Abstract
Background
Silent brain infarcts (SBIs) are highly prevalent in the aged population and relate to the occurrence of further stroke and dementia. Serum N‐glycome levels have been previously associated with aging and they might be related as well to the presence of SBIs and age‐related white matter hyperintensities.
Methods and Results
We determined the serum N‐glycome profile in a cohort study comprising 972 subjects and evaluated the relationship between N‐glycome levels and the presence and number of SBIs and with age‐related white matter hyperintensities grades, assessed by brain magnetic resonance imaging. Decreasing concentrations of bigalacto core‐α‐1,6‐fucosylated biantennary glycan and increasing concentrations of branching α‐1,3‐fucosylated triantennary glycan remained as independent predictors of SBIs (odds ratio 0.4, 95% CI 0.3–0.7 and odds ratio 1.8, 95% CI 1–3.2, respectively), after controlling for the presence of age and classic vascular risk factors. A similar pattern was found to be related to an increasing number of SBIs and white matter hyperintensities grade.
Conclusions
N‐glycome levels might be potentially useful as biomarkers for the presence of silent cerebrovascular disease.
Keywords: aging, biomarker, N‐glycome profile, silent brain infarct, white matter hyperintensities
Introduction
The term “silent brain infarcts” (SBIs) refers to cerebral infarcts that are found incidentally on neuroimaging, particularly brain magnetic resonance imaging (MRI), in the absence of symptoms suggesting a corresponding stroke event. The majority of SBIs are truly silent, but they can also be associated with subtle symptoms, neurological abnormalities, and cognitive impairment. More importantly, individuals with SBIs have a 2‐ to 4‐fold higher risk of having a future stroke or dementia than patients without them, independently of the presence of other vascular risk factors. Thus, they may also be referred to as “covert infarcts” or “MRI‐defined infarcts,” rather than “silent.” Their prevalence in population‐based studies ranges from 8% to 28% of healthy individuals. They are 5 times more frequent than stroke and therefore they constitute a significant burden of brain vascular disease.1 The majority of silent brain infarcts are lacunar infarcts and they usually coexist with other markers of cerebral small vessel disease, as white matter hyperintensities (WMHs).2
Age is the more clearly identified risk factor for SBIs, and according to a systematic review, the odds ratio for prevalent SBIs assessed per year of age ranges from 1.03 to 1.13 (or from 2.44–3.21 per decade). As a consequence, and given the progressive aging of the population in developed countries, the prevalence and consequences of SBIs are expected to reach epidemic proportions in the near future. Despite these numbers, their systematic screening is not recommended (brain MRI is expensive and its availability is limited in some settings). The use of plasma biomarkers for their identification could potentially overcome these limitations and help clinicians to establish preventive treatments to decrease the burden of stroke and dementia.
We have previously shown that urine albumin‐to‐creatinine ratio (or microalbuminuria) and lipoprotein‐associated phospholipase A2 (Lp‐PLA2) are associated with the risk of SBIs in hypertensive individuals. Subjects with microalbuminuria had twice the odds of SBIs, while Lp‐PLA2 activity was also associated with the presence of SBIs, but only in women.3, 4
Other potentially interesting candidate biomarkers would be N‐glycans. N‐glycans are oligosaccharide structures covalently linked (sugar–amino acid linkage) to an asparagine residue of a protein in a consensus sequence (GlcNAc‐β‐Asn). These sugar structures are highly heterogeneous and their biosynthesis is closely linked to the cellular metabolism, thereby they reflect the metabolic status of cells. They were first described in relation to aging in 1988, when their profile in plasma G immunoglobulins were studied in a group of 151 subjects.5 Later on, the entire serum N‐glycome was investigated and a consistent pattern of changes in the N‐glycan structures related to age was described within different European and Asian populations.6, 7 N‐glycome levels have also been described in relation to neurodegenerative diseases such as Alzheimer disease.8, 9
In the present study, we hypothesized that the serum glycome profile might reflect “brain aging” as well, and could be helpful for the identification of SBIs or other silent brain vascular lesions, such as WMHs.
Specifically in this study, we evaluated whether the serum N‐glycome relates to SBIs (and WMHs) and additionally, we assessed their predictive value independently of other clinical parameters and previously described biological markers to detect SBIs in a large cohort of stroke‐free individuals.
Methods
Study Population
Subjects for this study are those included within the ISSYS (Investigating Silent Strokes in hYpertensives: a magnetic resonance imaging Study). The basic design of this study is a cohort study in a randomly selected sample of 976 hypertensive individuals, aged 50 to 70 years old, with no history of previous clinical stroke or dementia. This study is aimed to determine the prevalence of silent brain infarcts and to assess their determinants. All participants were recruited in the city of Barcelona. A stratified randomization method was used to control for age, sex, and the center from which the subjects were recruited. Additional details on subject selection and baseline procedures have been published elsewhere.4, 10
Participants with prior stroke were excluded after it was confirmed by medical records and in those reporting suggestive stroke symptoms, according to the Stroke Symptom questionnaire.11 Other exclusion criteria were (1) impossibility to undergo a brain MRI (ie, patients carrying pacemakers, cochlear stimulators, etc), (2) suspected white coat hypertension syndrome, and (3) short‐term life expectancy preventing follow‐up examinations.
On baseline, clinical data were collected, including demographic and personal medical history, and the presence of vascular risk factors or an established cardiovascular disease.
