Abstract
Background
The American Heart Association developed criteria dubbed “Life's Simple 7” defining ideal cardiovascular health: not smoking, regular physical activity, healthy diet, maintaining normal weight, and controlling cholesterol, blood pressure, and blood glucose levels. The impact of achieving these metrics on survival after stroke is unknown. We aimed to determine cardiovascular health scores among stroke survivors in the United States and to assess the link between cardiovascular health score and all‐cause mortality after stroke.
Methods and Results
We assessed cardiovascular health metrics among a nationally representative sample of US adults with stroke (n=420) who participated in the National Health and Nutrition Examination Surveys in 1988–1994 (with mortality assessment through 2006). We determined cumulative all‐cause mortality by cardiovascular health score under the Cox proportional hazards model after adjusting for sociodemographic characteristics and comorbidities. No stroke survivors met all 7 ideal health metrics. Over a median duration of 98 months (range, 53–159), there was an inverse dose‐dependent relationship between number of ideal lifestyle metrics met and 10‐year adjusted mortality: 0 to 1: 57%; 2: 48%; 3: 43%; 4: 36%; and ≥5: 30%. Those who met ≥4 health metrics had lower all‐cause mortality than those who met 0 to 1 (hazard ratio, 0.51; 95% confidence interval, 0.28–0.92). After adjusting for sociodemographics, higher health score was associated with lower all‐cause mortality (trend P‐value, 0.022).
Conclusions
Achieving a greater number of ideal cardiovascular health metrics is associated with lower long‐term risk of dying after stroke. Specifically targeting “Life's Simple 7” goals might have a profound impact, extending survival after stroke.
Keywords: all‐cause mortality, American Heart Association, ideal cardiovascular health metrics, Life's Simple 7, Stroke
Introduction
The burden of stroke in life‐years lost, diminished quality of life, and medical costs is enormous1, 2, 3 and is expected to rise substantially over the next several decades.4 Up to 90% of strokes can be prevented through optimization of modifiable risk factors and adoption of a healthy lifestyle.5 Yet, nationally representative data suggest that the majority of the US population eat poorly and are sedentary.6, 7
In 2010, the American Heart Association (AHA), emphasizing a new approach targeted at embracing the benefits of healthy living to increase the chance of living free of cardiovascular disease (CVD) and stroke, introduced a set of ideal cardiovascular health metrics that can be used to monitor health factors and behaviors on individual and population levels.8 Based on evidence from randomized, clinical trials and epidemiological studies, the AHA identified 7 ideal cardiovascular health metrics (“Life's Simple 7”), which encompass 3 medical metrics (blood pressure [BP], total serum cholesterol, and blood glucose) and 4 behavioral metrics (smoking, body mass index [BMI], physical activity, and diet).8
Less than 2% of the US population meets all 7 cardiovascular health metrics.9, 10, 11, 12 Published studies of populations generally free of a history of symptomatic cardiovascular events show that individuals who meet a higher number of Life's Simple 7 health metrics tend to have lower cardiovascular and all‐cause mortality13, 14; however, the impact of meeting these health metrics on long‐term survival after a stroke is unknown. In this study, we assessed the prevalence of “ideal cardiovascular health” among stroke survivors in the United States, and the impact of meeting the Life's Simple 7 cardiovascular health metrics on outcome after stroke.
Methods
Study Design and Participants
National Health and Nutrition Examination Surveys (NHANES) are a series of cross‐sectional, national, stratified, multistage probability surveys conducted by the National Center for Health Statistics (NCHS) in representative samples of the civilian, noninstitutionalized US population. Detailed descriptions of the plan and operation of each survey have been published, and NHANES received approval from the NCHS Research Ethics Review Board.15 All participants were asked to sign an informed consent form.
We included adults aged ≥18 years with self‐reported stroke from NHANES III (1988–1994). In order to determine mortality status, we used the NHANES III Linked Mortality File 2006 in which NHANES III–eligible participants were matched, using a probabilistic matching algorithm, to the National Death Index through December 31, 2006.16
Primary Outcome: All‐Cause Mortality
The International Classification of Diseases Tenth Revision codes I00‐I99 were used to identify deaths from all causes. Participants not matched with a death record were considered alive through the entire follow‐up period.
