Abstract
Background
We hypothesized that a fully automated mobile health (mHealth) intervention with tracking and texting components would increase physical activity.
Methods and Results
mActive enrolled smartphone users aged 18 to 69 years at an ambulatory cardiology center in Baltimore, Maryland. We used sequential randomization to evaluate the intervention's 2 core components. After establishing baseline activity during a blinded run‐in (week 1), in phase I (weeks 2 to 3), we randomized 2:1 to unblinded versus blinded tracking. Unblinding allowed continuous access to activity data through a smartphone interface. In phase II (weeks 4 to 5), we randomized unblinded participants 1:1 to smart texts versus no texts. Smart texts provided smartphone‐delivered coaching 3 times/day aimed at individual encouragement and fostering feedback loops by a fully automated, physician‐written, theory‐based algorithm using real‐time activity data and 16 personal factors with a 10 000 steps/day goal. Forty‐eight outpatients (46% women, 21% nonwhite) enrolled with a mean±SD age of 58±8 years, body mass index of 31±6 kg/m2, and baseline activity of 9670±4350 steps/day. Daily activity data capture was 97.4%. The phase I change in activity was nonsignificantly higher in unblinded participants versus blinded controls by 1024 daily steps (95% confidence interval [CI], −580 to 2628; P=0.21). In phase II, participants receiving texts increased their daily steps over those not receiving texts by 2534 (95% CI, 1318 to 3750; P<0.001) and over blinded controls by 3376 (95% CI, 1951 to 4801; P<0.001).
Conclusions
An automated tracking‐texting intervention increased physical activity with, but not without, the texting component. These results support new mHealth tracking technologies as facilitators in need of behavior change drivers.
Clinical Trial Registration
URL: http://ClinicalTrials.gov/. Unique identifier: NCT01917812.
Keywords: accelerometer, activity tracker, automation, cardiovascular disease, digital health, eHealth, health technology, mHealth, mobile phone, pedometer, physical activity, prevention, smartphone, text messages, texting, wearable device, wearable sensor
Introduction
Physical activity is a central element of lifestyle guideline recommendations for the prevention of cardiovascular disease (CVD) and one of the health behaviors targeted by the American Heart Association's (AHA's) 2020 Strategic Impact Goals.1, 2 However, it is estimated that >50% of U.S. adults do not obtain ideal levels of physical activity, a statistic that has not significantly changed in National Health and Nutrition Examination Surveys since 1988–1994.3 Therefore, new approaches to physical activity promotion are needed.
Miniaturization and other advances in mobile health (mHealth) technology have enabled convenient and accurate tracking of physical activity through smartphone applications and wearable devices.4 Whereas in the past one would have to manually record daily activity when using a traditional pedometer, new technology automatically produces a digital activity log. In addition to streamlining self‐monitoring, the vast consumer adoption of these technologies has opened a new channel to deliver continuous feedback aimed at stimulating healthy behavior change.4, 5, 6, 7 The AHA's Scientific Statements on mHealth and interventions to promote activity have identified a critical need for rigorous studies of new mHealth techniques.7, 8
In this context, we conducted a randomized, clinical trial (“mActive”) testing the hypothesis that a fully automated, fully mobile, and physician‐designed mHealth intervention using new technologies to provide individual encouragement and foster feedback loops increases physical activity. The mHealth intervention had 2 core components—tracking and texting—and so we sought to use a trial design that would allow for specific evaluation of the effects of each of these components. Furthermore, we sought to conduct a practical trial maximizing convenience for the participants and investigators so that it would have greater potential to be scaled in future trial phases and adopted in routine care settings.
Methods
Trial Design
The mActive protocol was publically registered before enrollment (clinicaltrials.gov: NCT01917812) and was approved by the Johns Hopkins School of Medicine Institutional Review Board. We used sequential randomization to individually evaluate the tracking and texting components of the intervention. After establishing baseline activity during a blinded run‐in (week 1), in phase I (weeks 2 to 3) we randomized 2:1 to unblinded versus blinded tracking. The activity tracker itself did not show activity information, but continuously transmitted it in all participants. The activity data were only visible to those who were unblinded, as further described below. In phase II (weeks 4 to 5), we randomized unblinded participants 1:1 to “smart texts” versus no texts. Smart texts were automated, personalized, smartphone‐delivered coaching messages informed by real‐time activity and other factors, as described in detail below.
