Abstract
Background
Obstructive sleep apnea syndrome (OSAS) has been indicated to contribute to the development of cardiovascular disease; however, the underlying mechanism remains unclear. This study aimed to test the hypothesis that OSAS may be associated with cardiovascular disease by elevating serum levels of inflammatory markers and causing arterial stiffening and endothelial dysfunction.
Methods and Results
Related scientific reports published from January 1, 2006, to June 30, 2015, were searched in the following electronic literature databases: PubMed, Excerpta Medica Database, ISI Web of Science, Directory of Open Access Journals, and the Cochrane Library. The association of OSAS with serum levels of inflammatory markers, endothelial dysfunction, and arterial stiffening were investigated. Overall, 18 eligible articles containing 736 patients with OSAS and 424 healthy persons were included in this meta‐analysis. Flow‐mediated dilation in patients with moderate–severe OSAS was significantly lower than that in controls (standardized mean difference −1.02, 95% CI −1.31 to −0.73, P<0.0001). Carotid‐femoral pulse wave velocity (standardized mean difference 0.45, 95% CI 0.21–0.69, P<0.0001), augmentation index (standardized mean difference 0.57, 95% CI 0.25–0.90, P<0.0001), and serum levels of high‐sensitivity C‐reactive protein and C‐reactive protein (standardized mean difference 0.58, 95% CI 0.42–0.73, P<0.0001) were significantly higher in patients with OSAS than in controls.
Conclusion
OSAS, particularly moderate–severe OSAS, appeared to reduce endothelial function, increase arterial stiffness, and cause chronic inflammation, leading to the development of cardiovascular disease.
Keywords: arterial stiffening, C‐reactive protein, endothelial dysfunction, obstructive sleep apnea
Introduction
Obstructive sleep apnea syndrome (OSAS) is a common breathing‐related sleep disorder caused by repeated narrowing of the throat during sleep, and the airways can be either partially or completely blocked during OSAS.1 Recent studies show the association of OSAS with cardiovascular disease (CVD).2 Prospective community‐based studies have demonstrated that men with untreated severe OSAS had an increased risk for all‐cause and cardiovascular mortality that was independent of traditional CVD risk factors.3, 4, 5 The Sleep Heart Health Study found that incidence of stroke,6 coronary heart disease,7 and heart failure7 increased in persons with severe OSAS. A recent meta‐analysis further confirmed that the risk of all‐cause mortality increased in patients with severe OSAS compared with persons without OSAS.8
The mechanisms underlying OSAS‐associated CVD are not fully understood. Several factors, such as endothelial dysfunction, arterial stiffening, and systemic inflammation, may mediate OSAS‐associated CVD. Endothelial dysfunction contributes substantially to the pathogenesis of cardiovascular disorders.9, 10, 11 Arterial stiffening is strongly associated with the development of atherosclerosis.12 Epidemiological studies have demonstrated that high arterial stiffness is associated with increased risk of CVD and cardiovascular events.13, 14, 15 In addition, increasing evidence suggests that inflammation plays an important role in the development of cardiovascular complications in patients with OSAS.16, 17 High‐sensitivity C‐reactive protein/C‐reactive protein (hsCRP/CRP), a member of the pentraxin protein family,18 can mediate atherosclerosis‐associated low‐grade inflammation and is a product of the inflammatory reaction.19 Increased serum level of hsCRP/CRP is an independent risk predictor for CVD.20, 21 The proinflammatory cytokine tumor necrosis factor α (TNF‐α) plays an essential role in host defense and mediates the pathogenesis of atherosclerosis.22 Elevated TNF‐α levels predict the incidence of fatal and nonfatal cardiovascular events, such as heart failure and acute coronary syndrome.17, 23 Nevertheless, the effects of OSAS on endothelial dysfunction, arterial stiffening, and systemic inflammation are still unclear.
In a previous meta‐analysis, although serum inflammatory markers were investigated in patients with OSAS,24 the authors did not conduct metaregression to analyze the effects of potential modifiers, such as systolic and diastolic blood pressures, on the study end points. Systolic and diastolic blood pressures have been found to be associated with elevated inflammatory markers.25 The purpose of this meta‐analysis was to investigate the impact of OSAS on endothelial function, arterial stiffening, and serum levels of inflammatory markers hsCRP/CRP and TNF‐α and to examine the impact of potential modifiers on the effects of OSAS on the markers representing endothelial function, arterial stiffening, and inflammation. The findings of this study might shed new light on the mechanisms underlying OSAS‐associated CVD.