Definition of Life's Simple 7 Cardiovascular Health Metrics
The AHA definitions for ideal, intermediate, and poor health were used for BP, cholesterol, BMI, and physical activity8; however, modified definitions were used for fasting plasma glucose (FPG), smoking, and healthy diet score (Table 1). For BP, ideal health was defined as untreated BP <120/80 mm Hg; intermediate health was defined as BP 120 to 139/80 to 89 mm Hg or <120/80 mm Hg on antihypertensive medications; and poor health was defined as BP >140/90 mm Hg. Mean BP was the average of up to 3 standardized BP measurements taken during a single encounter based on a standardized protocol.17 For cholesterol, ideal health was defined as untreated total serum cholesterol <200 mg/dL; intermediate health was defined as untreated total serum cholesterol 200 to 239 mg/dL or treated to <200 mg/dL; and poor health was defined as total serum cholesterol >240 mg/dL. Hemoglobin A1c (HbA1c), rather than FPG, was used to describe diabetic health, because a sizeable percentage of participants in NHANES were not fasting. Ideal health was defined as HbA1c concentration <5.7% not on glucose‐lowering meds; intermediate health was defined as HbA1c 5.7% to 6.4% or <5.7% on glucose‐lowering medications; and poor health was defined as HbA1c ≥6.5%, consistent with previous analyses using NHANES data.13, 14 For smoking, ideal health was defined as never smoker; intermediate health was defined as former smoker; and poor health was defined as current smoker. NHANES III lacks information on when participants quit smoking. We used the categories of “never,” “former,” and “current” smoking across surveys for comparability. BMI was calculated from measured weight and height. For BMI, ideal health was defined as BMI <25 kg/m2; intermediate health was defined as BMI 25 to 29.9 kg/m2; and poor health was defined as BMI ≥30 kg/m2. For physical activity, participants were asked about the frequency and duration of participation in moderate and vigorous physical activity during the past 30 days. The weekly number of minutes of moderate or vigorous activity, and the weekly number of minutes of vigorous activity were summed. Ideal health was defined as ≥150 minutes/week of moderate or vigorous activity; intermediate health was defined as 1 to 149 minutes/week of moderate or vigorous activity; and poor health was defined as 0 minute/week of moderate or vigorous activity. The Healthy Eating Index (HEI) score was used to evaluate diet. We used the original HEI created by the US Department of Agriculture in 1995.18 The index was determined from dietary information collected by a single 24‐hour recall administered in person to participants attending the medical examination. The HEI includes 3 of the 5 primary criteria included in the AHA healthy dietary score: intake of fruits and vegetables, whole grains, and sodium. Not included are sugar‐sweetened beverages and fish consumption. Ideal health was defined as HEI >80; intermediate health was defined as HEI 50 to 80; and poor health was defined as HEI <50.
Table 1.
Definition of Life's Simple 7 Cardiovascular Health Metricsa
| Metric | Level of Cardiovascular Health | ||
|---|---|---|---|
| Poor | Intermediate | Ideal | |
| Blood pressure | Treated BP ≥140/90 mm Hg, and BP ≥140/90 mm Hg | SBP 120 to 139 mm Hg or DBP 80 to 89 mm Hg or treated to <120/80 mm Hg | <120/80 mm Hg, without BP‐lowering meds |
| Total cholesterol | ≥240 mg/dL | 200 to 239 mg/dL or treated to <200 mg/dL | <200 mg/dL, without lipid‐lowering medication |
| Glucose/diabetesb | HbA1c >6.4% | HbA1c 5.7% to 6.4% or treated with insulin or oral meds to HbA1C <5.7% | HbA1c <5.7%, without meds |
| Smokingc | Current smoker | Former smoker | Never smoker |
| Body weight | BMI ≥30 kg/m2 | 25 to 29.9 kg/m2 | <25 kg/m2 |
| Physical activity | No activity | 1 to 149 minutes moderate/vigorous per week | >=150 minutes moderate/vigorous per week |
| Dietd | HEI <50 | HEI 50 to 80 | HEI >80 |
AHA indicates American Heart Association; BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; HbA1C, hemoglobin A1c; HEI, Healthy Eating Index; SBP, systolic blood pressure.
The AHA definitions for poor, intermediate, and ideal health were used for blood pressure, cholesterol, BMI, and physical activity8; however, modified definitions were used for FPG, smoking, and health diet score.
Glucose/diabetes: AHA defined poor health as FPG ≥126 mg/dL or HgbA1C ≥7%, intermediate health as FPG 100 to 125 mg/dL or HgbA1C <7%, and ideal health as FPG <100 mg/dL.
Smoking: AHA defined poor health as current smoker, intermediate health as quit smoking <12 months, and ideal health as never smoker or quit smoking ≥12 months.
HEI score includes 3 of 5 primary criteria included in the AHA healthy dietary score: fruits and vegetables, whole grains, and sodium. Not included are sugar‐sweetened beverages and fish consumption.
The Life's Simple 7 cardiovascular health score was constructed by summing the number of ideal health metrics achieved, ranging from 0 to 7 (0 was the worst score and 7 was the optimal score). A point was given only when the participant met the criteria for ideal health. Participants with missing data on any of the 7 cardiovascular health metrics (BP, total serum cholesterol, HbA1C, smoking, BMI, physical activity, and HEI) were excluded from the analysis of the prevalence of “ideal cardiovascular health”.
Covariates in our association analyses included age, sex, race/ethnicity, poverty income ratio (PIR), education level, myocardial infarction (MI), and alcohol intake. Race/ethnicity was classified as non‐Hispanic white, non‐Hispanic black, Mexican American, and other. Socioeconomic status was assessed using PIR (≤2.00 classified as poor). Educational level was classified as ≤12 years or >12 years of formal education. Alcohol consumption was defined as having at least 12 drinks of any type of alcoholic beverage in the past year.