Systolic and diastolic office BP was measured with an oscillometric device (Omron M6 Comfort), and the mean of the last 2 of 3 determinations after 5‐minute rest was recorded. Diabetes mellitus was defined as fasting glucose levels over 7 mmol/L and/or the use of oral antidiabetic drugs or insulin. Smoking habit was collected and categorized into current, former, or never. Alcohol abuse was defined as intake of at least 280 g/week in males and 170 g/week in females. Body mass index was calculated as weight (kg) divided by height squared (m2). Coronary artery disease was recorded in participants with history of angina, myocardial infarction, or in those who underwent percutaneous coronary interventions or coronary artery bypass graft. Previous cardiovascular disease included coronary artery disease and peripheral artery disease (intermittent claudication, bypass surgery, aortic aneurysm).
This study was approved by our local institutional review Ethics Committee, and all subjects gave their informed consent to participate prior to the inclusion.
Brain MRI
A brain MRI with a pre‐established data acquisition protocol was performed within the next month after study entry. All examinations were performed with the same 1.5 Tesla MR (Signa HDx 1.5, General Electrics, Waukesha, WI), including axial T1, T2, and fluid‐attenuated inversion recovery weighted images.
SBIs were defined as previously,12 as lesions of ≥3 mm in their widest diameter, with cerebrospinal fluid–like signal characteristics in all pulse sequences, and with a hyperintense rim surrounding the lesion in fluid‐attenuated inversion recovery MRI. These lesions were differentiated from enlarged perivascular spaces based on their size, shape, and location. SBIs were dichotomized into 2 categories (present/absent) and also the number of SBIs was considered in our study.
WMHs were rated at fluid‐attenuated inversion recovery and defined as extensive or focal lesions that appeared as hyperintense lesions in the white matter (WM). They were graded in the subcortical deep WM and in periventricular regions, according to the Fazekas scale.13 For the analysis we considered WMHs as significant when lesions scored at least 2 points in this scale in the periventricular regions (ie, smooth halo or irregular lesions extending into the deep WM) or deep WM (ie, beginning of confluent foci or large confluent areas).
All images were primarily assessed by 2 neuroradiologists and in a second term by the same readers plus an experienced stroke neurologist. They were blinded to laboratory measurements and clinical data. Intrarater agreement was calculated for each reader in a training set before undertaking the present reading. Both intra‐ and inter‐rater agreement between different observers and imaging features ranged from 0.60 to 0.75. Disagreements in assessments were solved by consensus. Representative examples of these MRI features are shown in Figure 1.
Figure 1.
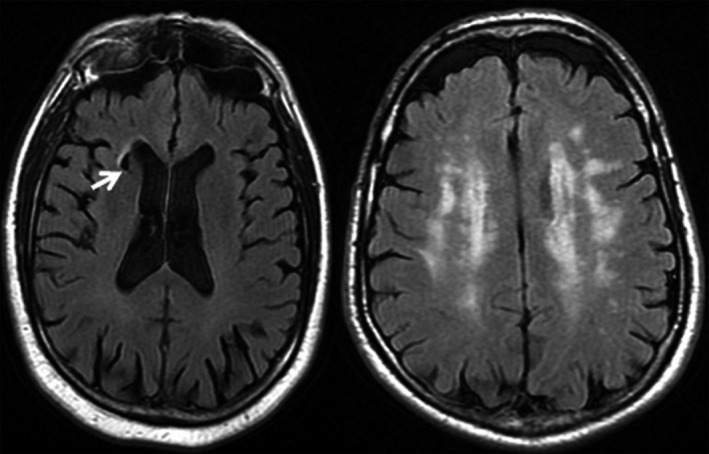
Representative examples of imaging markers of cerebral small‐vessel disease. From left to right: Brain infarct affecting caudate nuclei is shown by a white arrow (FLAIR MRI), extensive white matter changes (FLAIR MRI), 1.5 Tesla MR (Signa HDx 1.5; General Electric, Waukesha, WI). FLAIR indicates fluid‐attenuated inversion recovery; MRI, magnetic resonance imaging.
N‐glycome Profile Assessment
On the baseline visit of the ISSYS study, blood samples were obtained in all participants. Peripheral venous samples were collected in tubes with no anticoagulant and serum was extracted after 15‐minute centrifugation (1500 g) and then frozen at −80°C until the test was performed.
N‐glycan profiles were generated from 2‐μL serum samples by DSA‐FACE (DNA Sequencer Adapted‐Fluorophore Assisted Carbohydrate Electrophoresis) technology using a capillary electrophoresis (CE)‐based ABI3130 Genetic Analyzer (Applied Biosystems, Foster City, CA). Samples were only tested once since the intra‐ and interassay coefficients of variation of the glycan analyses have been reported previously to be <5%.6 Results are normalized values from peaks 1 to 9, which represent relative concentrations of the oligosaccharides that are more prominent in humans. Briefly, the corresponding structures are as follows: peak 1 corresponds to agalacto core‐α‐1,6‐fucosylated biantennary glycan (NGA2F); peak 2, to agalacto core‐α‐1,6‐fucosylated bisecting biantennary glycan (NGA2FB); peak 3 and peak 4 to single agalacto core‐α‐1,6‐fucosylated biantennary glycans (NG1A2F); peak 5, to bigalacto biantennary glycan (NA2); peak 6, to bigalacto core‐α‐1,6‐fucosylated biantennary glycan (NA2F); peak 7, to bigalacto core‐α‐1,6‐fucosylated bisecting biantennary glycan (NA2FB); peak 8, to triantennary glycan (NA3); and peak 9, to branching α‐1,3‐fucosylated triantennary glycan (NA3Fb).7 (Figure 2).
Figure 2.