Participants
We enrolled outpatients at an academic CVD prevention center in Baltimore, Maryland from January 17 to May 20, 2014. We included patients aged 18 to 69 years who were using a Fitbug‐compatible smartphone (ie, iPhone≥4S, Galaxy≥S3). Intending to mainly modify leisure‐time activity, we targeted individuals reporting <3 days/week of moderate or vigorous leisure‐time activity lasting ≥30 min/day by the long form of the International Physical Activity Questionnaire (IPAQ).9 Validation of the questionnaire up to 69 years of age served as the rationale for the trial's upper age limit. We did not have an eligibility restriction based on smartphone or Internet literacy, although all subjects confirmed having access to e‐mail for pertinent trial communication. We recorded demographic and clinical characteristics, including dog ownership, because it is thought to have a positive effect on the owner by modifying their cognitive beliefs about walking, providing motivation, and providing social support for walking.10 All patients continued to receive routine care and gave written informed consent. Face‐to‐face visits were not required after enrollment.
Interventions
Participants used their own smartphones. Digital physical activity tracking was performed using the Fitbug Orb (Chicago, IL) (Figure S1), a wearable, display‐free, triaxial accelerometer that pairs with low‐energy Bluetooth with compatible smartphones. The 3V lithium battery lasts around 6 months and thus did not require charging or replacement during the trial. Unblinded patients could continuously view their daily step count, activity time, and aerobic activity time through smartphone and Web interfaces (Figure S2). The Fitbug app also provided a history tab allowing review of data from previous days. Activity data were updated every 15 minutes if transmission occurred by beacon mode or were available any time if a participant activated a manual data push or streaming mode.
To enable real‐time activity data to inform smart texts, we linked the application programming interfaces of Fitbug and a smart texting system (Reify, Baltimore, MD). Smart text content was written by the physician investigators and reflected behavioral change theories,11 particularly of feedback loops and habit formation,12 integrated with cardiovascular knowledge and clinical experience. Smart texts took into account the importance of prescription writing and having a specific, proximal goal; we used a goal of 10 000 steps/day.8, 13, 14, 15 Each participant was a patient of a study physician with texts aiming to leverage the physician‐patient relationship, using the physician's name in texts. Messages underwent content iterations to optimize language during pretrial testing by the study team.
Smart texts were grouped as positive reinforcement messages, sent when a participant was on track to attain or had already attained his or her daily goal, and booster messages, to motivate individuals when they were not tracking to surpass their step goal. According to the preprogrammed algorithm, smart texts were sent 3 times/day (morning, mid‐day, and evening), with exact times customized to the participant's usual wake time, lunch time, and beginning of evening leisure time. On the day of enrollment, all participants completed an online questionnaire (Table S1) to provide information on 16 personal and clinical characteristics, which was later used for personalizing text messages within the texting arm. Specific examples of text messages are shown in Table S2.
Outcomes
We considered the AHA's Scientific Statement on assessing physical activity for clinical and research applications.16 Given the setting‐specific resources and technology capabilities, and common clinical focus on step count, we set our primary outcome measure as the mean change in accelerometer‐measured daily step count assessed from baseline through phase I and II. In addition to this continuous outcome measure, we examined attainment of the prescribed 10 000 steps/day goal. Secondary activity outcome measures were changes in total daily activity time and aerobic time. Aerobic time was defined as the time spent walking continuously for >10 minutes without breaking for >1 minute. Additionally, we assessed participant satisfaction through an online survey, with qualitative and quantitative elements, upon trial completion.
Statistical Analysis
We estimated that the sample size needed to detect at least a 2000‐step difference in means was 14 participants per group, or 42 participants total, assuming a within‐participant SD of 1800 steps/day, 2‐sided alpha of 0.05, and beta of 0.8. Allowing for 12% attrition, target enrollment was 48 participants. Our trial aimed to detect an increase of 2000 steps/day, given that this was previously associated with a ≈10% relative reduction in the long‐term incidence of CVD,17 and is generally felt to represent a clinically significant increase. However, the selection of 2000 steps/day does not signify that a smaller increase in physical activity could not also be clinically significant.
Baseline characteristics were summarized using descriptive statistics—frequency (percentage) for categorical data and mean (standard deviation) for continuous data. All outcomes were compared between treatment arms by intention to treat. We used repeated measures analysis of variance for mean comparison tests and calculated 95% confidence intervals (CIs). For comparisons of proportions, we used Fisher's exact test because of small cell sizes. Exploratory subgroup interaction testing was performed to examine for heterogeneity in treatment effect by age, sex, race, body mass index (BMI), diabetes, hypertension, dyslipidemia, coronary heart disease (CHD), dog ownership, marital status, employment, or baseline activity. Statistical analyses were performed using Stata software (version 11.1; StataCorp LP, College Station, TX).
Reporting
We followed the Consolidated Standards of Reporting Trials of Electronic and Mobile HEalth Applications and onLine TeleHealth (CONSORT‐EHEALTH).18 Most elements are reported here, with additional details on CONSORT‐EHEALTH items provided in Table S3.
Results
Participant and Data Flow
The trial flow diagram is shown in Figure 1. Of 50 eligible individuals, 48 (96%) enrolled. There was no early dropout and all participants in the intervention arms completed the protocol. One blinded participant had data transmission issues in phase II and elected not to complete the protocol. Daily activity data capture was 97.4%.