Statistical Analysis
We estimated the survey‐weighted prevalence of the baseline sociodemographic and medical characteristics of the cohort and by health metrics score subgroups (0–1, 2–3, and 4+). The distributions of each categorical variable were compared across subgroups using the Rao–Scott chi‐square test. We determined the proportion of stroke survivors who met each health metric and assessed the survey weighted prevalence of each score. To assess disparities in health metric scores by sociodemographic, we compared the distribution of sex, race/ethnicity, PIR, and education level between persons with low health metrics score (defined as 0–1 metrics in the ideal category) versus high health metrics score (≥4 metrics in the ideal category) using the Rao–Scott chi‐squared test. A cutoff of ≥4 was chosen based upon the sample size and effect size (data not shown). We used survey adjusted logistic regression to examine the odds of having low versus high score according to age, sex, race/ethnicity, PIR, and education level before and after simultaneously adjusting for each of the above demographic characteristics, as well as hypertension, diabetes, hypercholesterolemia, MI, and alcohol intake.
To evaluate the association between health metrics category and the risk of all‐cause mortality, we used the Cox regression model, before and after adjusting for covariates. To evaluate whether the relationship between health metrics category and the risk of all‐cause mortality differed by sex, race/ethnicity, income, and education, we included interaction terms in the above models. Trend P values were computed by including the health metrics score as a continuous variable in the models. We calculated the 10‐year adjusted incidence of mortality for each category of the health metric score by level of each sociodemographic factor and overall under the above Cox model. We computed the rate of all‐cause mortality per 1000 person‐years for each health metric category empirically with adjustment for age. First, we computed the age‐specific mortality rates by dividing the total number of deaths by the total number of person‐years follow‐up (multiplying by 1000 to obtain rate per 1000 person‐years) for age categories <65, 65 to 74, and ≥75 years. We computed the age‐adjusted mortality rate as the weighted average of the above age‐specific mortality rates, with the weights equal to the observed proportions of persons in each age category in the overall stroke cohort. In addition, we calculated the covariate‐adjusted rate of all‐cause mortality per 1000 person‐years for each health category under the Poisson regression model after simultaneously adjusting for age, sex, race/ethnicity, education level, and PIR, taking into account the NHANES survey design.
We excluded 65 (13%) of the 485 participants from the analyses because of incomplete health metric data. In order to assess the potential influence of missing data on the results, we compared covariates and each of 7 health metric items between persons with and without missing data to determine whether the distributions were comparable in the 2 groups. In addition, missing values were imputed using the Markov chain Monte Carlo imputation and additional sensitivity analyses were performed after adjusting for covariates. Data were analyzed using STATA software (version 11.2; StataCorp LP, College Station, TX) and R (version 3.0.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Participant Characteristics
A total of 485 participants of NHANES III (1988–1994) reported history of stroke. Of those, 65 (13%) had missing data for at least 1 health metric and therefore were excluded, leaving 420 with complete data on the 7 health metrics for analysis. Median follow‐up length was 98 months (range, 53–159). Table 2 shows the baseline sociodemographic and comorbid characteristics by health metric category (score 0–1, 2–3, and 4+) and overall. In the overall cohort, most participants were non‐Hispanic white (79.6%), 65 years of age or older (61.3%), and had ≤grade 12 education (76.6%).
Table 2.
Patient Sociodemographic and Comorbid Characteristics, NHANES 1988–1994
| Overall Cohort | 0 to 1 | 2 to 3 | 4+ | P Value | |
|---|---|---|---|---|---|
| N=420 | N=87 | N=263 | N=70 | ||
| Age, % | |||||
| 18 to 64 y | 38.7 | 25.3 | 58.2 | 16.5 | 0.14 |
| ≥65 years | 61.3 | 13.2 | 68.8 | 18.0 | |
| Sex, % | |||||
| Women | 51.4 | 20.3 | 66.2 | 13.5 | 0.10 |
| Men | 48.6 | 15.4 | 63.1 | 21.4 | |
| Race/ethnicity, % | |||||
| Non‐Hispanic white | 79.6 | 16.5 | 65.1 | 18.4 | 0.09 |
| Non‐Hispanic black | 10.8 | 34.3 | 53.4 | 12.3 | |
| Mexican American | 2.2 | 25.2 | 57.3 | 17.5 | |
| Other | 7.4 | 7.6 | 79.1 | 13.3 | |
| Poverty income ratio, % | |||||
| ≤2.00 | 50.7 | 18.6 | 69.6 | 11.8 | 0.025 |
| >2.00 | 40.5 | 19.8 | 58.5 | 21.7 | |
| Education level, % | |||||
| ≤12th grade | 76.6 | 20.5 | 63.6 | 16.0 | 0.058 |
| >12th grade | 23.4 | 9.6 | 68.5 | 22.0 | |
| Comorbidities, % | |||||
| Hypertensiona | <0.01 | ||||
| No | 31.7 | 2.2 | 69.7 | 28.1 | |
| Yes | 68.3 | 25.2 | 62.4 | 12.4 | |
| Diabetesb | <0.01 | ||||
| No | 72.1 | 10.9 | 67.6 | 21.5 | |
| Yes | 27.9 | 36.0 | 57.0 | 6.9 | |
| Hypercholesterolemiac | <0.01 | ||||
| No | 45.4 | 15.5 | 46.0 | 38.5 | |
| Yes | 54.6 | 22.3 | 69.1 | 8.7 | |
| Low HDLd | 0.06 | ||||
| No | 50.2 | 16.2 | 61.0 | 22.8 | |
| Yes | 49.8 | 19.6 | 68.4 | 11.9 | |
| Myocardial infarction | 0.44 | ||||
| No | 75.9 | 16.6 | 64.7 | 18.8 | |
| Yes | 24.1 | 22.2 | 64.8 | 13.0 | |
| Alcohole | 0.90 | ||||
| No | 75.3 | 18.5 | 64.8 | 16.7 | |
| Yes | 24.7 | 16.1 | 64.4 | 19.4 | |
BP indicates blood pressure; HDL, high‐density lipoprotein; NHANES, National Health and Nutrition Examination Survey.