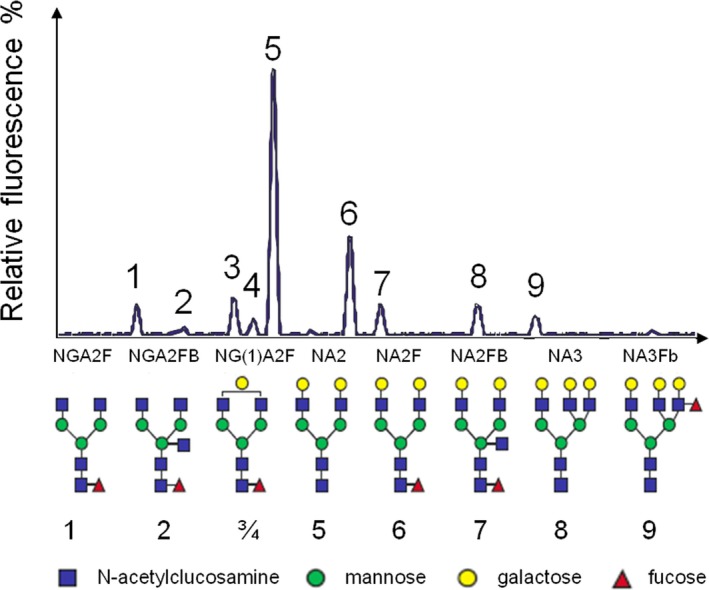
Typical human serum N‐glycome profile, and structural formulas of N‐glycans. Peak 1 is an agalactosylated, core‐α‐1,6‐fucosylated biantennary glycan (NGA2F), peak 2 is an agalactosylated, core‐α‐1,6‐fucosylated bisecting biantennary (NGA2FB), peaks 3 and 4 are monogalactosylated, core‐α‐1,6‐fucosylated biantennary (NG1A2F), peak 5 is a bigalactosylated, biantennary glycan (NA2), peak 6 is a bigalactosylated, core‐α‐1,6‐fucosylated biantennary (NA2F), peak 7 is a bigalactosylated, core‐α‐1,6‐fucosylated bisecting biantennary (NA2FB), peak 8 is a trigalactosylated, tri‐antennary (NA3), peak 9 is a branching α‐1,3‐fucosylated trigalactosylated tri‐antennary (NA3F(b) [Lewis X Ag: GlcNac‐galactose‐fucose]).
In the same participants, we assayed serum total and high‐density lipoprotein cholesterol (Olympus, Lismeehan, Ireland) and Lp‐PLA2 activity (PLAC test for activity, Diadexus, Inc) using enzyme assays on a single automated clinical chemistry analyzer (Olympus AU 2700). Finally, a single spot urine sample was also collected at the baseline visit and sent to a central laboratory for determining the urinary albumin‐to‐creatinine ratio.3, 4 Microalbuminuria was defined as the presence of urinary albumin‐to‐creatinine ratio higher than 21 mg/g in men and over 30 mg/g in women.14
Statistical Analyses
For descriptive purposes, normality for continuous variables was assessed by the Kolmogorov–Smirnov test. None of the N‐glycans structures was normally distributed in our sample, except NA2. For univariate analysis, continuous variables were compared using t test and ANOVA or Mann–Whitney U test and Kruskal–Wallis test when appropriate. Categorical variables were compared using Pearson χ2 and continuous data were analyzed using Pearson or Spearman correlation, depending on their distribution. We studied how the serum N‐glycome profile related to age, to the presence of traditional vascular risk factors, and to the presence/absence of SBIs and their number (considering 3 categories: none versus single versus multiple).
False discovery rate correction was applied for multiple comparisons and statistical significance was set at P values <0.05.
To assess for independent associations between N‐glycome structures and SBIs, adjusted models were constructed by means of forward stepwise logistic regression analysis including as covariates potential confounders such as age, sex, diastolic blood pressure, diabetes mellitus, tobacco, and high‐density lipoprotein cholesterol. Additionally, we added in the models Lp‐PLA2 activity and the presence of microalbuminuria, since both of them have been related to SBIs in our cohort before. Odds ratios and 95% CI will be presented.
To assess the improvement to prediction by adding variables (in this case, the serum N‐glycome structures) to a logistic regression model, likelihood ratio tests were used (Using R version 3.1.0 “Spring dance” software [Copyright (C) 2014 The R Foundation for Statistical Computing]).15 Also, we provided measures of global diagnostic accuracy for SBIs detection (area under the receiver operator curves) of the different models including or not the information on the serum N‐glycome and compared them by using the Delong's method16 implemented at MedCalc 12.4 software.
Finally, 2 more exploratory analyses were performed. First, we sought associations between serum N‐glycome levels and other brain vascular lesions, specifically with the presence of significant WMHs at subcortical deep and periventricular areas. Secondly, we studied whether the N‐glycome correlated with other markers that we previously related to SBIs (microalbuminuria and Lp‐PLA2), both in univariate and adjusted linear regression models (considering as outcomes the changes observed in the N‐glycome).
Results
We obtained results on the serum N‐glycome profile in almost all participants from the ISSYS study (n=972, 99.6%). Median age of the participants was 64 (59–67) years old and 59.4% were males. Other baseline clinical characteristics of the sample are shown in Table 1.
Table 1.
