Abstract
Background
Arterial stiffness, pressure pulsatility, and wave reflection are associated with cardiovascular disease. Left ventricular function is coupled to proximal aortic properties, but the association of central aortic stiffness and hemodynamics with incident clinical heart failure (HF) is not well described.
Methods and Results
Framingham Study participants without clinical HF (n=2539, mean age 64 years, 56% women) underwent applanation tonometry to measure carotid‐femoral pulse wave velocity (CFPWV), central pulse pressure, forward wave amplitude, and augmentation index. CFPWV was inverse‐transformed to reduce heteroscedasticity and multiplied by −1 to restore effect direction (iCFPWV). Over 10.1 (range 0.04–12.9) years, 170 HF events developed. In multivariable‐adjusted analyses, iCFPWV was associated with incident HF in a continuous, graded fashion (hazards ratio [HR] per SD unit [SDU] 1.29, 95% confidence interval [CI] 1.02–1.64, P=0.037). iCFPWV was associated with HF with reduced ejection fraction (HR=1.69/SDU, 95% CI 1.19–2.42, P=0.0037) in age‐ and sex‐adjusted models, which was attenuated in multivariable‐adjusted models (P=0.065). Central pulse pressure and forward wave amplitude were associated with HF in age‐ and sex‐adjusted models (per SDU, HR=1.20, 95% CI 1.06–1.37, P=0.006, and HR=1.15, 95% CI 1.01–1.31, P=0.036, respectively), but not in multivariable‐adjusted models (both P≥0.28). Augmentation index was not associated with HF risk (P≥0.19 in all models).
Conclusions
In our prospective investigation of a large community‐based sample of middle‐aged to elderly individuals, greater aortic stiffness (reflected by higher iCFPWV) was associated with increased risk of HF. Future studies may investigate the impact of modifying aortic stiffness in reducing the community burden of HF.
Keywords: aortic stiffness, epidemiology, heart failure, pressure pulsatility
Introduction
Elevated central arterial stiffness, a hallmark of aging,1, 2 is associated with adverse clinical outcomes, including coronary heart disease, stroke, and cardiovascular disease (CVD) mortality.3, 4, 5, 6 In animal and cross‐sectional human studies, greater arterial stiffness is closely related with both left ventricular (LV) systolic and diastolic dysfunction.7, 8, 9, 10 In addition, individuals with clinical heart failure (HF) with preserved11, 12, 13 or reduced ejection fraction14, 15 (HFPEF, HFREF, respectively) have elevated arterial stiffness. Wave reflection is a property of normal aortic function, but when elevated, it may increase hemodynamic load on the heart. Wave reflection also has been shown to be associated with incident HF.8, 16 However, prior investigations used diverse methodologies for the assessment of arterial stiffness, were predominantly cross‐sectional, and largely studied individuals with some degree of existing systolic and/or diastolic LV dysfunction. Comprehensive prospective studies relating vascular stiffness to heart failure risk in samples without prevalent CVD are sparse.16, 17
The pathogenesis of aortic stiffness‐related CVD may be due in part and also potentiated by abnormal hemodynamic interactions with the LV, particularly in patients with HFPEF.13 We hypothesized that tonometric measures of aortic stiffness and pressure pulsatility would be associated with incident HF in an ambulatory cohort free of baseline HF. We also posited that aortic stiffness measures were more strongly associated with incidence of HFPEF versus HFREF. We sought to test our hypotheses in the community‐based Framingham Heart Study sample.
Methods
Study Population
The Framingham Heart Study is a longitudinal epidemiological cohort study of adults in the community. The design and selection criteria of the Original and Offspring Cohorts have been described previously.18, 19 Briefly, the Original Cohort was examined every 2 years, beginning with its recruitment in 1948. The Offspring Cohort began in 1971, with serial examinations approximately every 4 to 8 years. Participants underwent routine medical history, physical examination, anthropometry, and electrocardiography. The present study included Original Cohort members attending their 26th examination cycle (2000–2001) and Offspring Cohort members participating in the 7th examination cycle (1998–2001). Applanation tonometry was performed in 310 of 558 and 2660 of 3539 Original and Offspring Cohort members, respectively. We excluded participants with prevalent HF (n=60), no follow‐up (n=6), and missing CFPWV (n=365). Tonometry did not begin with the onset of Offspring examination 7 and consequently was not completed in individuals who had their examinations early in the cycle. The characteristics of participants in whom tonometry was and was not completed are shown in Table 1. Participants who had tonometry were younger and had a slightly lower prevalence of CVD.