BP >140/90 mm Hg or on antihypertensive medications, or self‐report.
Hemoglobin A1C ≥6.5% or on diabetes medications, or self‐report.
Total serum cholesterol ≥240 mg/dL or on cholesterol medications.
HDL <50 mg/dL for women or <40 mg/dL for men.
Alcohol consumption in past year.
Performance on Life's Simple 7 Cardiovascular Health Metric
No participant met all 7 ideal health metrics; 17.9% scored 0 to 1; the majority (64.7%) scored 2 to 3; and 17.4% scored ≥4 (Table 3). Of the ideal health metric categories, 13.1% had untreated BP <120/80 mm Hg, 28.7% had untreated total cholesterol <200 mg/dL, 51.0% had HbA1c <5.7%, 37.4% had never smoked, 35.7% had BMI <25 kg/m2, 18.0% endorsed moderate‐to‐vigorous exercise for 150 minutes per week or more, and 22.3% consumed a healthy diet (HEI >80).
Table 3.
Weighted Prevalence of Meeting Life's Simple 7 Ideal Cardiovascular Health Metrics in Stroke Survivors, National Health and Nutrition Examination Survey (NHANES) 1988–1994
| Overall Cohort | |
|---|---|
| Blood pressure, mm Hg (%) | |
| <120/80 (untreated) | 13.1 |
| 120 to 139/80 to 89 or treated to goal | 41.7 |
| ≥140/90 | 45.2 |
| Total serum cholesterol, mg/dL (%) | |
| <200 mg/dL (untreated) | 28.7 |
| 200 to 239 mg/dL or treated to <200 mg/dL | 34.1 |
| ≥240 mg/dL | 37.2 |
| Hemoglobin A1C, % | |
| <5.7%, without meds | 51.0 |
| 5.7 to 6.4 or treated <5.7% | 30.6 |
| >6.4% | 18.3 |
| Smoking, % | |
| Never | 37.4 |
| Former | 40.5 |
| Current | 22.1 |
| Body mass index, kg/m2 (%) | |
| <25 | 35.7 |
| 25 to 29.9 | 37.3 |
| ≥30 | 26.9 |
| Physical activity, % | |
| ≥150 minutes per week | 18.0 |
| 1 to 149 minutes per week | 37.8 |
| No activity | 44.2 |
| Healthy diet, % | |
| Healthy eating Index >80 | 22.3 |
| Healthy eating index 50 to 80 | 63.5 |
| Healthy eating index <50 | 14.2 |
| Number of ideal health metrics, pooled, % | |
| 0 to 1 | 17.9 |
| 2 to 3 | 64.7 |
| 4+ | 17.4 |
| Number of ideal health metrics, % | |
| 0 | 3.1 |
| 1 | 14.8 |
| 2 | 35.7 |
| 3 | 29.0 |
| 4 | 13.3 |
| 5 | 2.8 |
| 6 | 1.3 |
| 7 | — |
Risk of Having Low Health Metric Score by Sociodemographic Characteristic
Women were more likely to have poor cardiovascular health scores (0–1) compared to men (odds ratio [OR], 2.08; 95% confidence interval [CI], 1.21–3.55), an association that persisted even after adjustment for race/ethnicity, PIR, education, and comorbidities (adjusted OR, 3.18; 95% CI, 1.01–10.05; Table 4). Blacks had over 3 times greater odds of having poor cardiovascular health than Whites (OR, 3.11; 95% CI, 1.37–7.09; adjusted OR, 3.63; 95% CI, 1.00–13.10), and after adjustment for covariates, those with less than or equal to a 12th‐grade education were more likely to have poor cardiovascular health than those with more than a 12th‐grade education (adjusted OR, 8.76; 95% CI, 2.08–36.82; Table 4). Mexican Americans trended toward poorer cardiovascular health compared to non‐Hispanic whites (adjusted OR, 5.24; 95% CI, 0.94–29.08; Table 4).
Table 4.