Materials and Methods
Guidelines and Data Collection
This meta‐analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines.26 Computerized and manual searches for relevant English articles published within the past 10 years were conducted using the following 5 electronic literature databases: PubMed, Excerpta Medica Database, ISI Web of Science, Directory of Open Access Journals, and the Cochrane Library. The reference lists of the retrieved articles and abstracts of scientific conference proceedings (Figure 1) were also manually searched. The Cochrane Database of Systematic Reviews and the Database of Abstracts of Reviews of Effectiveness were also searched to identify reviews that could lead to eligible trials.
Figure 1.
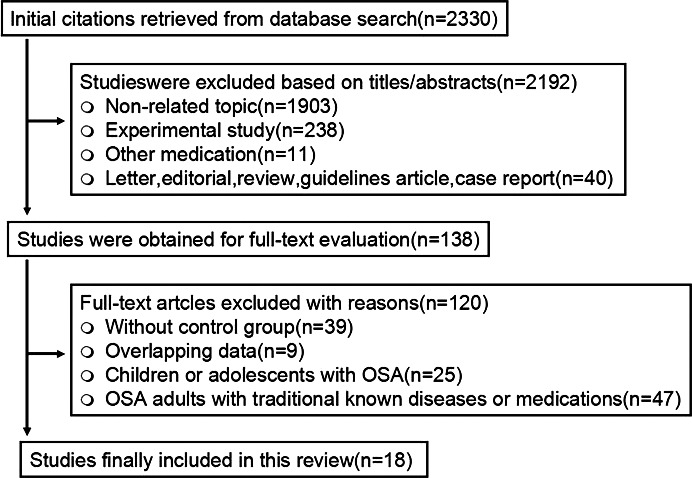
Flow diagram of study selection. OSA indicates obstructive sleep apnea.
Search Terms
The following search terms were used: (“sleep apnea”[All Fields] OR “sleep apnea”[All Fields]) AND (“arterial stiffness”[All Fields] OR “carotid‐femoral pulse wave velocity”[All Fields] OR “augmentation index”[All Fields]); (“sleep apnea”[All Fields] OR “sleep apnea” [All Fields]) AND (“endothelial dysfunction”[All Fields] OR “flow‐mediated dilation”[All Fields] OR “nitroglycerin‐induced dilation”[All Fields]); (“sleep apnea”[All Fields] OR “sleep apnea”[All Fields]) AND (“endothelial dysfunction”[All Fields] OR “tumor necrosis factor‐α”[All Fields] OR “C‐reactive protein”[All Fields] OR “high‐sensitivity C‐reactive protein”[All Fields] OR “inflammatory marker”[All Fields]). All studies that evaluated the effect of OSAS, per se, on endothelial function, arterial stiffening, and inflammation were screened. Two authors (J.W. and W.Y.) performed the literature search and assessed the eligibility of identified publications independently.
OSAS Definition, Study End Points, and Measurements
The apnea‐hypopnea index (AHI) was defined as the average number of episodes of apneas and hypopneas per hour of sleep. OSAS was defined in events per hour as AHI ≥5. Participants were categorized into the following 4 subgroups according to their AHI values: healthy persons (AHI <5) and patients with OSAS that was mild (AHI 5 to <15), moderate (AHI 15 to <30), or severe (AHI ≥30). Moderate–severe OSAS was defined as AHI ≥15; mild–moderate OSAS was defined as AHI 5 to <30. The primary end point was the difference in serum levels of CRP and TNF‐α, arterial stiffness, and vascular endothelial function between patients with OSAS and controls. The control group included persons without OSAS.
Endothelial function was evaluated by brachial artery dilatation in response to hyperemia (represented by flow‐mediated dilation [FMD]) or nitroglycerin (NTG; represented by NTG‐induced dilation), which was calculated as the percentage change in arterial diameter on stimulation relative to resting values. Arterial stiffness was determined by carotid‐femoral pulse wave velocity (PWV), which reflects the amount of time that the pulse wave generated by the contracting heart needs to travel to the periphery and to be reflected back.2, 27 Augmentation index (AIx) was also determined to estimate arterial stiffness. The inflammatory response in patients was evaluated by serum levels of hsCRP/CRP and TNF‐α. The definition and units of the indices of endothelial function, arterial stiffness, and inflammatory markers are displayed in Table 1.