Table 1.
Clinical Characteristics of FHS Participants by Tonometry Acquisition
| Characteristic | No Tonometry (n=1127) | Had Tonometry (n=2970) | P Value |
|---|---|---|---|
| Age, y | 69±13 | 64±12 | <0.0001 |
| Male sex, % | 42 | 44 | 0.28 |
| Body mass index, kg/m2 | 28.2±5.0 | 27.9±5.3 | 0.22 |
| Prevalent CVD, % | 20 | 17 | 0.01 |
| Systolic blood pressure, mm Hg | 128±19 | 129±20 | 0.22 |
| Diastolic blood pressure, mm Hg | 72±10 | 73±10 | <0.0001 |
| Use of antihypertensive medications, % | 40 | 37 | 0.11 |
| Diabetes, % | 12 | 11 | 0.43 |
| Current smoking, % | 12 | 13 | 0.44 |
| Total cholesterol, mg/dL | 198±36 | 199±37 | 0.63 |
| HDL cholesterol, mg/dL | 51±16 | 54±17 | <0.0001 |
Continuous data presented as mean±SD. CVD indicates cardiovascular disease; FHS, Framingham Heart Study; HDL, high‐density lipoprotein.
The study was approved by the Boston University Medical Center institutional review board. All study participants provided written informed consent.
Arterial Tonometry Data Acquisition and Analysis
Participants were studied in the supine position after ≈5 minutes of rest. Supine brachial systolic and diastolic blood pressures were obtained using an oscillometric device. Arterial tonometry with simultaneous ECG was obtained from brachial, radial, femoral, and carotid arteries using a commercially available tonometer (SPT‐301; Millar Instruments, Houston, TX). Transit distances were assessed by the subtraction method, using body surface measurements from the suprasternal notch to each pulse recording site.4 Tonometry and ECG data were digitized during the primary acquisition (1000 Hz), transferred to CD‐ROMs, shipped to the core lab (Cardiovascular Engineering, Inc, Norwood, MA) and analyzed by operators who were blinded to clinical data.
Details of the tonometry data analysis have been reported.20 Tonometry waveforms were signal‐averaged using the ECG R‐wave as a fiducial point. The brachial waveform was calibrated using cuff systolic and diastolic pressure and the integrated calibrated waveform was used to derive mean arterial pressure. Diastolic and integrated mean brachial pressures were then used to calibrate carotid pressure tracings. Central pulse pressure (CPP) was derived from calibrated carotid pressure waveforms. CFPWV was calculated from tonometry waveforms and body surface measurements, which were adjusted for parallel transmission in the brachiocephalic artery and aortic arch, using the suprasternal notch as a fiducial point. We used the carotid pressure waveform to measure forward wave amplitude and augmentation index (AI). AI was calculated as the augmented pressure divided by CPP. Forward wave amplitude was estimated from the primary pressure wave defined as the difference between pressure at the waveform foot and pressure at the first systolic inflection point or peak of the carotid pressure waveform.
Clinical Follow Up
Clinical covariates were assessed at the respective examinations of the Original and Offspring cohorts. Diabetes mellitus was defined as a fasting glucose ≥126 mg/dL, nonfasting blood glucose ≥200 mg/dL, or the use of insulin or oral hypoglycemic medications. Prevalent CVD, including coronary heart disease, stroke, and transient ischemic attack was adjudicated by a 3‐investigator committee based upon review of Framingham Heart Study (FHS) physician assessment and all pertinent medical records. A history of coronary heart disease was defined as myocardial infarction (diagnostic ECG, cardiac biomarkers, and clinical presentation), coronary insufficiency (unstable angina), or stable angina. We excluded participants with missing covariates.