Weighted Prevalence and OR of Having Low Health Score (0–1) vs High Health Score (4+) Stratified by Sex, Race, Poverty Index Ratio, and Education
| Score 0 to 1 (%) | Score 4+ (%) | Crude | Adjusteda | |||
|---|---|---|---|---|---|---|
| OR (0–1 vs 4+) | P Value | OR (0–1 vs 4+) | P Value | |||
| Age | ||||||
| 18 to 64 y | 25.3 | 16.5 | Reference | Reference | ||
| ≥65 years | 13.2 | 18.0 | 0.48 (0.20–1.12) | 0.09 | 0.69 (0.28–1.71) | 0.42 |
| Sex | ||||||
| Men | 15.4 | 21.4 | Reference | — | Reference | — |
| Women | 20.3 | 13.5 | 2.08 (1.21–3.55) | 0.008 | 3.18 (1.01–10.05) | 0.048 |
| Race/ethnicity | ||||||
| Non‐Hispanic white | 16.5 | 18.4 | Reference | — | Reference | — |
| Non‐Hispanic black | 34.3 | 12.3 | 3.11 (1.37–7.09) | 0.007 | 3.63 (1.00–13.10) | 0.049 |
| Mexican American | 25.2 | 17.5 | 1.61 (0.69–3.78) | 0.27 | 5.24 (0.94–29.08) | 0.058 |
| Other | 7.6 | 13.3 | 0.64 (0.22–1.87) | 0.42 | 1.18 (0.17–8.43) | 0.87 |
| Poverty index ratio | ||||||
| >2.00 | 19.8 | 21.7 | Reference | — | Reference | — |
| ≤2.00 | 18.6 | 11.8 | 1.73 (0.90–3.35) | 0.103 | 1.23 (0.40–3.81) | 0.72 |
| Education | ||||||
| >12th grade | 9.6 | 22.0 | Reference | — | Reference | — |
| ≤12th grade | 20.5 | 16.0 | 2.95 (0.81–10.77) | 0.102 | 8.76 (2.08–36.82) | 0.003 |
Adjusted for sex, race, poverty index ratio, education, hypertension, diabetes, hypercholesterolemia, myocardial infarction, and alcohol intake. OR indicates odds ratio.
Association Between Life's Simple 7 and Mortality
Over a median of 98 months (53–159 months) of follow‐up, there were 320 deaths from all causes. The absolute rates of all‐cause mortality among participants who had cardiovascular health metric scores of 0 to 1, 2, 3, and 4+ were 80.3±9.7, 70.2±5.1, 62.7±5.3, and 53.0±7.5 deaths per 1000 person‐years, respectively (trend, P<0.0001), after adjusting for age, sex, race/ethnicity, PIR, and education (Table 5). Meeting a greater number of cardiovascular health metrics was associated with a lower risk of cumulative 10‐year all‐cause mortality (56.6%±7.5%, 47.6%±5.2%, 43.0%±6.5%, and 34.6%±6.7%; P=0.025) after adjusting for covariates. Compared to the low cardiovascular health metric group (0–1), participants who achieved at least 4 ideal cardiovascular health metrics had 49% reduction in the rate of all‐cause mortality after a stroke (hazard ratio [HR], 0.51; 95% CI, 0.28–0.92). The age‐standardized mortality rate according to number of health metrics revealed a strong inverse linear, dose‐dependent relationship between meeting greater numbers of health metrics and mortality (FigureA). There was an inverse dose‐dependent relationship between cardiovascular health score and all‐cause mortality (FigureB). The association between higher cardiovascular health scores and reduced risk of all‐cause mortality persisted regardless of sex, race/ethnicity, income, or education; however, because sample sizes in each of the subgroups were smaller, there was limited ability to detect significant results in most of the subgroups (data not shown).
Table 5.
Mortality Rate and HRs According to Number of Health Metrics in NHANES Participants With Self‐Reported History of Stroke
| Number of Cardiovascular Health Metrics | Trend P Value | ||||
|---|---|---|---|---|---|
| 0 to 1 | 2 | 3 | 4+ | ||
| No. of deaths/at risk | 66/87 | 126/161 | 79/102 | 49/70 | — |
| No. of person years | 738.5 | 1406.3 | 868.4 | 627.7 | — |
| Crude mortality rate per 1000 person‐years | 75.9±20.5 | 77.1±13.7 | 67.5±14.5 | 54.4±14.5 | — |
| Complete case analysis | |||||
| Age‐adjusted mortality rate per 1000 person‐years | 97.9±12.9 | 90.5±8.1 | 76.6±8.6 | 63.1±9.2 | <0.01 |
| Age, sex, race, PIR, education‐adjusted mortality rate per 1000 person‐years | 80.3±9.7 | 70.1±5.1 | 62±5.2 | 53.0±7.5 | <0.01 |
| 10‐years adjusted mortality, %a | 56.6%±7.5% | 47.6%±5.2% | 42.9%±6.5% | 34.6%±6.7% | 0.025 |
| Adjusted HR (95% CI)a | Reference | 0.77 (0.50–1.20) | 0.67 (0.40–1.13) | 0.51 (0.28–0.92) | 0.022 |
| Imputed analysis | |||||
| Age, sex, race, PIR, education‐adjusted mortality rate per 1000 person‐yearsb | 83.0±8.6 | 72.4±4.9 | 59.8±4.8 | 57.6±7.7 | <0.01 |
| 10‐years adjusted mortality, %a, b | 59.8%±6.6% | 50.2%±4.1% | 41.8%±4.5% | 38.4%±6.2% | 0.016 |
| Adjusted HR (95% CI)a, b | Reference | 0.74 (0.51–1.07) | 0.57 (0.37–0.90) | 0.52 (0.30–0.93) | 0.016 |
CI indicates confidence interval; PIR, poverty income ratio; HR, hazard ratio; NHANES, National Health and Nutrition Examination Survey.
Adjusting for age, sex, race, PIR, and education.
Missing values were imputed using Markov chain Monte Carlo imputation method.