Figure 1.
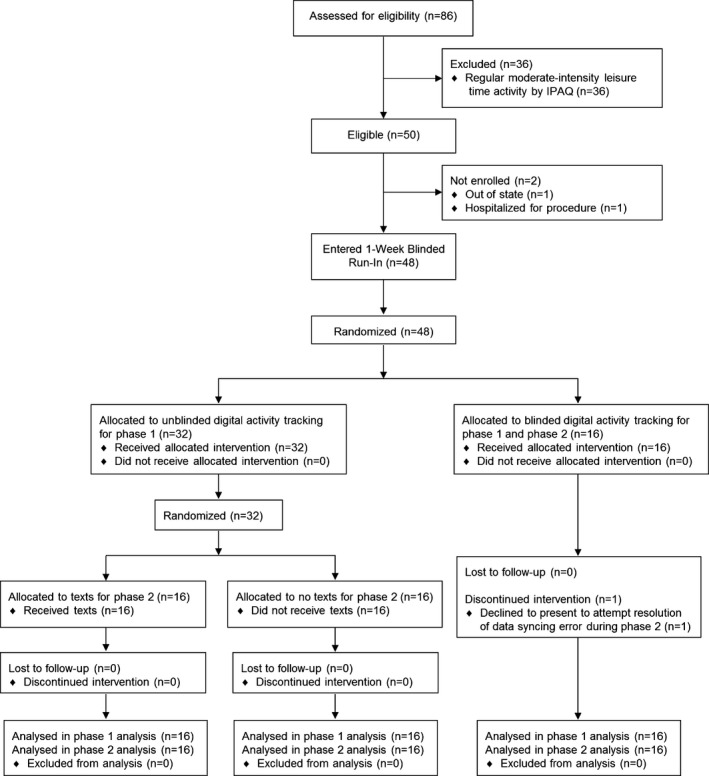
mActive trial flow diagram. IPAQ indicates International Physical Activity Questionnaire, Long‐Form.
Baseline Characteristics
There were no significant baseline differences between groups (Table 1). Overall, participants were age 58±8 years, nearly evenly split by sex, and 21% nonwhite. Eighty‐eight percent were employed, primarily in management and professional occupations. The majority of participants were obese, 23% had diabetes, and 29% CHD. Baseline activity levels were 9670±4350 steps/day, 93±45 min/day, and 13±18 aerobic min/day.
Table 1.
Baseline Characteristics of mActive Trial Participants
| All Participants (n=48) No. (%) | Unblind (n=32) | Blind (n=16) No. (%) | ||
|---|---|---|---|---|
| No Texts (n=16) No. (%) | Texts (n=16) No. (%) | |||
| Age, y, mean±SD | 58±8 | 58±8 | 55±8 | 60±7 |
| Sex | ||||
| Men | 26 (54) | 9 (56) | 8 (50) | 9 (56) |
| Women | 22 (46) | 7 (44) | 8 (50) | 7 (44) |
| White race | 38 (79) | 12 (75) | 12 (75) | 14 (88) |
| Dog owner | 21 (44) | 9 (56) | 5 (31) | 7 (44) |
| Married | 35 (73) | 13 (81) | 9 (56) | 13 (81) |
| Employed | 42 (88) | 13 (81) | 15 (94) | 13 (81) |
| Management, professionala | 30 (63) | 9 (56) | 9 (56) | 12 (75) |
| Service | 5 (10) | 2 (13) | 3 (19) | 0 (0) |
| Sales, office | 5 (10) | 2 (13) | 2 (13) | 1 (6) |
| Construction, maintenance | 2 (4) | 0 (0) | 1 (6) | 1 (6) |
| CHD | 14 (29) | 5 (31) | 2 (13) | 7 (44) |
| Diabetes | 11 (23) | 5 (31) | 2 (13) | 4 (25) |
| Smoker | 1 (2) | 1 (6) | 0 (0) | 0 (0) |
| Hypertension | 24 (50) | 8 (50) | 5 (31) | 11 (69) |
| Dyslipidemia | 39 (81) | 14 (88) | 12 (75) | 13 (81) |
| BMI kg/m2, mean±SD | 31±6 | 30±5 | 30±7 | 33±7 |
| ≥30 | 26 (54) | 7 (44) | 9 (56) | 10 (63) |
| IPAQb | ||||
| Low | 10 (21) | 2 (12) | 3 (19) | 5 (31) |
| Moderate | 21 (44) | 7 (44) | 9 (56) | 5 (31) |
| High | 17 (35) | 7 (44) | 4 (25) | 6 (38) |
BMI indicates body mass index; CHD, coronary heart disease; IPAQ, International Physical Activity Questionnaire, Long‐Form.