Assessment of Heart Failure
HF events are adjudicated by a 3‐physician committee after extensive review of inpatient and outpatient medical records, using established clinical criteria.21 The date of onset was noted as the first episode of heart failure symptoms noted by a physician or hospitalization. HFREF and HFPEF were defined as left ventricular ejection fraction <45% and ≥45%, respectively, as defined by echocardiography or radionuclide angiography within the year of incident HF diagnosis.22 The most common etiology of a significant change in ejection fraction within a short time period is a myocardial infarction. Thus, if an interim myocardial infarction between the time of LV imaging and incident HF occurred, the ejection fraction was considered unclassified, due to possible significant change in ejection fraction following myocardial infarction.
Statistical Methods
We used t tests and chi‐squared tests to compare continuous and categorical clinical covariates, respectively, among individuals with and without tonometry (Table 1). Participants from both cohorts were pooled and stratified by cohort type in each of the analyses to assess the associations of each of the tonometry variables with the outcome of incident HF. CFPWV, the primary variable of interest, was inverted to reduce heteroscedasticity, sex‐standardized, and multiplied by −1 to restore the directionality of effect (standardized CFPWV, termed iCFPWV). CPP, forward wave amplitude, and AI did not have heteroscedasticity and were not transformed prior to statistical analysis. Figures 1, 2, 3, 4 through 5 demonstrate the variance of each tonometry variable with age. Continuous relationships between CFPWV and incident HF were depicted by a penalized cubic spline.23 Cumulative incidence curves described associations between tertiles of CFPWV and incident HF, adjusting for the competing risk of non‐HF death.24 We used proportional hazards regression models to assess the associations between tonometry measures and incident HF. The Fine‐Gray method was used to account for the competing risk of death. Models were first fitted adjusting for age and sex, and then adjusting for additional clinical covariates (body mass index, mean arterial pressure, total cholesterol, HDL cholesterol, history of antihypertensive medications, diabetes, current smoking, and prevalent CVD). We only analyzed complete cases without missing variables in multivariable models. We further conducted separate, similar sensitivity analyses for the association of CFPWV with HFPEF and HFREF, whereby participants with unclassified LV ejection fraction were counted as non‐events. Lastly, we compared the hazards of CFPWV for HFPEF with that of HFREF.
Figure 1.
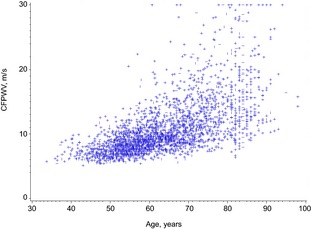
Plot of carotid femoral pulse wave velocity (CFPWV) with age. The variance of untransformed CFPWV increased markedly at older ages, whereas the variances of the other tonometry variables with age (Figures 2, 3, 4 through 5) were not as notable. Y axis is standardized measure of CFPWV.
Figure 2.
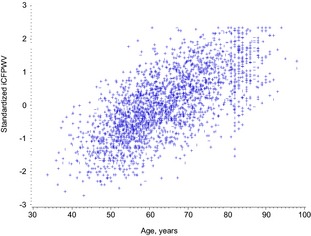
Plot of inverse‐transformed carotid‐femoral pulse wave velocity (iCFPWV) with age. Y axis is standardized measure of iCFPWV.
Figure 3.
Plot of central pulse pressure (CPP) with age. Y axis is standardized measure of CPP.
Figure 4.
Plot of forward wave amplitude with age. Y axis is standardized measure of forward wave amplitude.
Figure 5.
Plot of augmentation index (AI) with age. Y axis is standardized measure of AI.
Results
Baseline clinical characteristics of our study sample by cohort and heart failure status, including untransformed CFPWV, are shown in Table 2. A total of 2539 participants (mean age 64+12 years, 56% women) were included in the analyses. Median follow‐up time was 10.1 years (limits 0.04–12.9 years). Incident HF occurred in 170 participants (Original Cohort, n=69; Offspring Cohort, n=101; n=86 women), among whom 77 (43%) developed HFPEF and 61 (34%) developed HFREF, with 32 unclassified cases (23%).
Table 2.