Figure 1.
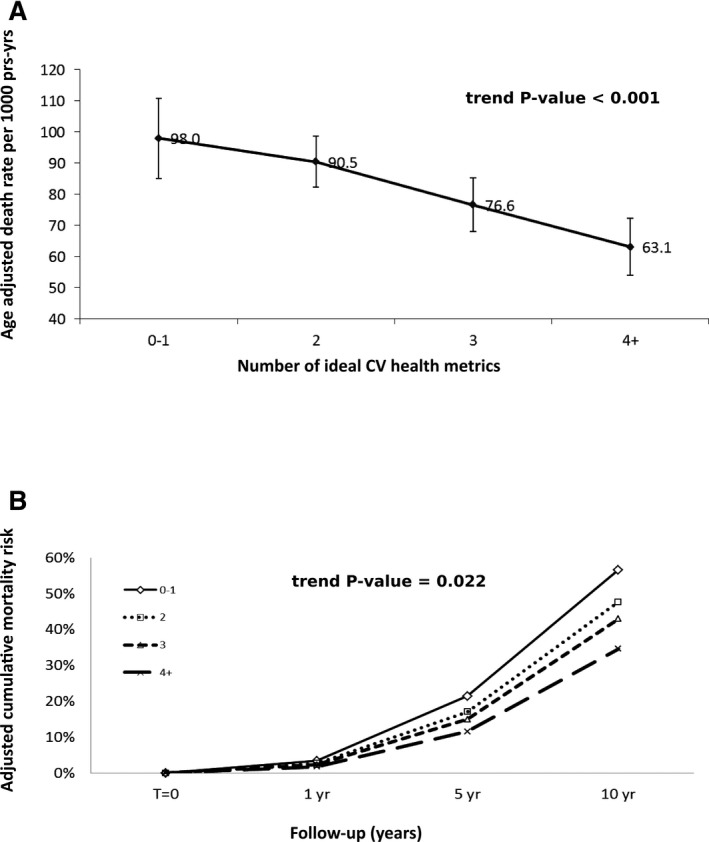
A, Age‐standardized mortality rate per 1000 person‐years according to ideal cardiovascular health score. B, Adjusted cumulative all‐cause mortality risk across time by ideal cardiovascular health score. Adj indicates adjusted; py, person‐years; yr, year(s).
Missing Data Analysis
To reduce the possibility that missing data could influence the directionality of the cardiovascular health score and mortality relationship, we imputed the missing values using the Markov chain Monte Carlo imputation method and adjusted for covariates. The imputed analysis again demonstrated an inverse dose‐dependent relationship between cardiovascular health score and mortality (P<0.001). The results were in close agreement with the previous results using the complete cases (Table 5).
The variables with >5% missing values were HEI (5.4% missing), cholesterol (7.2% missing), and HbA1C (6.8% missing). Comparing covariates in persons who had complete data for all 7 health metrics versus those who did not, incomplete cases were more likely to be women (70% vs 51%), non‐Hispanic black (37% vs 11%), and older (mean age 73 vs 66 years). In addition, they were more likely to have elevated BP (57% vs 44%) and to be physically inactive (80% vs 41%; Table 6).
Table 6.
Comparison of Covariates and Health Metrics Between Participants With and Without Complete Data for the 7 Health Metrics
| Incomplete Case | Complete Cases | ||||||
|---|---|---|---|---|---|---|---|
| N=65 | N=420 | ||||||
| N | Percenta | Percentb | N | Percenta | Percentb | P Value | |
| Age | 0.06 | ||||||
| 18 to 64 y | 12 | 20.2 | 4.9 | 119 | 38.7 | 95.1 | |
| ≥65 years | 53 | 79.8 | 11.3 | 301 | 61.3 | 88.7 | |
| Sex | 0.03 | ||||||
| Men | 28 | 29.9 | 5.7 | 215 | 48.6 | 94.3 | |
| Women | 37 | 70.1 | 11.8 | 205 | 51.4 | 88.2 | |
| Race/ethnicity | |||||||
| Non‐Hispanic white | 7 | 3.2 | 12.7 | 67 | 2.2 | 87.3 | <0.01 |
| Non‐Hispanic black | 26 | 59.9 | 6.9 | 237 | 79.6 | 93.1 | |
| Mexican American | 32 | 36.9 | 25.0 | 100 | 10.8 | 75.0 | |
| Other | 0 | 0.0 | — | 16 | 7.4 | 100.0 | |
| Poverty income ratio | 0.24 | ||||||
| ≤200% | 40 | 61.0 | 10.5 | 244 | 50.7 | 89.5 | |
| >200% | 15 | 26.9 | 6.1 | 129 | 40.5 | 93.9 | |
| Education level | 0.18 | ||||||
| ≤12 grade | 61 | 89.0 | 10.2 | 350 | 76.6 | 89.8 | |
| >12 grade | 4 | 11.0 | 4.4 | 70 | 23.4 | 93.9 | |
| Blood pressure, mm Hg | 0.04 | ||||||
| <120/80 (untreated) | 6 | 5.9 | 3.9 | 34 | 13.8 | 96.1 | |
| 120 to 139/80 to 89 | 21 | 37.0 | 7.7 | 176 | 42.1 | 92.3 | |
| ≥140/90 | 35 | 57.1 | 10.9 | 210 | 44.0 | 89.1 | |
| Total serum cholesterol, mg/dL | 0.30 | ||||||
| <200 mg/dL (untreated) | 7 | 22.9 | 4.0 | 136 | 29.0 | 96.0 | |
| 200 to 239 mg/dL | 9 | 23.2 | 3.4 | 144 | 34.7 | 96.6 | |
| ≥240 mg/dL | 14 | 53.9 | 7.2 | 140 | 36.3 | 92.8 | |
| Hemoglobin A1C | 0.36 | ||||||
| <5.7%, without medications | 9 | 32.0 | 3.1 | 194 | 52.05 | 96.9 | |
| 5.7 to 6.4 or treated <5.7% | 14 | 37.7 | 6.2 | 133 | 30.26 | 93.8 | |
| >6.4% | 9 | 30.3 | 8.3 | 93 | 17.69 | 95.2 | |
| Smoking | 0.18 | ||||||
| Never | 25 | 37.8 | 9.0 | 171 | 37.40 | 92.3 | |
| Former | 31 | 50.2 | 11.0 | 171 | 39.56 | 89.1 | |
| Current | 9 | 12.0 | 4.8 | 78 | 23.04 | 94.1 | |
| Body mass index, kg/m2 | 0.34 | ||||||
| <25 | 22 | 32.6 | 7.7 | 148 | 36.01 | 92.3 | |
| 25 to 29.9 | 24 | 48.4 | 10.9 | 165 | 36.32 | 89.1 | |
| ≥30 | 13 | 19.0 | 5.9 | 107 | 27.66 | 94.1 | |
| Physical activity | <0.01 | ||||||
| ≥150 minutes per week | 9 | 12.6 | 6.4 | 72 | 18.10 | 93.6 | |
| 1 to 149 minutes per week | 6 | 7.6 | 1.8 | 127 | 40.75 | 98.2 | |
| No activity | 50 | 79.8 | 15.9 | 221 | 41.15 | 84.1 | |
| Healthy diet | 0.81 | ||||||
| Healthy Eating Index >80 | 6 | 24.2 | 5.2 | 76 | 22.17 | 94.8 | |
| Healthy Eating Index 50 to 80 | 24 | 57.7 | 4.3 | 281 | 63.79 | 95.7 | |
| Healthy Eating Index <50 | 9 | 18.1 | 6.0 | 63 | 14.04 | 94.0 | |
Survey‐weighted percentage over subgroups.
Survey‐weight percentage over complete vs noncomplete cases.
Discussion
