor for poor outcome. J Cereb Blood Flow Metab. 2010;30:1577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Origin Trial Investigators , Gerstein HC, Bosch J, Dagenais GR, Diaz R, Jung H, Maggioni AP, Pogue J, Probstfield J, Ramachandran A, Riddle MC, Ryden LE, Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. [DOI] [PubMed] [Google Scholar]
- 40. Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendic S, Ryden L, Malmberg K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet. 2002;359:2140–2144. [DOI] [PubMed] [Google Scholar]