Table 1.
Definition and Units of the Indices of Endothelial Function, Arterial Stiffness, and Inflammatory Markers
| Index | Definition | Equation | Measurement |
|---|---|---|---|
| AIx | The percentage increase in pressure after the peak of blood flow in the vessel. Units: %, pulse pressure | (Ps−Pi)/(Ps−Pd) | We assess the reproducibility of AIx and PWV determined using the Sphygmocor system |
| PWV | Velocity of the pressure pulse along an arterial segment. Units: m/s | Distance/transit time (df−dc)/transit time | We assess the reproducibility of AIx and PWV determined using the Sphygmocor system |
| PWV, aortic (carotid‐femoral) | Velocity assumed to represent aortic PWV using noninvasive measurements of the carotid and femoral arterial pulses. Units: m/s |
df: suprasternal notch to femoral distance dc: suprasternal notch to carotid distance |
We assess the reproducibility of AIx and PWV determined using the Sphygmocor system |
| FMD | The percentage increase in vessel diameter from baseline in response to reactive hyperemia. Units: %, vessel diameter from baseline | Absolute change/baseline×100 absolute change in diameter (maximum minus baseline) | Vessel diameter was measured by a real‐time computerized edge detection system |
| Nitroglycerin‐induced dilation | The percentage increase in vessel diameter from baseline in response to reactive nitroglycerin. Units: %, vessel diameter from baseline | Absolute change/baseline×100 absolute change in diameter (maximum minus baseline) | Vessel diameter was measured by a real‐time computerized edge detection system |
| TNF‐α | Inflammatory marker. Units: pg/mL | Quantitative sandwich enzyme immunoassay kits were used to measure TNF‐a | |
|
hsCRP CRP |
Inflammatory marker. Units: mg/dL | hsCRP/CRP concentration was measured using a latex‐particle enhanced turbidimetric immunoassay |
AIx indicates augmentation index; CRP, C‐reactive protein; dc, distance from suprasternal notch to carotid artery; df, distance from suprasternal notch to femoral artery; FMD, flow‐mediated dilation; hsCRP, high‐sensitivity C‐reactive protein; Pd, diastolic pressure; Pi, augmentation inflection point; Ps, systolic pressure; PWV, pulse wave velocity; TNF‐α, tumor necrosis factor‐α.
Data Extraction and Quality Assessment
Two reviewers (J.W and W.Y.) extracted the following information from the eligible studies independently: first author's surname, year of publication, country of origin, study design, sample size of OSAS and control groups, means and standard deviations of FMD, NTG‐induced dilation, carotid‐femoral PWV, AIx, and serum levels of hsCRP/CRP and TNF‐α in both groups. If studies contained multiple categories of OSAS or body mass index (BMI), data were extracted at each category as independent studies. The 11 items recommended by the US Agency for Healthcare Research and Quality (AHRQ) for assessing the quality of cross‐sectional studies were used to assess the quality of the included studies.28
Study Inclusion and Exclusion Criteria
Studies with a focus on the investigation of the association of OSAS with serum levels of inflammatory markers (hsCRP/CRP and TNF‐α), endothelial function, and arterial stiffening were included. The following exclusion criteria were used: (1) duplicate publication; (2) report in the format of an abstract, review, comment, or editorial; (3) preclinical study; (4) lack of sufficient original data; (5) lack of a control group; (6) patients with OSAS and aged <18 years; (7) patient inclusion criteria in the report explicitly describing that patients with OSAS had traditionally known diseases, including hypertension, diabetes, dyslipidemia, cardiovascular diseases, neuromuscular diseases, inflammatory diseases, restrictive pulmonary disease, chronic renal disease, and/or were taking antihypertensive, antidiabetic, lipid‐lowering, antidepressant, or anti‐inflammation medication; (8) study participants in the report were patients with OSAS and obesity.