Description of Demographic and Clinical Baseline Factors in the Total Sample and in Those With or Without SBIs
| Included Patients (n=972) | Absence of SBI (n=874) | Presence of SBI (n=98) | P Value | |
|---|---|---|---|---|
| Age, y | 64 (59–67) | 64 (58–67) | 65 (62–68) | 0.001 |
| Sex, female | 50.5% | 53% | 28.6% | <0.001 |
| Diabetes mellitus | 23.5% | 22.8% | 29.6% | 0.13 |
| HDL cholesterol | 47.5 (40–57) | 47.9 (40.3–57.4) | 44.6 (39–51.9) | <0.001 |
| Mean SBP, mm Hg | 142 (132–153) | 142 (132–153) | 142 (131–152) | 0.76 |
| Mean DBP, mm Hg | 78±9 | 78±9 | 80±10 | 0.02 |
| Tobacco use | 15.1% | 15.3% | 13.3% | 0.59 |
| Alcohol abuse | 6.5% | 6.7% | 4.8% | 0.79 |
| Any established CVD | 12.3% | 11.2% | 22.4% | 0.001 |
| CAD | 6.7% | 8.7% | 18.6% | 0.002 |
| LpPLA2 activity, nmol/mL per minute | 181 (153–215) | 179 (151–215) | 195.5 (158–232) | 0.03 |
| Microalbuminuria | 12.9% | 11.5% | 26.4% | <0.001 |
Data are expressed in median (interquartile range), mean±SD, and percentage as appropriate. CAD indicates coronary artery disease; CVD, cardiovascular disease; DBP, diastolic blood pressure; HDL, high‐density lipoproteins; Lp‐PLA2, lipoprotein‐associated phospholipase A2; SBI, silent brain infarct; SBP, systolic blood pressure.
Serum N‐glycome Profile, Age, and Vascular Risk Factors
As previously described, several N‐glycans were associated with age in this population (NGA2F, NGA2FB, NA2F, and NA3). Specifically, a weak positive correlation was observed with age for NGA2F and NGA2FB (r=0.14, P<0.001 and r=0.17, P<0.001, respectively), whereas a negative correlation was found for NA2F and NA3 (r=−0.12, P<0.001 and r=−0.06, P=0.051). Also, as described before, age was correlated with the log ratio of NGA2F to NA2F or GlycoAgeTest (r=0.17, P<0.001) (also named GlycoAgeTest).6
Associations between different N‐Glycans and demographic and cardiovascular risk factors are shown in Table 2. Significant differences were observed among some of the peaks regarding sex, vascular risk factors such as diabetes, dyslipidemia, and tobacco and alcohol intake. Moreover, some peaks were associated with obesity measurements such as body mass index or waist circumference.
Table 2.
Relations Between Age, Vascular Risk Factors, and Serum N‐glycans
| Characteristics | Peak 1 | Peak 2 | Peak 3 | Peak 4 | Peak 5 | Peak 6 | Peak 7 | Peak 8 | Peak 9 |
|---|---|---|---|---|---|---|---|---|---|
| Age |
R=0.145 P<0.001† |
R=0.168 P<0.001† |
R=−0.020 P=0.527 |
R=−0.035 P=0.273 |
R=0.001 P=0.695 |
R=−0.122 P<0.001† |
R=−0.032 P=0.316 |
R=−0.059 P=0.069 |
R=−0.019 P=0.563 |
| Sex | |||||||||
| Women | 9.7 (7.8–11.8) **, † | 1.8 (1.5–2.3) **, † | 6 (5.2–7) | 4.6 (4–5.2) | 41.5±4.3 | 17.1 (15.7–18.6) | 6 (5.2–7) | 8 (6.3–9.3) **, † | 2.1 (1.4–2.9) |
| Men | 9.1 (7.4–11.5) | 1.7 (1.4–2.2) | 6.1 (5.2–7) | 4.8 (4.1–5.4)**, † | 41.7±4.5 | 17.9 (16.5–19.7)**, † | 6.2 (5.3–7.2) | 6.6 (5.4–8) | 2.9 (2.1–4) **, † |
| Diabetes | |||||||||
| No | 9.5 (7.7–1.2) | 1.8 (1.4–2.2) | 6.4 (5.3–7)**, † | 4.7 (4.1–5.4)**, † | 41.5±4.3 | 17.5 (16–19.2) | 6.1 (5.3–7.1) | 7.2 (5.9–8.8) | 2.6 (1.7–3.6)** |
| Yes | 9.5 (7.7–1.2) | 2 (1.5–2.4)**, † | 5.5 (4.9–6.5) | 4.4 (3.9–5.1) | 41.8 ±4.7 | 18 (16–19.2) | 6.2 (5.3–7.3) | 7.6 (6.3–9) | 2.3 (1.7–3.2) |
| HDL |
R=0.021 P=0.516 |
R=−0.017 P=0.603 |
R=0.046 P=0.185 |
R=−0.031 P=0.343 |
R=0.017 P=0.589 |
R=−0.044 P=0.171 |
R=0.036 P=0.259 |
R=0.106 P<0.001† |
R=−0.167 P<0.001† |
| Active smoker | |||||||||
| No | 9.7 (7.8–11.7)**‡ | 1.8 (1.5–2.3) | 6. 1 (5.2–7)**, † | 4.7 (4.1–5.4)**, † | 41.2±4.3 | 17.6 (16.2–19.3)**, † | 6 (5.2–7) | 7.3 (6.1–8.9) | 2.3 (1.6–3.3) |