Characteristics of FHS Participants by Cohort and Development of HF in Follow Up
| Baseline Characteristic | Original Cohort (n=272) | Offspring Cohort (n=2267) | ||
|---|---|---|---|---|
| No HF (n=203) | HF (n=69) | No HF (n=2166) | HF (n=101) | |
| Age, y | 85±3 | 84±3 | 61±9 | 71±8 |
| Body mass index, kg/m2 | 26.2±4.3 | 25.9±4.0 | 27.3±4.6 | 29.0±4.8 |
| Prevalent CVD, % | 37 | 43 | 11 | 39 |
| Systolic blood pressure, mm Hg | 141±21 | 148±18 | 127±19 | 138±21 |
| Diastolic blood pressure, mm Hg | 68±11 | 66±10 | 74±10 | 73±11 |
| Mean arterial pressure, mm Hg | 92±14 | 95±13 | 91±12 | 96±13 |
| Use of antihypertensive medications, % | 65 | 62 | 31 | 58 |
| Diabetes, % | 4 | 6 | 9 | 30 |
| Current smoking, % | 4 | 4 | 13 | 9 |
| Total cholesterol, mg/dL | 190±37 | 177±31 | 202±37 | 187±38 |
| HDL cholesterol, mg/dL | 56±17 | 55±19 | 55±17 | 48±16 |
| Tonometry measures | ||||
| Carotid‐femoral pulse wave velocity, m/s | 15.7±5.6 | 17.3±6.2 | 10.0±3.5 | 13.3±4.7 |
| Central pulse pressure, mm Hg | 65.2±21.8 | 69.6±18.0 | 50.3±16.1 | 60.7±21.5 |
| Forward wave amplitude, mm Hg | 54.2±17.9 | 56.4±16.0 | 40.5±12.5 | 48.6±18.5 |
| Augmentation index, % | 13.0±12.0 | 16.1±12.5 | 15.0±12.4 | 14.4±17.1 |
Data presented as mean ±SD or percentage. CVD, prior coronary heart disease, myocardial infarction, angina, stroke, transient ischemic attack. CVD indicates cardiovascular disease; FHS, Framingham Heart Study; HDL, high‐density lipoprotein; HF, heart failure.
Relations of Arterial Stiffness, Pressure Pulsatility, and Wave Reflection with Incident HF
Standardized, transformed CFPWV (iCFPWV) are presented to describe the relations of arterial stiffness with HF. Each standard deviation higher iCFPWV conferred a 50% (P=0.0002) and ≈30% (P=0.037) increased risk of incident HF in age‐ and sex‐adjusted and multivariable‐adjusted analyses, respectively (Table 3). iCFPWV was associated with risk for incident HF in a continuous manner (Figure 6). The incidence of HF rose across iCFPWV tertiles (Figure 7). An analysis of sex‐interactions between iCFPWV and incident HF was non‐significant (P=0.90).
Table 3.
Relations of Tonometry Measures With Incident HF
| Model | Age‐ and Sex‐Adjusted | Multivariable‐Adjusted | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| iCFPWV, s/m | 1.50 (1.21–1.85) | <0.001 | 1.29 (1.02–1.64) | 0.037 |
| Central pulse pressure, mm Hg | 1.20 (1.06–1.37) | 0.006 | 1.10 (0.93–1.29) | 0.28 |
| Forward wave amplitude, mm Hg | 1.15 (1.01–1.31) | 0.036 | 1.02 (0.88–1.19) | 0.79 |
| Augmentation index, % | 1.10 (0.95–1.29) | 0.21 | 1.11 (0.95–1.31) | 0.19 |
HR for incident HF expressed per standard deviation increment in tonometry variable. SD for the pooled sample were as follows: iCFPWV SD=31.1 s/m, Central pulse pressure SD=17.7 mm Hg, Forward wave amplitude SD=14.1 mm Hg, Augmentation index SD=12.6%. Multivariable model adjusted for age, sex, body mass index, mean arterial pressure, total cholesterol, HDL cholesterol, history of antihypertensive medications, diabetes mellitus, current smoking, and baseline cardiovascular disease. HDL indicates high‐density lipoprotein; HF, heart failure; HR, hazards ratio; iCFPWV, inverse‐transformed carotid femoral pulse wave velocity; SD, standard deviations.
Figure 6.