Types of employment per U.S. Census Bureau definitions.
Represents total activity; all subjects reported low leisure time activity per the IPAQ.
Primary Outcome: Change in Steps/Day
Physical activity trajectories were different among the 3 trial groups. The blinded group showed a progressive downward trend over the whole time period, particularly in the change from phase I to II. This downward drift was not observed in either of 2 other trial groups. The unblinded arm trajectory was characterized by a maintenance of baseline activity levels, whereas the biggest shift in trajectory was noted in the text‐receiving arm. This group had a clear upward trend in physical activity in response to smart texts.
In phase I (Table 2), blinded control participants obtained a mean of 616 fewer steps/day (6% decrease) whereas unblinded participants increased their steps/day by a mean of 408 (4% increase). The between‐group differential was nonsignificantly higher in the unblinded group versus blinded controls by 1024 steps/day (95% CI, −580 to 2628; P=0.21).
Table 2.
Changes in Activity Outcomes With Phase I and II Interventions
| Unblind (n=32) Mean Change±SD | Blind (n=16) Mean Change±SD | Unblind–Blind Mean Difference (95% CI), P Value | |
|---|---|---|---|
| Phase I | |||
| Steps, count/daya | 408±2701 | −616±2385 | 1024 (−580 to 2628), P=0.21 |
| Activity time, min/day | 2±27 | −6±26 | 8 (−9 to 25), P=0.33 |
| Aerobic time, min/day | −3±12 | −11±14 | 8 (0–16), P=0.05 |
|
Texts (n=16) |
No Texts (n=16) |
Blind (n=16) |
Texts–No Texts | Texts–Blind | No Texts–Blind | |
|---|---|---|---|---|---|---|
| Mean Change±SD | Mean Difference (95% CI), P‐value | |||||
| Phase II | ||||||
| Steps, count/daya | 2334±1714 | −200±1653 | −1042±2202 | 2534 (1318–3750), P<0.001 | 3376 (1951–4801), P<0.001 | 842 (−564 to 2248), P=0.23 |
| Activity time, min/day | 21±20 | 0±17 | −8±23 | 21 (8–34), P=0.003 | 29 (13–45), P<0.001 | 8 (−7 to 23), P=0.28 |
| Aerobic time, min/day | 13±11 | −1±8 | −3±10 | 14 (7–21), P<0.001 | 16 (7–23), P<0.001 | 2 (−6 to 8), P=0.71 |
Primary outcome. CI indicates confidence interval.
In phase II (Table 2), the blinded group further decreased its activity by 1042 steps/day (11% decrease) whereas the unblinded–no texts group decreased by 200 steps/day (<1% change). In contrast, the unblinded‐texts group obtained 2334 more steps/day (25% increase). The differential in activity levels was significant; participants receiving texts increased their daily steps over those not receiving texts by 2534 (95% CI, 1318–3750; P<0.001) and over blinded controls by 3376 (95% CI, 1951 to 4801; P<0.001).
At baseline, 23 (48%) participants had a daily step count ≥10 000 steps/day, 9 (56%) in the blinded group and 14 (44%) in the unblinded group (Figure 2). In phase I, the ≥10 000 step/day goal was attained by 1 less participant in both the blinded group (8 of 16; 50%) and unblinded group (13 of 32; 41%). In phase II, the number of participants meeting the 10 000 steps/day goal was 7 (44%) in both the blinded‐ and unblinded–no texts groups. In contrast, 13 (81%) participants who were unblinded and receiving smart texts achieved a daily step count ≥10 000 steps/day (37% absolute increase and 84% relative increase over other groups; P=0.02).
Figure 2.
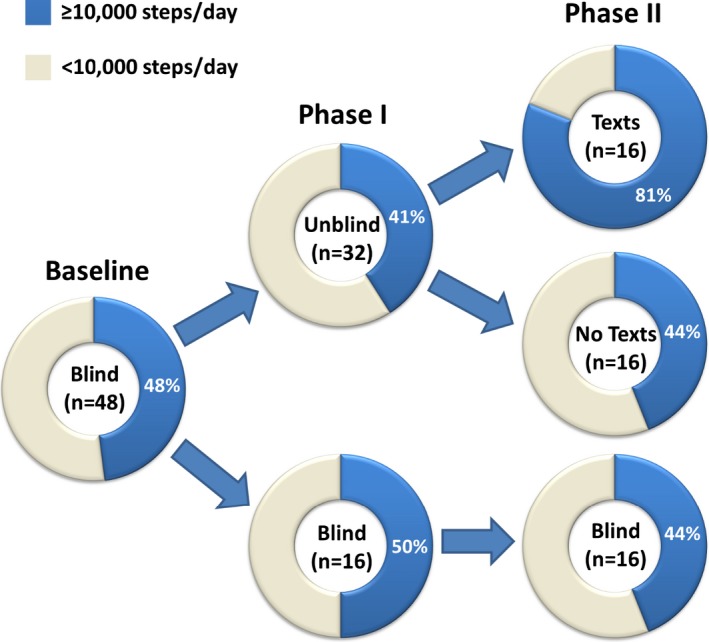
Proportions attaining the 10 000 steps/day goal at baseline, in phase I, and phase II, by intervention group. Forty‐eight percent of participants attained the 10 000 step/day goal at baseline. No significant change or between‐group difference was observed in phase I, whereas in phase II, the unblinded‐texts group showed a 37% absolute increase and 84% relative increase over other groups in 10 000 steps/day attainment (P=0.02).