| Yes | 8.3 (6.8–10.5) | 1.8 (1.5–2.3) | 4.8 (5.5–6.3) | 4.2 (3.8–4.8) | 43.4±4.4**, † | 16.5 (15.2–18) | 6.4 (5.5–7.3)**, † | 7.1 (6.4–8.5) | 3.5 (2.6–4.7)**, † |
| Alcohol abuse | |||||||||
| No | 9.2 (7.6–1.1) | 1.8 (1.5–2.3) | 6 (5.2–6.9) | 4.8 (4.1–5.4) | 41.7±4.4 | 17.5 (16–19.2) | 6.3 (5.3–7.2) | 7 (5.8–8.5) | 2.7 (1.9–3.6) |
| Yes | 9 (7.3–1.1) | 1.8 (1.5–2.1) | 5.5 (5–6.6) | 4.5 (4–4.9) | 42±4.2 | 17.7 (16–19.5) | 6.3 (5.8–7.2) | 7.9 (6.4–9.3) | 3.4 (2.5–4.1)** |
| BMI, kg/m2 |
R=0.124 P<0.001† |
R=0.112 P<0.001† |
R=−0.069 P=0.035 |
R=−0.008 P=0.802 |
R=−0.057 P=0.078 |
R=−0.068 P=0.037 |
R=−0.023 P=0.480 |
R=0.069 P=0.034 |
R=−0.022 P=0.492 |
| CAD | |||||||||
| No | 9.5 (7.7–11.6) | 1.8 (1.5–2.3) | 6 (5.2–7) | 4.7 (4.1–5.4) | 41.6±4.3 | 17.5 (16–19.3) | 6.1 (5.2–7.1) | 7.3 (6–8.9) | 2.6 (1.7–3.6) |
| Yes | 9.2 (7.7–11.4) | 1.8 (1.5–2.2) | 6.2 (5.2–6.9) | 4.7 (4.1–5.4) | 41.5±5 | 17.9 (16.6–19.5) | 6.2 (5.3–7.5) | 7.1 (5.7–8.5) | 2.4 (1.7–3.3) |
| Any established CVD | |||||||||
| No | 9.5 (7.7–1.7) | 1.8 (1.5–2.3) | 6.1 (5.2–7) | 4.7 (4.1–5.4) | 41.5±4.4 | 17.5 (16–19.3) | 6 (5–7) | 7.3 (6–8.9) | 2.6 (1.7–3.6) |
| Yes | 9.17 (7.7–1.1) | 1.8 (1.6–2.2) | 5.9 (5.2–6.7) | 4.6 (4–5.3) | 42±4.8 | 17.7 (16.4–19.3) | 6.3 (5.4–7.5)* | 7.1 (5.8–8.4) | 2.5 (1.8–3.5) |
Cells show P values for the association between each N‐glycan structure and baseline characteristics. For age, body mass index and waist circumference, correlation coefficients (r) are also shown. BMI indicates body mass index; waist circumference is expressed in centimeters; CAD, coronary artery disease; CVD, cardiovascular disease; HDL, high‐density lipoproteins.
*P<0.050; **P<0.010, †associations that remained significant after False Discovery Rate correction.
Serum N‐glycome Profile and SBIs
From all participants with valid data on serum N‐glycome, 98 subjects presented 1 or more SBIs on MRI (10.1%). Among all factors associated with SBIs in the univariate analysis (Table 1) and as reported before,4 age, male sex, and the presence of microalbuminuria were independent predictors of SBIs in our sample.
Regarding the serum N‐glycome, we found that NA2F and NA3Fb were associated in opposite directions with the presence of SBIs, as shown in Figure 3A. NA2F and NA3Fb were also associated with the multiplicity of SBI (comparing none, single infarct, and multiple infarcts) as shown in Figure 3B. Furthermore, in order to evaluate whether these associations were independent of age, the presence of other risk factors, and potential confounders, we performed logistic regression analysis. In order to do so, cutoffs for these peaks were selected based on their sensitivity and specificity on SBIs diagnosis, determined by configuring receiver operator characteristics curves (NA2F≤16.66: sensitivity=50%, specificity=67% and NA3Fb≥2.11: sensitivity=79.6%, specificity=38.6%). As shown in Table 3, both N‐glycan structures remained as independent predictors of SBIs in fully adjusted models, together with increasing age, male sex, increasing diastolic blood pressure, and the presence of microalbuminuria.
Figure 3.
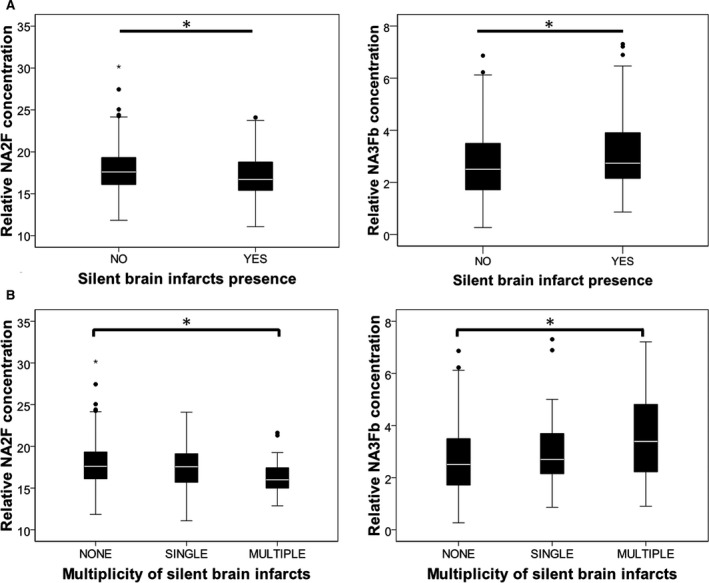
Boxplots representing the relation between NA2F and NA3Fb and the presence (A) and number (B) of SBIs. NA2F: bigalacto core‐α‐1,6‐fucosylated biantennary glycan; NA3Fb: branching α‐1,3‐fucosylated triantennary glycan. *P<0.01. Whiskers were calculated by the formula (Q1‐1.5 [interquartile range]) and (Q3‐1.5 [interquartile range]). NA2F indicates peak 6, to bigalacto core‐α‐1,6‐fucosylated biantennary glycan; NA3Fb, peak 9, to branching α‐1,3‐fucosylated triantennary glycan; SBIs, silent brain infarcts.