Statistical Analysis
Standardized mean differences (SMDs) with 95% CIs of FMD, NTG‐induced dilation, carotid‐femoral PWV, AIx, and serum levels of hsCRP/CRP and TNF‐α in patients with OSAS compared with those in the control group were calculated. The inverse variance method was used to estimate the weight of each study for SMD calculation. A Z test was performed to determine the statistical significance of SMDs. Study heterogeneity was evaluated by Cochran's Q test and the I2 index. P<0.10 in the Q test was considered significantly heterogeneous. A fixed‐effects model was used when study heterogeneity was not significant; a random‐effects model was used when study heterogeneity was significant. When significant study heterogeneity was detected, metaregression was conducted to explore the association between predictor variables and SMD, in other words, to examine whether the impact of OSAS on FMD, NTG‐induced dilation, carotid‐femoral PWV, CRP/hsCRP, and TNF‐α could be modified by potentially meaningful predictors, such as demographic parameters, blood pressure, and blood lipid profile. A Q model statistic was used to estimate the association. Funnel plot and Egger's linear regression test were used to estimate publication bias. All P values are 2‐sided. All statistical analyses were conducted using the Stata statistical analysis software (version 13.0; Stata Corp).
Results
Study Characteristics and Quality Assessment
The flow diagram of study selection is displayed in Figure 1. A total of 18 cross‐sectional articles met the inclusion criteria.18, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 Of the 18 reports, 3 contained 2 categories of OSAS31, 33, 36 (mild–moderate and severe), 1 contained 2 categories of OSAS45 (mild and moderate–severe OSAS), and 1 contained 3 categories of OSAS43 (mild, moderate, and severe OSAS). We extracted data for each category of OSAS. Overall, 736 patients with OSAS and 424 healthy persons were included in the meta‐analysis. Study characteristics including basic epidemiological data, end points, potential modifiers, patient exclusion criteria, and comments are described in Table 2. Of the 18 included studies, 9 reported the impact of OSAS per se on endothelial function (measured by FMD and NTG‐induced dilation),29, 30, 31, 32, 34, 35, 36, 38, 39 5 reported the impact of OSAS per se on arterial stiffening (determined by carotid‐femoral PWV and AIx),36, 37, 39, 40, 42 and 7 investigated the effects of OSAS per se on serum inflammatory markers (hsCRP/CRP and TNF‐α).18, 31, 33, 41, 43, 44, 45 The ARHQ methodology checklist of quality assessment of the included cross‐sectional studies is displayed in Table 3. All included cross‐sectional studies described the source of data and patient inclusion and exclusion criteria clearly, provided detailed explanation for excluded data, and presented multiple measurements for the primary study end points.
Table 2.
Characteristics of the Included Studies
| Author (Year) | Study Population | Endothelial Function | Arterial Stiffening | Inflammatory Markers | Mean EES | Potential Modifier | Patient Exclusion Criteria | Comments |
|---|---|---|---|---|---|---|---|---|
| Tanriverdi29 (2006) |
OSA: 40 Con: 24 |
OSA: 4.57±1.3 (FMD) Con: 6.34±0.83 (FMD) OSA: 10.48±2.1 (NTG) Con: 9.97±2.39 (NTG) |
NR | NR | NR | Age, sex, BMI, SBP, DBP, smoking, TC, HDL, TG, FBG | (1) Impaired cardiorespiratory function (2) CAD (3) valvulopathy, permanent atrial fibrillation or congenital heart disease (4) hypertension, diabetes, dyslipidemia, drug treatment and (5) chronic severe alcoholism | |
| Sohl30 (2007) |
OSA: 14 Con: 10 |
OSA: 5.8±1.8 (FMD) Con: 7.7±1.5 (FMD) |
NR | NR | NR | Age, sex, BMI, SBP, DBP, TC, FBG | Smokers, OSA with other diseases, and taking medications | |
| Chung31 (mild–moderate) (2007) |
OSA: 28 Con: 22 |
OSA: 7.6±2.7 (FMD) Con: 8.1±1.7 (FMD) |
NR |
OSA: 0.115±0.128 (hsCRP) Con: 0.063±0.083 (hsCRP) |