Secondary Outcomes: Total and Aerobic Activity Times
In phase I (Table 2), the unblinded and blinded groups were not significantly different in modifying their total activity times, whereas there was a borderline significant smaller decrease in aerobic time in unblinded patients (differential 8 minutes; 95% CI, 0 to 16; P=0.05). In phase II, activity times continued to decrease in the blinded group and remained relatively stable in the unblinded–no texts group. In contrast, the unblinded‐texts group increased its total activity time by 21 min/day (23% increase) and aerobic time by 13 min/day (160% increase), which was highly statistically significant compared to the other groups.
Exploratory Subgroup Interaction Testing
For the interaction of the phase I intervention (unblinding) with a given patient factor, P values for interaction were as follows: 0.36 for age; 0.53 for sex; 0.67 for race; 0.17 for BMI; 0.46 for diabetes; 0.83 for hypertension; 0.51 for dyslipidemia; 0.63 for CHD; 0.70 for dog ownership; 0.99 for marital status; 0.46 for employment; and 0.20 for baseline activity. Interaction testing was similarly null for the secondary outcomes of total activity time and aerobic time. The respective P values for the interaction of the phase II intervention (smart texts) were also null for most patient factors as follows: 0.59 for age; 0.60 for race; 0.35 for BMI; 0.45 for diabetes; 0.29 for hypertension; 0.41 for dyslipidemia; 0.14 for dog ownership; 0.68 for marital status; 0.72 for employment; and 0.93 for baseline activity.
However, there was a trend for significant interaction by sex (P=0.06), with women showing a 3754 steps/day increase (95% CI, 2189 to 5319) in response to smart texts as compared with men, who showed a 1562 steps/day increase (95% CI, −209 to 3334). Similar results were observed for the interaction of total time and sex (P=0.05), with responsiveness to text messaging predominantly found in women (35 min/day; 95% CI: 20 to 50) rather than in men (10 min/day; 95% CI, −10 to 30). In contrast, an interaction was not observed between aerobic time and sex (P=0.58).
There was a significant interaction by CHD status (P=0.03). Those with coronary disease increased their physical activity by 5068 steps/day (95% CI, 1371 to 8765) in response to smart texts as compared to 1777 steps/day (95% CI, 489 to 3065 steps/day) in others. There was a similar interaction by CHD status for the secondary outcome of total activity time (P=0.04). The group with versus without known coronary disease increased physical activity time by 50 min/day (95% CI, 4 to 95) versus 14 min/day (95% CI, 0 to 28). For the outcome of aerobic time, however, no significant interaction was present (P=0.90).
Satisfaction
On post‐trial surveys, participants largely expressed feelings of satisfaction and enthusiasm for future trial participation. Quantitatively, participants assigned the activity tracker a mean score of 4.0 of 5.0, and text messages 3.8 of 5.0 (4=good; 5=great).
Discussion
In mActive, coupling smart texts with activity tracking led to the best physical activity outcomes. Nearly twice as many participants in the text‐receiving arm achieved the 10 000 steps/day goal compared with the other groups. Aerobic activity levels were disproportionately low compared with steps and total activity time, but appeared most responsive to intervention. The mActive trial lends support to the notion of new mHealth devices as facilitators, not drivers, of behavior change,6 because sequential randomization suggested that unblinding to device data did not significantly modify behavior, whereas coupling it with smart texts did.