In this national sample of US adults from NHANES III (1988–1994) with follow‐up mortality assessment through 2006, no stroke survivors met all 7 criteria for ideal cardiovascular health. There was a strong dose‐dependent inverse relationship between the number of ideal cardiovascular health metrics achieved and all‐cause mortality after a stroke regardless of sex, race/ethnicity, income, or education. Stroke survivors who met 4 or more health metrics had a significant 49% reduction in all‐cause mortality. This is the first study to delineate the distribution of cardiovascular health metrics among stroke survivors and to show the graded inverse correlation between number of ideal cardiovascular health and mortality after stroke.
Our findings of poor performance on cardiovascular health metrics are similar to several studies in different populations, including Northern Manhattan; Forsyth County, North Carolina; Jackson, Mississippi; Washington County, Maryland; Minneapolis, Minnesota; and Allegheny County, Pennsylvania.9, 10, 11, 12 Recent analyses have similarly demonstrated marked reductions in mortality5, 13, 14, 19 and 20‐year fatal and nonfatal CVD event rates10 with higher cardiovascular health scores.
Of the 7 ideal cardiovascular health metrics, stroke survivors performed worst on behavioral health metric items, namely, physical activity and diet. Only 1 of 5 individuals endorsed engaging in moderate or vigorous physical activity at least 150 minutes per week, and similar proportions had an ideal diet score. These findings highlight the difficulty stroke survivors have in achieving behavioral health benchmarks and the need for concurrent targeted interventions.
This study has several limitations. First, in NHANES, stroke is determined by self‐report; the survey does not collect data regarding stroke type, duration poststroke, stroke severity, or poststroke functional status, all factors that could play roles in stroke mortality and were not controlled for. Self‐reported stroke has a positive predictive value of 79%, a sensitivity of 80%, and specificity of 99%, suggesting that self‐report can reliably be used in epidemiological analysis.20 Second, NHANES is a cross‐sectional sample of the US population; therefore, the study assessed the health metrics at the time of the interview and examination, not at the time of the stroke. In addition, because of the study's cross‐sectional nature, we were unable to capture temporal changes in each of the participant's cardiovascular health metrics. Third, NHANES only captures noninstitutionalized individuals and those who can comprehend and respond to surveys, resulting in a possible bias toward a healthier population. Fourth, only participants with complete data for all 7 components of cardiovascular health were included in prevalence estimates for ideal cardiovascular health, which could introduce unaccounted selection bias. The results of the missing data using Markov chain Monte Carlo imputation, however, demonstrated close agreement with the analysis using the complete cases, further strengthening the validity of our findings. Fifth, there remains uncertainty about ideal goals for BP, cholesterol level, and physical activity after stroke. Sixth, the sample size was relatively small in sub‐subgroup analyses, which may have limited our ability to detect significant trends attributable to lack of power. Because of the sample size, a cutoff of ≥4 ideal health metrics was chosen to compare to participants with ≤1 ideal health metric in order to have significant effect size. It is likely that if another threshold were chosen, a gradation of effect may be observed with more individuals falling into the higher categories of ideal health behaviors. Nevertheless, our findings are consistent with literature using cut‐off points at ≥513 or ≥6,14 showing that the number of LS7 ideal health metrics was significantly and inversely related to all‐cause mortality among stroke survivors. Finally, whereas the National Death Index has long been validated to calculate population mortality estimates,21 the matching algorithm inevitably introduced selection bias in not matching special populations, such as immigrants, undocumented residents, and nonwhite minorities.