Table 3.
Association of N‐glycome Structures with SBIs in the Multivariate Analysis
| Variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age per 1‐year increase | 1.09 (1.05–1.14) | 1.08 (1.03–1.13) | 1.08 (1.03–1.13) |
| Sex, male | 2.84 (1.77–4.57) | 2.61 (1.58–4.32) | 2.5 (1.46–4.28) |
| Mean office DBP per 1 mm Hg increase | 1.03 (1.00–1.05) | ns | 1.3 (1.00–1.05) |
| Microalbuminuria | — | 2.50 (1.45–4.31) | 1.87 (1.04–3.36) |
| NA2F≥16.657 | — | — | 0.42 (0.26–0.69) |
| NA3Fb>2.1134 | — | — | 1.81 (1.00–3.28) |
Model 1 is adjusted by age, sex, mean (DBP), diabetes, tobacco, and high‐density lipoprotein cholesterol. Model 2 is additionally adjusted by microalbuminuria and lipoprotein‐associated phospholipase A2 activity nmol/mL per minute. Model 3 includes the same covariates as model 2 plus the relative amounts of NA2F and NA3Fb below and above their calculated cutoffs. ns, means office DBP was not included in model 2. DBP indicates diastolic blood pressure; NA2F, peak 6, to bigalacto core‐α‐1,6‐fucosylated biantennary glycan; NA3Fb, peak 9, to branching α‐1,3‐fucosylated triantennary glycan; OR, odds ratio; SBI, silent brain infarct.
Moreover, the addition of these serum N‐glycans to the clinical model was significantly associated with an improvement on the prediction of SBIs (χ2 of the likelihood ratio test=11.394, P value <0.001), and with a nonsignificant increase in the global diagnostic accuracy (area under the curve increased from 69.2% to 72.6%, P=0.083). The area under the curve of the model containing only clinical variables plus Lp‐PLA2 and the presence of microalbuminuria was also smaller (ie, less predictive capacity) than the fully adjusted model containing the N‐glycome structures (area under the curve=70.1%).
Regarding other brain vascular lesions, such as WMH, we found the same N‐glycan structures (P value=0.012 for NA2F, and P value=0.015 for NA3Fb) related to the presence of significant WMHs at the periventricular areas, whereas no associations between the serum N‐glycome and WMH at deep subcortical areas were observed. In this case, only NA2F remained independently associated with the presence of significant periventricular WMHs (odds ratio considering NA2F≤16.66=0.47, from 0.30–0.74, P=0.001) together with age (odds ratio per year increase=1.12, 1.07–1.18, P<0.001) and the mean diastolic blood pressure (odds ratio per mm Hg increase=1.04, 1.02–1.07, P=0.001).
Finally, we conducted an exploratory analysis to evaluate how the biomarkers associated with SBIs in the ISSYS study (Lp‐PLA2 activity, microalbuminuria, and NA2F and NA3Fb) were related to each other. We found that NA3Fb was significantly higher in those with microalbuminuria (2.95 [2.11–3.90] versus 2.49 [1.71–3.48], P<0.05), whereas NA2F was positively correlated with Lp‐PLA2 activity (Spearman R=0.221, P value<0.001). However, these associations were no longer present after we controlled for age, sex, or smoking habit, suggesting that they were not independent from the existence of vascular risk factors.
Discussion
In this study, we described for the first time that the serum N‐glycome relate to the presence of silent brain lesions (SBIs, WMHs), independently of age and of the presence of vascular risk factors. To our knowledge, this is the first study investigating serum N‐glycans as markers of silent cerebrovascular disease. Remarkably, they were independent of age, which is the main risk factor for these conditions, and also from other biological markers, such as the presence of microalbuminuria and Lp‐PLA2 activity levels.