If sustained, the change in physical activity from the mActive tracking‐texting intervention may be clinically significant. A comparable step count increase over a mean duration of 18 weeks was associated with significant decreases in BMI and systolic blood pressure.14 During a mean of 6 years, each 2000 step/day increase in physical activity was associated with a ≈10% relative reduction in the incidence of CVD.17 Sustaining regular physical activity over the long term is associated with a more favorable circulating metabolome19 and decreased markers of inflammation.20 Benefits of cardiorespiratory fitness may extend to non‐CVD diseases, such as cancer, depression, and dementia, with a dose‐dependent reduction in all‐cause mortality.21, 22, 23, 24
However, the influence of behavioral counseling may be limited in the current health care delivery model wherein clinicians tend to see patients for brief visits every several months or annually.25 Aimed to fill the gap, the mActive system is physician designed and intended to leverage the clinician‐patient relationship while functioning automatically without day‐to‐day involvement of the physician. Compatible with the importance of smart text support, pedometer‐based interventions without smart texts did not benefit insurance company employees in Finland26 or adults with diabetes in southwest England.27
mActive also appears to provide a significant advance over previous Web‐based approaches, which have produced mixed results.8, 28, 29, 30 We are aware of another group aiming to send mobile phone text messages31 based on activity tracking; distinct from the mActive mobile design, it relies on computer access points for data transmission. mActive moves the field forward by providing the first fully mobile, fully automated, physician‐designed, integrated tracking‐texting intervention, with potential to modify behavior in real time, while also being potentially reproducible, affordable, and widely scalable. Enhancing such prospects, activity tracking apps built into smartphones are now common, and our technique could be applied using them. Our technique might also enhance the development of cardiac rehabilitation programs using mHealth.32 Overall, our resource‐efficient strategy may facilitate more continuous monitoring and feedback between the health system and patients.
mActive presents an attractive new paradigm for patient‐oriented research as a union of the strengths of mHealth and randomization. We suspect that the convenience offered by the truly mobile mActive design may have benefitted recruitment, retention, and data capture. The trial met its recruitment target, enrolled 96% of eligible patients (as compared to the typical statistic of around 50%33), and all patients but 1 completed the protocol. Without specifically targeting recruitment of women, the mActive trial had nearly equal representation of men and women. The mActive trial recruitment experience supports the data‐driven hypothesis33 that a trial design fostering convenience can enhance trial participation. Furthermore, there was a very high level of physical activity outcome completeness obtained using a digital approach.
Limitations
Given its limited size and scope, mActive may be categorized as a pilot trial. As such, it is most appropriately interpreted as exploratory evidence to guide a definitive pivotal trial in the future.34 The trial provides a solid basis to build upon given that it was conducted using the rigorous scientific methodology characteristic of pivotal randomized, clinical trials, including public registration and incorporation of randomization and blinding. Whereas our trial detected an effect only when smart texts were added to activity tracking, with a larger sample size, less within‐person variability (within‐person SD exceeded our estimate of 1800 steps/day), or a longer duration of intervention, activity tracking alone may have led to a statistically significant increase in physical activity. Greater power could also allow more‐definitive examination of potential moderators of treatment. Our subgroup analyses were exploratory in nature and underpowered, but produced intriguing signals by sex and coronary disease status, which can be considered by future studies. To address the limited duration of follow‐up in mActive, we plan to perform long‐term follow‐up of participants for physical activity outcomes and risk factors (eg, BMI and blood pressure). Additionally, we are creating additional smart text content to allow for longer‐term interventions.
Generalizability remains uncertain for multiple reasons. Participants were adult smartphone users from a single preventive cardiology clinic and may have been highly motivated because of obesity, other CVD risk factors, or known CVD. Behavioral influences also potentially arose from attention on participation in a physical activity trial, awareness of being observed (Hawthorne effect), and investigator expectations (Rosenthal effect). In total, these factors may explain the unexpectedly high physical activity levels at baseline and throughout the trial in the trial participants as a whole (further discussion in Table S3, 19‐ii).
Finally, it may be considered a limitation that we did not use human coaches as part of the intervention. There may be added value in what a human coach could provide, for example, through motivational interviewing. On the other hand, it could be considered a strength given that the automated approach may be more reproducible and more easily scaled at lower cost.
Conclusion
In ambulatory cardiology patients who are smartphone users, a novel mHealth intervention coupling smart texts to digital tracking significantly increased near‐term physical activity. The effect was dependent on the text message component of the intervention. This pilot trial gives insight into the efficacy and feasibility of components of the mHealth intervention and provides an initial step forward in a rapidly growing area in critical need of high‐quality clinical evidence. Ultimately, therapeutic implications await larger, multicenter, definitive pivotal trials involving more‐diverse groups of patients.
Sources of Funding
This trial was funded, in part, by an unrestricted grant to Blaha from the PJ Schafer Cardiovascular Research Fund, a 501(c)(3) nonprofit organization. Martin was supported by a National Institutes of Health training grant (T32HL07024) for which Coresh served as the PI. Martin received additional support from the Pollin Cardiovascular Prevention Fellowship and the Marie‐Josée and Henry R Kravis Endowed Fellowship. Furthermore, Martin received a modest monetary award in conjunction with the Howard C. Silverman prize for originality and creativity in medical research, which was awarded by the Johns Hopkins Division of Cardiology based on the preliminary design of the mActive trial. He also received a modest monetary award from the American Heart Association's Council on Lifestyle and Cardiometabolic Health with the Steven N. Blair Award for Excellence in Physical Activity Research. Long‐term follow‐up of mActive trial participants is being supported by the Aetna Foundation. Blumenthal was supported by the Kenneth Jay Pollin Professorship in Cardiology.