NR | Age, sex, BMI, SBP, DBP, TC, HDL, TG, FBG | Participants aged >60 years, those suffering from inflammatory diseases, or cardiovascular diseases and those taking antihypertensives or antihyperlipidemic or diabetes medications | The report contains 2 categories of OSA (mild–moderate OSA and severe OSA). We extracted data for each category of OSA. |
| Chung31 (severe) (2007) |
OSA: 40 Con: 22 |
OSA: 6.5±2 (FMD) Con: 8.1±1.7 (FMD) |
NR |
OSA: 0.12±0.146 (hsCRP) Con: 0.063±0.083 (hsCRP) |
NR | Age, sex, BMI, SBP, DBP, TC, HDL, TG, FBG | Participants aged >60 years, those suffering from inflammatory diseases, or cardiovascular diseases and those taking antihypertensives or antihyperlipidemic or diabetes medications | The report contains 2 categories of OSA (mild–moderate OSA and severe OSA). We extracted data for each category of OSA. |
| Jelic32 (2008) |
OSA: 30 Con: 15 |
OSA: 4.01±2.99 (FMD) Con: 9.52±2.79 (FMD) |
NR | NR |
OSA: 15 Con: 8 |
Age, sex, BMI, SBP, DBPEES, TC, FBG | Patients with hypertension, CAD, heart failure, a history of stroke, diabetes mellitus, chronic obstructive or restrictive pulmonary disease, chronic renal disease, dyslipidemias, pharmacologically treated depression, or tobacco use within the past 10 years. Patients receiving medications or nutritional supplements and nightshift workers | |
| Kapsimalis33 (mild–moderate) (2008) |
OSA: 26 Con: 15 |
NR | NR |
OSA: 0.26±0.2 (CRP) Con: 0.19±0.1 (CRP) |
OSA: 10.4 Con: 7 |
Age, sex, BMI, EES, FBG | Women, those taking glucose lowering agents/medications and with a history of diabetes mellitus or cardiac, renal, liver, and chronic inflammatory diseases | The report contains 2 categories of OSA (mild–moderate OSA and severe OSA). We extracted data for each category of OSA. |
| Kapsimalis33 (severe) (2008) |
OSA: 26 Con: 15 |
NR | NR |
OSA: 0.35±0.3 (CRP) Con: 0.19±0.1 (CRP) |
OSA: 12.2 Con: 7 |
Age, sex, BMI, EES, FBG | Women, those taking glucose lowering agents/medications and with a history of diabetes mellitus or cardiac, renal, liver, and chronic inflammatory diseases | The report contains 2 categories of OSA (obese and nonobese patients). We included only the nonobese group. |
| Noda40 (2008) |
OSA: 45 Con: 71 |
NR |
OSA: 23.5±8.7 (AIx) Con: 18.6±9 (AIx) |
NR | Age, sex, BMI, SBP, DBP, smoking, HR | Patients had medications and a history of cardiovascular disease or diabetes mellitus | ||
| Constantinidis41 (2008) |
OSA: 11 Con: 15 |
NR | NR |
OSA: 105±87.7 (TNF‐α) Con: 48.5±36.7 (TNF‐α) |
NR | Age, sex, BMI | OSA patients were prior surgery for OSA, chronic inflammation, systemic disease and acute infection within the previous 2 weeks. None of the patients had been under steroidal, anti‐inflammatory, sympatheticomimetic or sympatheticolitic medications | The report contains 2 categories of OSA (mild–moderate OSA and severe OSA). We extracted data for each category of OSA. |
| Jelic34 (2009) |
OSA: 16 Con: 16 |
OSA: 3.3±2.1 (FMD) Con: 7.4±3.9 (FMD) |
NR | NR |
OSA: 12 Con: 4 |
Age, sex, BMI, SBP, DBPEES, TC, FBG | Patients with dyslipidemias, diabetes mellitus, cardiovascular, neurological, pulmonary, and renal diseases were ineligible for the study. Former and current smokers and patients receiving medications or nutritional supplements | |
| Bayram35 (2009) |
OSA: 29 Con: 17 |
OSA: 7.19±1.78 (FMD) Con: 10.93±2.59 (FMD) OSA: 13.75±1.01 (NTG) Con: 14.25±1.83 (NTG) |
NR | NR | NR | Age, BMI, SBP, DBP, HR smoking, TC, HDL, LDL, TG, FBG | Patients were known to have hypertension or other cardiovascular disease, diabetes mellitus, dyslipidemia, alcoholism, neuromuscular disease, renal failure, COPD, or malignancy; were not previously diagnosed with or treated for OSA; and did use medications | Patients with an AHI of 15 or greater per hour (moderate–severe OSA) were included in the study. Participants with an AHI of at least 5 but <15 per hour were not included in the study. |