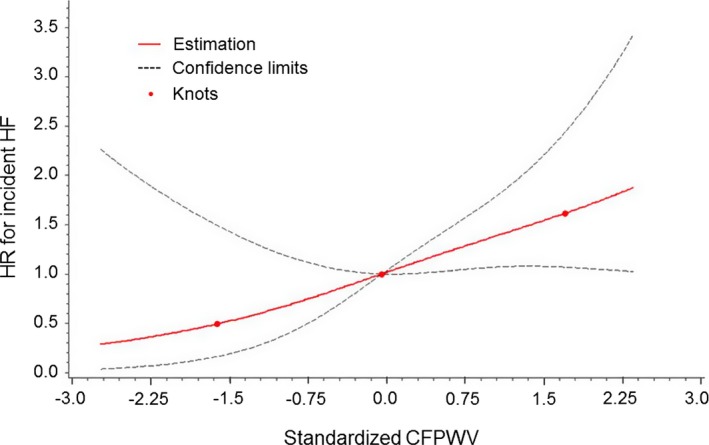
Continuous association of iCFPWV with risk for incident HF. The risk for incident HF increased continuously with increasing standardized iCFPWV. The x‐axis represents standardized values for inverse‐transformed CFPWV: mean −104.2 s/m maps to 0 and 1 unit on x‐axis (1 SD) corresponds with 31.1 s/m. Each 1 SD increase in iCFPWV corresponds with 1.29‐fold higher hazard for incident HF. A test for non‐linearity was nonsignificant (P=0.63). iCFPWV indicates inverse‐transformed carotid femoral pulse wave velocity; HF, heart failure; HR, hazard ratio.
Figure 7.
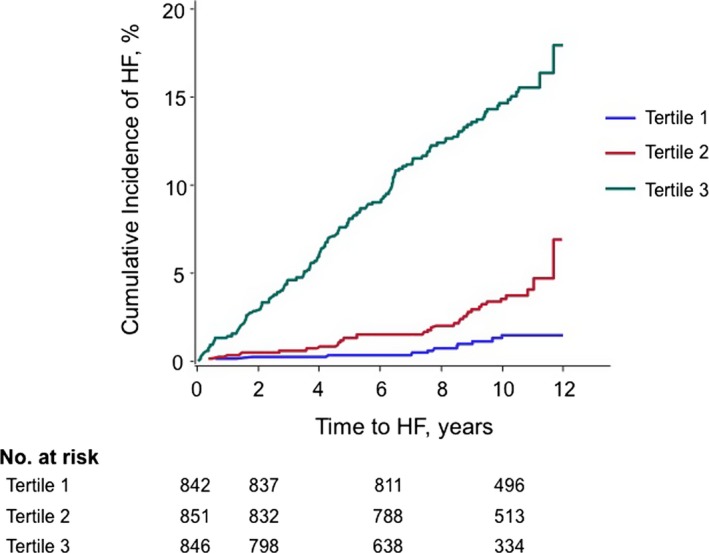
Risk of heart failure by tertile of sex‐standardized iCFPWV. The cumulative incidence of HF rose with greater tertile of iCFPWV. Sex‐standardized iCFPWV tertiles: Tertile 1: <−0.5; Tertile 2: −0.5 to 0.4; Tertile 3: >0.4. iCFPWV indicates inverse‐transformed carotid femoral pulse wave velocity; HF, heart failure.
In the age‐ and sex‐adjusted model, CPP and forward wave amplitude were nominally associated with incident HF, but there were no statistically significant associations between HF and CPP or forward wave amplitude after adjustment for additional covariates (Table 3). In stepwise multivariable analysis, total cholesterol level (P=0.0001) and diabetes (0.003) were the covariates that attenuated the association between either CPP or forward wave amplitude and HF (observed in age‐ and sex‐adjusted models). No significant relation was seen between AI and incident HF in either age‐ and sex‐adjusted or multivariable‐adjusted models.