Disclosures
Digital physical activity tracking devices were provided in kind by Fitbug, a private for‐profit company. This trial was investigator initiated and Fitbug did not provide cash payments for the research or writing of the manuscript. Fitbug did not participate in the analysis of the data or influence the conclusions.
Supporting information
Figure S1. Fitbug orb and accessories.
Figure S2. Fitbug smartphone app.
Table S1. Factors in Personalization of Text Messages
Table S2. Example Text Messages
Table S3. CONSORT‐EHEALTH Checklist
(J Am Heart Assoc. 2015;4:e002239 doi: 10.1161/JAHA.115.002239)
Accompanying Figures S1, S2 and Tables S1 through S3 are available at http://jaha.ahajournals.org/content/4/11/e002239/suppl/DC1
Presented as an abstract at the Epidemiology and Prevention and Lifestyle and Cardiometabolic Health Scientific Sessions, March 3–6, 2015, in Baltimore, MD, and at the American Heart Association Scientific Sessions, November 7–11, 2015, in Orlando, FL.
References
- 1. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;2014(129):S76–S99. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 3. Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd‐Jones DM. Cardiovascular health behavior and health factor changes (1988‐2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Case MA, Burwick HA, Volpp KG, Patel MS. Accuracy of smartphone applications and wearable devices for tracking physical activity data. JAMA. 2015;313:625–626. [DOI] [PubMed] [Google Scholar]
- 5. Steinhubl SR, Muse ED, Topol EJ. Can mobile health technologies transform health care? JAMA. 2013;310:2395–2396. [DOI] [PubMed] [Google Scholar]
- 6. Patel MS, Asch DA, Volpp KG. Wearable devices as facilitators, not drivers, of health behavior change. JAMA. 2015;313:459–460. [DOI] [PubMed] [Google Scholar]
- 7. Burke LE, Ma J, Azar KM, Bennett GG, Peterson ED, Zheng Y, Riley W, Stephens J, Shah SH, Suffoletto B, Turan TN, Spring B, Steinberger J, Quinn CC. Current Science on Consumer Use of Mobile Health for Cardiovascular Disease Prevention: a Scientific Statement From the American Heart Association. Circulation. 2015;132:1157–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Artinian NT, Fletcher GF, Mozaffarian D, Kris‐Etherton P, Van Horn L, Lichtenstein AH, Kumanyika S, Kraus WE, Fleg JL, Redeker NS, Meininger JC, Banks J, Stuart‐Shor EM, Fletcher BJ, Miller TD, Hughes S, Braun LT, Kopin LA, Berra K, Hayman LL, Ewing LJ, Ades PA, Durstine JL, Houston‐Miller N, Burke LE. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:406–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. [DOI] [PubMed] [Google Scholar]
- 10. Levine GN, Allen K, Braun LT, Christian HE, Friedmann E, Taubert KA, Thomas SA, Wells DL, Lange RA. Pet ownership and cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2013;127:2353–2363. [DOI] [PubMed] [Google Scholar]
- 11. Cowan LT, Van Wagenen SA, Brown BA, Hedin RJ, Seino‐Stephan Y, Hall PC, West JH. Apps of steel: are exercise apps providing consumers with realistic expectations?: a content analysis of exercise apps for presence of behavior change theory. Health Educ Behav. 2013;40:133–139. [DOI] [PubMed] [Google Scholar]
- 12. Aarts H, Paulussen T, Schaalma H. Physical exercise habit: on the conceptualization and formation of habitual health behaviours. Health Educ Res. 1997;12:363–374. [DOI] [PubMed] [Google Scholar]
- 13. Metkus TS Jr, Baughman KL, Thompson PD. Exercise prescription and primary prevention of cardiovascular disease. Circulation. 2010;121:2601–2604. [DOI] [PubMed] [Google Scholar]
- 14. Bravata DM, Smith‐Spangler C, Sundaram V, Gienger AL, Lin N, Lewis R, Stave CD, Olkin I, Sirard JR. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. [DOI] [PubMed] [Google Scholar]
- 15. Grandes G, Sanchez A, Sanchez‐Pinilla RO, Torcal J, Montoya I, Lizarraga K, Serra J; PEPAF Group . Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694–701. [DOI] [PubMed] [Google Scholar]
- 16. Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, Richardson CR, Smith DT, Swartz AM. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–2279. [DOI] [PubMed] [Google Scholar]
- 17. Yates T, Haffner SM, Schulte PJ, Thomas L, Huffman KM, Bales CW, Califf RM, Holman RR, McMurray JJ, Bethel MA, Tuomilehto J, Davies MJ, Kraus WE. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383:1059–1066. [DOI] [PubMed] [Google Scholar]