We have described that relative concentrations of N‐glycans are related to traditional vascular risk factors in our population. These findings are in agreement with the results from a Croatian population study that reported the relationship of different N‐glycans with several vascular risk factors such as age, sex, smoking, lipid levels, and body fat measurements. As in our case, age and vascular risk factors explained only a small amount of the variability in the N‐glycan profile, that otherwise has been strongly associated with the genetic background and/or specific physiopathological conditions.17
An increasing number of studies have investigated the role of blood biomarkers associated with age‐related WMHs in the past and a smaller number focused on SBIs, but very few of them have been replicated in other populations thus far.18 The most well‐known biomarkers are related to inflammation and endothelial dysfunction: C‐reactive protein,19 interleukin‐6,20 homocysteine,21 and urinary albumin‐to‐creatinine ratio or microalbuminuria.22
Despite the heterogeneous set of proteins and lipids to which N‐glycans structures can be attached, previous reports have described specific patterns in relation to several pathological conditions, such as NA2 for nonalcoholic steatohepatitis,23 NA2FB for liver cirrhosis,24 NA3Fb for hepatocellular carcinoma25 or NG1A2F for type 2 diabetes mellitus.26
In our study, even though sensitivity and specificity of NA2F and NA3Fb cutoff values were low, they improved prediction of SBIs when added to affordable clinical variables, but the diagnostic accuracy of these models (72.6%) still needs to be enhanced to be more accurate and useful from a clinical point of view as surrogate. Possibly this can be achieved by combining them with other biological markers involved in different molecular pathways, and including all possible interactions among all biomarkers might also be a key aspect in the analyses. Indeed, the study of several biomarkers contained in a single panel seems to be a promising strategy to diagnose SBIs, as others have reported before. As examples, a panel of biomarkers involving inflammation, hemostasis, neurohormonal activity, and endothelial function was described to be associated with subclinical vascular brain lesions.27 More recently, Shoamanesh and collaborators tested a panel of 15 markers related to systemic inflammation, vascular inflammation, and oxidative stress and found differential associations of these markers with hemorrhagic (cerebral microbleeds) and ischemic (WMHs and SBIs) manifestations of cerebral small vessel disease.28
Interestingly, we found a different N‐glycome profile concerning SBIs and WMHs and additionally, N‐glycans were only related to periventricular but not deep WMHs. This might be relevant, since it is uncertain whether or not periventricular and deep WMHs differ regarding pathogenesis, risk factors, and clinical consequences.29
Our study has some strengths and limitations. As strengths, it has been performed in a large cohort and we obtained results on serum N‐glycans in almost the whole original sample, limiting selection biases. Also, we described the serum N‐glycan levels as risk factors for SBIs and other brain vascular lesions such as WMHs and assessed their diagnostic accuracy over clinical features and other previously described biological markers.
This study also has some limitations.
The major sources of N‐glycosylated proteins in serum are the B‐cells (immunoglobulins), but other cells such as macrophages (cytokines) and other cell types can also contribute. It is well known that small changes in the cell environment can be associated with large changes in the N‐glycans produced by a cell type.30 Therefore, the N‐glycosylation patterns that we described here associated with SBIs and white matter hyperintensities might be signatures of changes in the structure and function of proteins involved in cerebrovascular disease, suggesting that they could be promising markers to identify specific therapeutic targets and for their future use as biomarkers.
Conclusions
We have measured and described for the first time an association between the serum N‐glycome and SBIs in a large cohort of stroke‐free individuals. If validated in other samples and combined with other biomarkers in a panel, these N‐glycans could be useful to assess the risk of cerebral small vessel disease in asymptomatic individuals.
Sources of Funding
This research has been cofunded with grants from the ISCIII and FEDER (PI10/0705, PI14/1535, CM10/00063, and CP09/136), from the Catalonian Society of Hypertension, the Càtedra‐UAB Novartis de Medicina de Familia and IDIAP Jordi Gol, from the Fundació Josep Palau Francàs. Neurovascular Research Laboratory takes part in the Spanish stroke research network INVICTUS (RD12/0014/0005) (A.V.‐B., I.R.‐L., A.P., J.M., P.D.); and from FWO Flanders and IUAP, Belspo Belgium (C.L., S.D., and V.V.).
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002669 doi: 10.1161/JAHA.115.002669)
References
- 1. Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6:611–619. [DOI] [PubMed] [Google Scholar]
- 2. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. [DOI] [PubMed] [Google Scholar]
- 3. Riba‐Llena I, Penalba A, Pelegri D, Vilar A, Jarca CI, Filomena J, Montaner J, Delgado P. Role of lipoprotein‐associated phospholipase A2 activity for the prediction of silent brain infarcts in women. Atherosclerosis. 2014;237:811–815. [DOI] [PubMed] [Google Scholar]
- 4. Delgado P, Riba‐Llena I, Tovar JL, Jarca CI, Mundet X, Lopez‐Rueda A, Orfila F, Llussa J, Manresa JM, Alvarez‐Sabin J, Nafria C, Fernandez JL, Maisterra O, Montaner J. Prevalence and associated factors of silent brain infarcts in a Mediterranean cohort of hypertensives. Hypertension. 2014;64:658–663. [DOI] [PubMed] [Google Scholar]