Relations of Arterial Stiffness With HF Subtype
There was no statistically significant relation seen between iCFPWV and incident HFPEF in an age‐ and sex‐adjusted model (HR=1.35 per SD increment, 95% CI 0.98–1.85, P=0.054), which was attenuated in a model adjusting for additional clinical covariates (HR=1.22 per SD increment, 95% CI 0.85–1.73, P=0.28). iCFPWV was associated positively with a significant risk for HFREF (HR=1.69 per SD increment, 95% CI 1.19–2.42, P=0.0037) in age‐ and sex‐adjusted models, which was attenuated with further adjustment for clinical covariates (HR=1.46 per SD increment, 95% CI 0.98–2.18, P=0.065). There were no significant differences comparing the hazards of iCFPWV for HFPEF with that for HFREF (age‐ and sex‐adjusted P=0.58, multivariable‐adjusted P=0.71). These results suggest that there are comparable risks for HFREF versus HFPEF conferred by CFPWV.
Discussion
In our community‐based sample of middle‐aged and older adults, we observed several important findings. Higher iCFPWV, a measure of greater global aortic stiffness, was associated with incident HF, after adjustment for standard risk factors including mean arterial blood pressure, consistent with the hypothesis that the contribution of stiffness to development of HF is beyond that conferred by these risk factors. iCFPWV was associated with HF in a continuous manner. Moreover, greater iCFPWV was associated with both HFPEF and HFREF, although the findings did not achieve statistical significance, in part due to a modest number of HF events.
Aortic Stiffness and Incident HF
Elevated aortic stiffness is associated with cardiovascular events, including coronary heart disease, stroke, and CVD death in population studies.3, 4, 5, 6 CFPWV was associated with LV systolic and diastolic function cross‐sectionally in a high‐risk population.8 In addition, HF was included in the composite CVD definition in a prior FHS study of CFPWV in prediction of CVD events.4 However, to our knowledge, the present investigation is one of the few to examine the relations of CFPWV, the gold standard measure of global aortic stiffness, and HF incidence prospectively in an unselected community‐based sample.
We observed a modest, albeit statistically non‐significant, association between aortic stiffness and HFREF. A common etiology for HFREF is coronary heart disease and myocardial infarction. CFPWV, as an index of aortic stiffness, is associated with coronary disease, including in those without apparent CVD.3, 25 Common pathophysiologic mechanisms may underlie the associations of aortic stiffness with both HFREF and HFPEF. For example, the myocardial oxygen supply‐demand mismatch conferred by both LV hypertrophy and lower central diastolic blood pressure (often concomitant with increased aortic stiffness) may result in reduced coronary perfusion and subendocardial ischemia.26, 27 Elevated aortic stiffness may also create intimal damage, leading to development and accelerated progression of coronary atherosclerosis. The results of studies from FHS and others have substantiated these hypotheses, demonstrating that increased aortic stiffness is associated with greater atherosclerotic burden.25, 28, 29
Community‐based studies have demonstrated that ventricular and arterial stiffening occur in parallel with aging,30 and multiple lines of evidence suggest that ventriculo‐arterial interactions contribute to the pathophysiology of both HFPEF and HFREF. Patients with HF with either normal12, 13 or reduced31 EF have elevated aortic stiffness compared with reference subjects without HF. Physiological studies in hypertensive cohorts have shown that greater degrees of LV diastolic dysfunction are associated progressively with higher arterial stiffness.32 Greater aortic stiffness results in an earlier reflected wave return, which augments systolic blood pressure, places an unfavorable load on the LV during systole, and may delay ventricular diastolic relaxation.33 We did not observe a significant association of aortic stiffness with incident HFPEF, which may have been attributable to low event rates and the relative health of our sample. While middle‐aged women may have greater LV wall stress than men,34 we did not observe effect modification by sex in the relation between arterial stiffness and incident HF. Overall, our study extends prior observations of the cross‐sectional relations of aortic stiffness with HF by demonstrating prospectively a continuous relation between aortic stiffness, measured by CFPWV, and incident clinical HF, across the full spectrum from HFREF to HFPEF.
Pressure Pulsatility, Wave Reflection, and Left Ventricular Dysfunction
The associations between pressure pulsatility and wave reflection with HF are less clear. The inclusion of CVD risk factors in multivariable models significantly attenuated the observed relations seen between CPP and forward wave amplitude with incident HF in age‐ and sex‐adjusted models. Brachial pulse pressure was associated with incident HF previously in the older FHS cohort in models adjusting for common CVD risk factors, which did not include measures of blood pressure.35 In contrast, we studied central pulse pressure, included a younger cohort with fewer HF events, and additionally accounted for mean arterial pressure and blood pressure‐lowering medications, which may partially explain differences in results.