| Drager37 (2010) |
OSA: 43 Con: 18 |
NR |
OSA: 9.8±1.2 (cfPWV) Con: 8.7±0.7 (cfPWV) |
NR | NR | Age, sex, BMI, SBP, DBP, FBG, TC, HDL, LDL, TG, HR | Women, smoking, hypertension, diabetes, heart failure, renal diseases, and patients using any medication | We recruited consecutive men patients who had a recent diagnosis of moderate‐severe OSA (AHI ≥15 events/hour by polysomnography). |
| Chung36 (mild–moderate) (2010) |
OSA: 39 Con: 29 |
OSA: 6.7±2 (FMD) Con: 8.1±2.6 (FMD) |
OSA: 9.0±1.4 (cfPWV) Con: 8.8±1.2 (cfPWV) |
NR | NR | Age, sex, BMI, smoking, TC, HDL, TG, FBG, SBP | Participants who had inflammatory diseases, COPD, or cardiovascular diseases. Patients who were taking antihypertensives, antihyperlipidemics or hypoglycemics | The report contains 2 categories of OSA (mild–moderate OSA and severe OSA). We extracted data for each category of OSA. |
| Chung36 (severe) (2010) |
OSA: 44 Con: 29 |
OSA: 6.1±2.1 (FMD) Con: 8.1±2.6 (FMD) |
OSA: 9.8±1.6 (cfPWV) Con: 8.8±1.2 (cfPWV) |
NR | NR | Age, sex, BMI, smoking, TC, HDL, TG, FBG, SBP, DBP | Participants who had inflammatory diseases, COPD, or cardiovascular diseases. Patients who were taking antihypertensives, antihyperlipidemics or hypoglycemics | The report contains 2 categories of OSA (mild–moderate OSA and severe OSA). We extracted data for each category of OSA. |
| Guven18 (2012) |
OSA: 47 Con: 29 |
NR | NR |
OSA: 0.403±0.358 (hsCRP) Con: 0.241±0.195 (hsCRP) |
NR | Age, sex, BMI | Patients with a history of cerebrovascular or cardiovascular events, arterial hypertension, COPD or other pulmonary disorders, infectious upper airway disorders, diabetes mellitus, malignancy, hyperlipidemia, hypercholesterolemia, and smoking Patients taking medications that might affect their sleeping pattern | |
| Panoutsopoulos38 (2012) |
OSA: 20 Con: 18 |
OSA: 6.72±0.86 (FMD) Con: 8.3±4.1 (FMD) |
NR | NR | NR | Age, sex, BMI, SBP, DBPTC, HDL, LDL, HD, TG, FBG, smoking | Female patients with suspected OSA, receiving medications, and known suffer from diabetes mellitus, arterial hypertension, dyslipidemia, or inflammatory, cardiovascular, neuromuscular or pulmonary diseases | |
| Bruno39 (2013) |
OSA: 20 Con: 20 |
OSA: 3.7±2.1 (FMD) Con: 6.1±3 (FMD) OSA: 7.7±3.6 (NTG) Con: 6.1±3.8 (NTG) |
OSA: 7.9±1.8 (cfPWV) Con: 7.6±1.4 (cfPWV) |
NR | NR | Age, sex, BMI, SBP, DBP, FBG, TC, smoking | Participants have any traditional cardiovascular risk factors and established cardiovascular or renal disease; obesity; a history of arterial hypertension, diabetes mellitus; severe hypercholesterolemia | Patients with newly diagnosed moderate–severe OSA (AHI ≥15 events/h), were recruited |
| Jones42 (2013) |
OSA: 20 Con: 20 |
NR |
OSA: 19.3±10.9 (AIx) Con: 12.6±10.2 (AIx) OSA: 7.6±1.2 (cfPWV) Con: 7.4±1.2 (cfPWV) |
NR |
OSA: 16 Con: 4 |
Age, sex, BMI, SBP, DBP, smoking, TC, FBG | Previous continuous positive airway pressure, respiratory failure, medications, sleepiness when driving, professional driving, contraindications to magnetic resonance imaging, and intercurrent illness, a history of cardiovascular disease hypertension and diabetes mellitus | Patients with newly diagnosed moderate–severe OSA (AHI ≥15), were recruited |
| Ciccone45 (mild) (2014) |
OSA: 26 Con: 40 |
NR | NR |
OSA: 0.132±0.048 (hsCRP) Con: 0.108±0.053 (hsCRP) OSA: 14.42±3.29 (TNF‐α) Con: 12.52±3.48 (TNF‐α) |
OSA: 10.55 Con: 2.11 |
Age, sex, BMI, SBP, DBP, EES, HR | COPD, history of smoking, congestive heart failure, hypertension, previous myocardial infarction, unstable angina, prior coronary intervention, arrhythmias, use of cardioactive drugs, chronic renal disease, diabetes mellitus, morbid obesity, any chronic inflammatory disease, and systemic infections at the time of the study or within 2 weeks | The report contains 2 categories of OSA (mild and moderate–severe OSA). We extracted data for each category of OSA. |