- 5. Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. Age‐related galactosylation of the N‐linked oligosaccharides of human serum IgG. J Exp Med. 1988;167:1731–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vanhooren V, Dewaele S, Libert C, Engelborghs S, De Deyn PP, Toussaint O, Debacq‐Chainiaux F, Poulain M, Glupczynski Y, Franceschi C, Jaspers K, van der Pluijm I, Hoeijmakers J, Chen CC. Serum N‐glycan profile shift during human ageing. Exp Gerontol. 2010;45:738–743. [DOI] [PubMed] [Google Scholar]
- 7. Ding N, Nie H, Sun X, Sun W, Qu Y, Liu X, Yao Y, Liang X, Chen CC, Li Y. Human serum N‐glycan profiles are age and sex dependent. Age Ageing. 2011;40:568–575. [DOI] [PubMed] [Google Scholar]
- 8. Hwang H, Zhang J, Chung KA, Leverenz JB, Zabetian CP, Peskind ER, Jankovic J, Su Z, Hancock AM, Pan C, Montine TJ, Pan S, Nutt J, Albin R, Gearing M, Beyer RP, Shi M, Zhang J. Glycoproteomics in neurodegenerative diseases. Mass Spectrom Rev. 2010;29:79–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen CC, Engelborghs S, Dewaele S, Le Bastard N, Martin JJ, Vanhooren V, Libert C, De Deyn PP. Altered serum glycomics in Alzheimer disease: a potential blood biomarker? Rejuvenation Res. 2010;13:439–444. [DOI] [PubMed] [Google Scholar]
- 10. Riba‐Llena I, Jarca CI, Mundet X, Tovar JL, Orfila F, Lopez‐Rueda A, Nafria C, Fernandez JL, Castane X, Domingo M, Alvarez‐Sabin J, Fernandez‐Cortinas I, Maisterra O, Montaner J, Delgado P. Investigating silent strokes in hypertensives: a magnetic resonance imaging study (ISSYS): rationale and protocol design. BMC Neurol. 2013;13:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berger K, Hense HW, Rothdach A, Weltermann B, Keil U. A single question about prior stroke versus a stroke questionnaire to assess stroke prevalence in populations. Neuroepidemiology. 2000;19:245–257. [DOI] [PubMed] [Google Scholar]
- 12. Zhu YC, Dufouil C, Tzourio C, Chabriat H. Silent brain infarcts: a review of MRI diagnostic criteria. Stroke. 2011;42:1140–1145. [DOI] [PubMed] [Google Scholar]
- 13. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti‐Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti‐Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O'Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 15. Vickers AJ, Cronin AM, Begg CB. One statistical test is sufficient for assessing new predictive markers. BMC Med Res Methodol. 2011;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 17. Knezevic A, Gornik O, Polasek O, Pucic M, Redzic I, Novokmet M, Rudd PM, Wright AF, Campbell H, Rudan I, Lauc G. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N‐glycans. Glycobiology. 2013;20:959–969. [DOI] [PubMed] [Google Scholar]
- 18. Vilar‐Bergua A, Riba‐Llena I, Nafria C, Bustamante A, Llombart V, Delgado P, Montaner J. Blood and CSF biomarkers in brain subcortical ischemic vascular disease: Involved pathways and clinical applicability. J Cereb Blood Flow Metab. 2015. doi: 10.1038/jcbfm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anan F, Shimomura T, Kaku T, Kaneda K, Imagawa M, Tsukagawa H, Masaki T, Nawata T, Yonemochi H, Eshima N, Saikawa T, Yoshimatsu H. High‐sensitivity C‐reactive protein level is a significant risk factor for silent cerebral infarction in patients on hemodialysis. Metabolism. 2008;57:66–70. [DOI] [PubMed] [Google Scholar]
- 20. Kikuchi H, Anan F, Kaneda K, Nawata T, Eshima N, Saikawa T, Yoshimatsu H. Interleukin‐6 and silent cerebral infarction in hemodialysis patients: a cross‐sectional study. Eur J Neurol. 2011;18:625–630. [DOI] [PubMed] [Google Scholar]
- 21. Rosenberg GA, Bjerke M, Wallin A. Multimodal markers of inflammation in the subcortical ischemic vascular disease type of vascular cognitive impairment. Stroke. 2014;45:1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wada M, Nagasawa H, Kurita K, Koyama S, Arawaka S, Kawanami T, Tajima K, Daimon M, Kato T. Microalbuminuria is a risk factor for cerebral small vessel disease in community‐based elderly subjects. J Neurol Sci. 2007;255:27–34. [DOI] [PubMed] [Google Scholar]
- 23. Chen C, Schmilovitz‐Weiss H, Liu XE, Pappo O, Halpern M, Sulkes J, Braun M, Cohen M, Barak N, Tur‐Kaspa R, Vanhooren V, Van Vlierberghe H, Libert C, Contreras R, Ben‐Ari Z. Serum protein N‐glycans profiling for the discovery of potential biomarkers for nonalcoholic steatohepatitis. J Proteome Res. 2009;8:463–470. [DOI] [PubMed] [Google Scholar]
- 24. Liu XE, Desmyter L, Gao CF, Laroy W, Dewaele S, Vanhooren V, Wang L, Zhuang H, Callewaert N, Libert C, Contreras R, Chen C. N‐glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46:1426–1435. [DOI] [PubMed] [Google Scholar]
- 25. Vanhooren V, Liu XE, Franceschi C, Gao CF, Libert C, Contreras R, Chen C. N‐glycan profiles as tools in diagnosis of hepatocellular carcinoma and prediction of healthy human ageing. Mech Ageing Dev. 2009;130:92–97. [DOI] [PubMed] [Google Scholar]
- 26. Testa R, Vanhooren V, Bonfigli AR, Boemi M, Olivieri F, Ceriello A, Genovese S, Spazzafumo L, Borelli V, Bacalini MG, Salvioli S, Garagnani P, Dewaele S, Libert C, Franceschi C. N‐glycomic changes in serum proteins in type 2 diabetes mellitus correlate with complications and with metabolic syndrome parameters. PLoS One. 2015;10:e0119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pikula A, Beiser AS, DeCarli C, Himali JJ, Debette S, Au R, Selhub J, Toffler GH, Wang TJ, Meigs JB, Kelly‐Hayes M, Kase CS, Wolf PA, Vasan RS, Seshadri S. Multiple biomarkers and risk of clinical and subclinical vascular brain injury: the Framingham Offspring Study. Circulation. 2012;125:2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shoamanesh A, Preis SR, Beiser AS, Vasan RS, Benjamin EJ, Kase CS, Wolf PA, DeCarli C, Romero JR, Seshadri S. Inflammatory biomarkers, cerebral microbleeds, and small vessel disease: Framingham Heart Study. Neurology. 2015;84:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O'Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vanhooren V. Navarrete Santos A, Voutetakis K, Petropoulos I, Libert C, Simm A, Gonos ES, Friguet B. Protein modification and maintenance systems as biomarkers of ageing. Mech Ageing Dev. 2015;151:71–84. [DOI] [PubMed] [Google Scholar]