We did not observe a relation of AI, a commonly used index of wave reflection, with incident HF, consistent with other studies, including population‐based samples.16, 17, 36 An arterial forward wave is partially reflected at areas of impedance mismatch, such as branching or vessel narrowing. Augmentation pressure, the increase in aortic pressure above that contributed by the forward waveform, has been thought to be primarily determined by the backward reflected wave. However, invasive hemodynamics suggest that the magnitude of augmented pressure is influenced by LV contraction and relaxation accounting for the reflected wave.37 Likewise, LV dynamics likely affects CPP and AI, the ratio of augmented pressure to CPP. As individuals with impaired LV function are those who manifest clinical HF symptoms, CPP and AI are not reliable measures of wave reflection particularly in this group. In contrast, CFPWV, as a measure of central aortic stiffness, is relatively less confounded by LV dysfunction,38 rendering these findings more robust.
Strengths and Limitations
The strengths of our study include its prospective design, comprehensive assessment of arterial stiffness and wave reflection, and meticulous follow‐up of participants with HF events that were adjudicated through review of medical records and symptoms. Our modest number of HF events, perhaps related to the relative health of our sample, limited our ability to examine the development of HFPEF and HFREF separately. Due to the limited number of events, we also did not have adequate power to examine the relations of arterial stiffness with HF in a subsample without baseline CVD. Future studies may investigate the association of arterial stiffness and pressure pulsatility with HF severity, in which standardized criteria were unavailable across FHS examinations. Lastly, our sample consisted of a majority of people who were white and of European ancestry, limiting the generalizability of our findings to other ethnic groups.
Conclusions
In our prospective study of a community‐based sample of ambulatory older adults, elevated aortic stiffness as evidenced by higher iCFPWV was associated with the development of HF. Replication of our findings would emphasize the need for evaluating underlying biological mechanisms and for studying the impact of lowering arterial stiffness on HF risk in the community.
Sources of Funding
The Framingham Heart Study is funded by the National Heart, Lung and Blood Institute (HHSN268201500001I, NO1‐HC‐25195, HL076784, AG028321, HL070100, HL060040, HL080124, HL071039, HL077447, 6R01‐NS‐17950, and HL107385). This work was also supported by the American Heart Association (13SDG14250015 to Tsao), and National Institutes of Health (1K23 HL118259 to Tsao).
Disclosures
Mitchell is the owner of Cardiovascular Engineering, Inc, a company that develops and manufactures devices to measure vascular stiffness.
(J Am Heart Assoc. 2015;4:e002189 doi: 10.1161/JAHA.115.002189)
References
- 1. Alecu C, Gueguen R, Aubry C, Salvi P, Perret‐Guillaume C, Ducrocq X, Vespignani H, Benetos A. Determinants of arterial stiffness in an apparently healthy population over 60 years. J Hum Hypertens. 2006;20:749–756. [DOI] [PubMed] [Google Scholar]
- 2. Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Cross‐sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–2636. [DOI] [PubMed] [Google Scholar]
- 3. Mattace‐Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM, Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. [DOI] [PubMed] [Google Scholar]
- 4. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutton‐Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG, Spurgeon H, Kritchevsky S, Pahor M, Bauer D, Newman A. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well‐functioning older adults. Circulation. 2005;111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 6. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. [DOI] [PubMed] [Google Scholar]
- 7. Fernandes VR, Polak JF, Cheng S, Rosen BD, Carvalho B, Nasir K, McClelland R, Hundley G, Pearson G, O'Leary DH, Bluemke DA, Lima JA. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. [DOI] [PubMed] [Google Scholar]
- 8. Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008;21:1194–1202. [DOI] [PubMed] [Google Scholar]
- 9. Shapiro BP, Lam CS, Patel JB, Mohammed SF, Kruger M, Meyer DM, Linke WA, Redfield MM. Acute and chronic ventricular‐arterial coupling in systole and diastole: insights from an elderly hypertensive model. Hypertension. 2007;50:503–511. [DOI] [PubMed] [Google Scholar]