- 18. Eysenbach G; CONSORT‐EHEALTH Group. CONSORT‐EHEALTH: improving and standardizing evaluation reports of web‐based and mobile health interventions. J Med Internet Res. 2011;13:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kujala UM, Makinen VP, Heinonen I, Soininen P, Kangas AJ, Leskinen TH, Rahkila P, Wurtz P, Kovanen V, Cheng S, Sipila S, Hirvensalo M, Telama R, Tammelin T, Savolainen MJ, Pouta A, O'Reilly PF, Mantyselka P, Viikari J, Kahonen M, Lehtimaki T, Elliott P, Vanhala MJ, Raitakari OT, Jarvelin MR, Kaprio J, Kainulainen H, Ala‐Korpela M. Long‐term leisure‐time physical activity and serum metabolome. Circulation. 2013;127:340–348. [DOI] [PubMed] [Google Scholar]
- 20. Hamer M, Sabia S, Batty GD, Shipley MJ, Tabak AG, Singh‐Manoux A, Kivimaki M. Physical activity and inflammatory markers over 10 years: follow‐up in men and women from the Whitehall II cohort study. Circulation. 2012;126:928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Willis BL, Gao A, Leonard D, Defina LF, Berry JD. Midlife fitness and the development of chronic conditions in later life. Arch Intern Med. 2012;172:1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Herring MP, Puetz TW, O'Connor PJ, Dishman RK. Effect of exercise training on depressive symptoms among patients with a chronic illness: a systematic review and meta‐analysis of randomized controlled trials. Arch Intern Med. 2012;172:101–111. [DOI] [PubMed] [Google Scholar]
- 23. Defina LF, Willis BL, Radford NB, Gao A, Leonard D, Haskell WL, Weiner MF, Berry JD. The association between midlife cardiorespiratory fitness levels and later‐life dementia: a cohort study. Ann Intern Med. 2013;158:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA. 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 25. Moyer VA; U.S. Preventive Services Task Force . Behavioral counseling interventions to promote a healthful diet and physical activity for cardiovascular disease prevention in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157:367–371. [DOI] [PubMed] [Google Scholar]
- 26. Reijonsaari K, Vehtari A, Kahilakoski OP, van Mechelen W, Aro T, Taimela S. The effectiveness of physical activity monitoring and distance counseling in an occupational setting ‐ results from a randomized controlled trial (CoAct). BMC Public Health. 2012;12:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andrews RC, Cooper AR, Montgomery AA, Norcross AJ, Peters TJ, Sharp DJ, Jackson N, Fitzsimons K, Bright J, Coulman K, England CY, Gorton J, McLenaghan A, Paxton E, Polet A, Thompson C, Dayan CM. Diet or diet plus physical activity versus usual care in patients with newly diagnosed type 2 diabetes: the Early ACTID randomised controlled trial. Lancet. 2011;378:129–139. [DOI] [PubMed] [Google Scholar]
- 28. Irvine AB, Gelatt VA, Seeley JR, Macfarlane P, Gau JM. Web‐based intervention to promote physical activity by sedentary older adults: randomized controlled trial. J Med Internet Res. 2013;15:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bosak KA, Yates B, Pozehl B. Effects of an internet physical activity intervention in adults with metabolic syndrome. West J Nurs Res. 2010;32:5–22. [DOI] [PubMed] [Google Scholar]
- 30. Wijsman CA, Westendorp RG, Verhagen EA, Catt M, Slagboom PE, de Craen AJ, Broekhuizen K, van Mechelen W, van Heemst D, van der Ouderaa F, Mooijaart SP. Effects of a web‐based intervention on physical activity and metabolism in older adults: randomized controlled trial. J Med Internet Res. 2013;15:e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jethwani K. Text to Move Study. Available at: http://clinicaltrials.gov/show/nct01569243. Accessed October 31, 2015.
- 32. Beatty AL, Fukuoka Y, Whooley MA. Using mobile technology for cardiac rehabilitation: a review and framework for development and evaluation. J Am Heart Assoc. 2013;2:e000568. doi:10.1161/JAHA.113.000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin SS, Ou FS, Newby LK, Sutton V, Adams P, Felker GM, Wang TY. Patient‐ and trial‐specific barriers to participation in cardiovascular randomized clinical trials. J Am Coll Cardiol. 2013;61:762–769. [DOI] [PubMed] [Google Scholar]
- 34. Loscalzo J. Pilot trials in clinical research: of what value are they? Circulation. 2009;119:1694–1696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Fitbug orb and accessories.
Figure S2. Fitbug smartphone app.
Table S1. Factors in Personalization of Text Messages
Table S2. Example Text Messages
Table S3. CONSORT‐EHEALTH Checklist