| Ciccone45 (moderate–severe) (2014) |
OSA: 54 Con: 40 |
NR | NR |
OSA: 0.132±0.048 (hsCRP) Con: 0.108±0.053 (hsCRP) OSA: 22.83±3.85 (TNF‐α) Con: 12.52±3.48 (TNF‐α) |
OSA: 11.25 Con: 2.11 |
Age, sex, BMI, SBP, DBP, EES, HR | COPD, history of smoking, congestive heart failure, hypertension, previous myocardial infarction, unstable angina, prior coronary intervention, arrhythmias, use of cardioactive drugs, chronic renal disease, diabetes mellitus, morbid obesity, any chronic inflammatory disease, and systemic infections at the time of the study or within 2 weeks | The report contains 2 categories of OSA (mild and moderate–severe OSA). We extracted data for each category of OSA. |
| Korkmaz43 (mild) (2015) |
OSA: 27 Con: 40 |
NR | NR |
OSA: 0.4±0.026 (CRP) Con: 0.31±0.018 (CRP) |
NR | Age, sex, BMI, TC, FBG, TG | If they had any malignancy, diabetes mellitus, dyslipidemia, cardiovascular disease or hypertension, chronic inflammatory processes, thyroid dysfunction, chronic hepatic disease, renal failure, or any acute‐subacute infectious disease within the past 2 months, or if they were taking drugs and if data such as blood sample testing results were incomplete or if they were unable to undergo complete PSG testing | The report contains 3 categories of OSA (mild, moderate, and severe OSA). We extracted data for each category of OSA. |
| Korkmaz43 (moderate) (2015) |
OSA: 37 Con: 40 |
NR | NR |
OSA: 0.81±1.94 (CRP) Con: 0.31±0.018 (CRP) |
NR | Age, sex, BMI, TC, FBG, TG | If they had any malignancy, diabetes mellitus, dyslipidemia, cardiovascular disease or hypertension, chronic inflammatory processes, thyroid dysfunction, chronic hepatic disease, renal failure, or any acute–subacute infectious disease within the past 2 months, or if they were taking drugs and if data such as blood sample testing results were incomplete or if they were unable to undergo complete PSG testing | The report contains 3 categories of OSA (mild, moderate, and severe OSA). We extracted data for each category of OSA. |
| Korkmaz43 (severe) (2015) |
OSA: 43 Con: 40 |
NR | NR |
OSA: 0.52±0.69 (CRP) Con: 0.31±0.018 (CRP) |
NR | Age, sex, BMI, TC, FBG, TG | If they had any malignancy, diabetes mellitus, dyslipidemia, cardiovascular disease or hypertension, chronic inflammatory processes, thyroid dysfunction, chronic hepatic disease, renal failure, or any acute–subacute infectious disease within the past 2 months, or if they were taking drugs and if data such as blood sample testing results were incomplete or if they were unable to undergo complete PSG testing | The report contains 3 categories of OSA (mild, moderate, and severe OSA). We extracted data for each category of OSA. |
| Andaku44 (2015) |
OSA: 11 Con: 10 |
NR | NR |
OSA: 0.21±0.06 (hsCRP) Con: 0.11±0.008 (hsCRP) |
OSA: 6.55 Con: 6.9 |
Age, sex, BMI, TC, HDL, LDL, TG, FBG | Participants who had BMI >30, age >60 years, severe chronic pulmonary or CVD, diabetes mellitus or previous OSA treatment; metabolic syndrome (3 of the 5 following factors were present: (1) waist circumference ≥102 cm for men; (2) TG ≥150 mg/dL or patient on specific drug treatment; (3) HDL <40 mg/dL for men or patient on specific drug treatment; (4) arterial blood pressure ≥130 or 85 mm Hg, respectively, for SBP and DBP, or patient on antihypertensive drug treatment; (5) fasting glucose ≥100 mg/dL or patient on specific drug treatment) | The study included male patients with moderate to severe OSA and distributed into 2 groups according to EDS. We included only the non‐EDS OSA group. |