- 10. Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle‐dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. [DOI] [PubMed] [Google Scholar]
- 12. Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. [DOI] [PubMed] [Google Scholar]
- 13. Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular‐vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patrianakos AP, Parthenakis FI, Karakitsos D, Nyktari E, Vardas PE. Proximal aortic stiffness is related to left ventricular function and exercise capacity in patients with dilated cardiomyopathy. Eur J Echocardiogr. 2009;10:425–432. [DOI] [PubMed] [Google Scholar]
- 15. Rerkpattanapipat P, Hundley WG, Link KM, Brubaker PH, Hamilton CA, Darty SN, Morgan TM, Kitzman DW. Relation of aortic distensibility determined by magnetic resonance imaging in patients > or =60 years of age to systolic heart failure and exercise capacity. Am J Cardiol. 2002;90:1221–1225. [DOI] [PubMed] [Google Scholar]
- 16. Chirinos JA, Kips JG, Jacobs DR Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis). J Am Coll Cardiol. 2012;60:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15‐year all‐cause and cardiovascular mortalities: a community‐based study. Hypertension. 2010;55:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dawber TR, Meadors GF, Moore FE Jr. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 20. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. [DOI] [PubMed] [Google Scholar]
- 22. Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Desquilbet L, Mariotti F. Dose‐response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–1057. [DOI] [PubMed] [Google Scholar]
- 24. Gaynor JJ, Feuer EJ, Tan CC, Wu DH, Little CR, Straus DJ, Clarkson BD, Brennan MF. On the use of cause‐specific failure and conditional failure probabilities. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 25. Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent S. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. [DOI] [PubMed] [Google Scholar]
- 26. Safar ME. Pulse pressure in essential hypertension: clinical and therapeutical implications. J Hypertens. 1989;7:769–776. [DOI] [PubMed] [Google Scholar]
- 27. Watanabe H, Ohtsuka S, Kakihana M, Sugishita Y. Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol. 1993;21:1497–1506. [DOI] [PubMed] [Google Scholar]
- 28. Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Berent R, Eber B. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–189. [DOI] [PubMed] [Google Scholar]
- 29. van Popele NM, Mattace‐Raso FU, Vliegenthart R, Grobbee DE, Asmar R, van der Kuip DA, Hofman A, de Feijter PJ, Oudkerk M, Witteman JC. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens. 2006;24:2371–2376. [DOI] [PubMed] [Google Scholar]
- 30. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age‐ and gender‐related ventricular‐vascular stiffening: a community‐based study. Circulation. 2005;112:2254–2262. [DOI] [PubMed] [Google Scholar]
- 31. Giannattasio C, Achilli F, Failla M, Capra A, Vincenzi A, Valagussa F, Mancia G. Radial, carotid and aortic distensibility in congestive heart failure: effects of high‐dose angiotensin‐converting enzyme inhibitor or low‐dose association with angiotensin type 1 receptor blockade. J Am Coll Cardiol. 2002;39:1275–1282. [DOI] [PubMed] [Google Scholar]
- 32. Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ooi H, Chung W, Biolo A. Arterial stiffness and vascular load in heart failure. Congest Heart Fail. 2008;14:31–36. [DOI] [PubMed] [Google Scholar]
- 34. Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC, Asklepios I. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle‐aged adults: the Asklepios study. Hypertension. 2013;61:296–303. [DOI] [PubMed] [Google Scholar]
- 35. Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. [DOI] [PubMed] [Google Scholar]
- 36. Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NR, Thom SA, Hughes AD, Investigators A. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo‐Scandinavian Cardiac Outcome Trial) substudy. J Am Coll Cardiol. 2010;56:24–30. [DOI] [PubMed] [Google Scholar]
- 37. Fok H, Guilcher A, Li Y, Brett S, Shah A, Clapp B, Chowienczyk P. Augmentation pressure is influenced by ventricular contractility/relaxation dynamics: novel mechanism of reduction of pulse pressure by nitrates. Hypertension. 2014;63:1050–1055. [DOI] [PubMed] [Google Scholar]
- 38. McDonald DA. Regional pulse‐wave velocity in the arterial tree. J Appl Physiol. 1968;24:73–78. [DOI] [PubMed] [Google Scholar]