Our study has several strengths, including a nationally representative sample of US adults with a long follow‐up for mortality, rigorous and validated survey and examination procedures, adjustment for numerous possible confounders, and robust estimations of absolute mortality and cumulative 10‐year mortality rates, with multiple models adjusting for various potential confounders.
Future directions may include prospectively assessing the relationship between cardiovascular health metric and mortality after stroke, or extending the analysis to NHANES cycles beyond 1988–1994 (with linked mortality data beyond 2006) to increase sample size to provide the power needed for subgroup analyses. Whereas the present study demonstrated a graded mortality benefit from meeting a higher number of ideal cardiovascular health metrics after stroke, the extent to which each of the health metrics contributed to the mortality benefit is unclear. Future studies may focus on quantifying the population‐attributable risk of each of the respective 7 cardiovascular health metrics in stroke survivors. Furthermore, future studies comparing the gain in health benefits associated with a change from poor to intermediate health with the gain associated with a change from intermediate to ideal health for each metric may yield helpful insights that will be of value to clinicians in optimizing the cardiovascular health of their patients. In the meantime, these results suggest the need for targeted interventions aimed at improving cardiovascular health metrics in this vulnerable population.
Source of Funding
Towfighi is supported by 1U54NS081764‐01 from the National Institute of Neurological Disorders and Stroke, 11SDG7590160 from the American Heart Association, and by the Roxanna Todd Hodges Foundation.
Disclosures
Ovbiagele is supported by Award Number U01 NS079179 from the National Institute of Neurological Disorders and Stroke. Markovic reports no disclosures.
(J Am Heart Assoc. 2015;4:e001470 doi: 10.1161/JAHA.114.001470)
References
- 1. Hartmann A, Rundek T, Mast H, Paik MC, Boden‐Albala B, Mohr JP, Sacco RL. Mortality and causes of death after first ischemic stroke: the Northern Manhattan Stroke Study. Neurology. 2001;57:2000–2005. [DOI] [PubMed] [Google Scholar]
- 2. U.S. Burden of Disease Collaborators . The state of US health, 1990‐2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Medical expenditure panel survey: Household component summary data table: Table 4: Total expenses and percent distribution for selected conditions by source of payment: United States, 2011. Available at: http://meps.ahrq.gov/data_stats/quick_tables_results.jsp?component=1&subcomponent=0&tableSeries=2&year=-1&SearchMethod=1&Action=Search. Accessed April 1, 2014.
- 4. Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, Lackland DT, Lichtman JH, Mohl S, Sacco RL, Saver JL, Trogdon JG. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44:2361–2375. [DOI] [PubMed] [Google Scholar]
- 5. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez‐Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 6. McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office. Adv Nutr. 2011;2:293–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd‐Jones DM. Cardiovascular health behavior and health factor changes (1988‐2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lloyd‐Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 9. Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the Northern Manhattan study. Circulation. 2012;125:2975–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community‐based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kulshreshtha A, Vaccarino V, Judd SE, Howard VJ, McClellan WM, Muntner P, Hong Y, Safford MM, Goyal A, Cushman M. Life's Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44:1909–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation. 2012;125:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB. Trends in cardiovascular health metrics and associations with all‐cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National center for health statistics . National health and nutrition examination survey: Questionnaires, datasets, and related documentation. Available at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed April 1, 2014.
- 16. The Third National Health and Nutrition Examination Survey (NHANES III) linked mortality file, mortality follow‐up through 2006: Matching methodology. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality.htm. Accessed April 1, 2014.
- 17. National Health and Nutrition Examination Survey III. Available at: http://www.cdc.gov/nchs/nhanes/nhesiii.htm. Accessed April 1, 2014.
- 18. Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–1108. [DOI] [PubMed] [Google Scholar]
- 19. Towfighi A, Markovic D, Ovbiagele B. Impact of a healthy lifestyle on all‐cause and cardiovascular mortality after stroke in the USA. J Neurol Neurosurg Psychiatry. 2012;83:146–151. [DOI] [PubMed] [Google Scholar]
- 20. Engstad T, Bonaa KH, Viitanen M. Validity of self‐reported stroke : the Tromso Study. Stroke. 2000;31:1602–1607. [DOI] [PubMed] [Google Scholar]
- 21. Articles describing the performance of the national death index. Available at: http://www.cdc.gov/nchs/data/ndi/ndi_bibliography_performance.pdf. Accessed July 20, 2015.