AHI indicates apnea hypopnea index (events per hour); BMI, body mass index (kg/m2); CAD, coronary artery disease; cfPWV, carotid‐femoral pulse wave velocity (m/s); Con, control; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; CVD, cardiovascular disease; DPB, diastolic blood pressure (mm Hg); EDS, excessive daytime sleepiness; EES, Epworth sleepiness score; FBG, fasting blood glucose (mg/dL); FMD, flow‐mediated dilatation (%); HDL, high‐density lipoprotein (mg/dL); HR, heart rate (beats per minute); hsCRP, high‐sensitivity C‐reactive protein (mg/dL); LDL, low‐density lipoprotein (mg/dL); NR, not reported; NTG, nitroglycerin‐induced dilation; OSA, obstructive sleep apnea; PSG, polysomnography; SBP, systolic blood pressure (mm Hg); TC, total cholesterol (mg/dL); TG, triglycerides (mg/dL); TNF‐α, tumor necrosis factor‐α (pg/mL).
Table 3.
The US Agency for Healthcare Research and Quality Checklist for Quality Assessment of Cross‐sectional Studies
| Author, Year | Define the Source of Information (Survey, Record Review) | List Inclusion and Exclusion Criteria for Exposed and Unexposed Participants (Cases and Controls) or Refer to Previous Publications | Indicate Time Period Used for Identifying Patients | Indicate Whether or Not Participants Were Consecutive If Not Population‐Based | Indicate Whether Evaluators of Subjective Components of Study Were Masked to Other Aspects of the Status of the Participants | Describe any Assessments Undertaken for Quality Assurance Purposes (eg, Test/Retest of Primary Outcome Measurements) | Explain Any Patient Exclusions From Analysis | Describe How Confounding was Assessed and/or Controled | If Applicable, Explain How Missing Data Were Handled in the Analysis | Summarize Patient Response Rates and Completeness of Data Collection | Clarify What Follow‐up, If Any, was Expected and the Percentage of Patients for Which Incomplete Data or Follow‐up was Obtained |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tanriverdi,29 2006 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Solh,30 2007 | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Chung,31 2007 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | No |
| Noda,40 2008 | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear | No |
| Kapsimalis,33 2008 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | No |
| Constantinidis,41 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Jelic,32 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear |
| Jelic,342009 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear |
| Bayram,35 2009 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Chung,36 2010 | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear | No |
| Drager,37 2010 | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Panoutsopoulos,38 2012 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Guven,18 2012 | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | Yes | Unclear | No |
| Bruno,39 2013 | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Jones,42 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| Ciccone,45 2014 | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Unclear | No |
| Korkmaz,43 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Andaku,44 2015 | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Unclear | No |
Patients With Moderate–Severe OSAS Showed Significantly Lower FMD Than Controls
The Q‐statistic (P<0.0001) test and the I2 index (74.8%) demonstrated significant heterogeneity of the eligible studies; therefore, a random‐effects model was used to calculate the SMD of FMD. The analysis revealed that FMD in patients with OSAS was significantly lower than that in controls (SMD −1.21, 95% CI −1.60 to −0.83, P<0.0001) (Figure 2A). Subgroup analysis of moderate–severe OSAS (AHI ≥15) showed that patients with moderate–severe OSAS had significantly lower FMD than controls (SMD −1.02, 95% CI −1.31 to −0.73, P<0.0001) (Figure 2B) without significant study heterogeneity (Q statistic P=0.154, I2=42.9%) and publication bias (P=0.243). In contrast to FMD, NTG‐induced dilation was similar in the OSAS and control groups (SMD 0.11, 95% CI −0.22 to 0.44, P=0.306) (Figure 2C) without significant heterogeneity (Q statistic P=0.164, I2 =44.7%) and publication bias (P=0.856).
Figure 2.
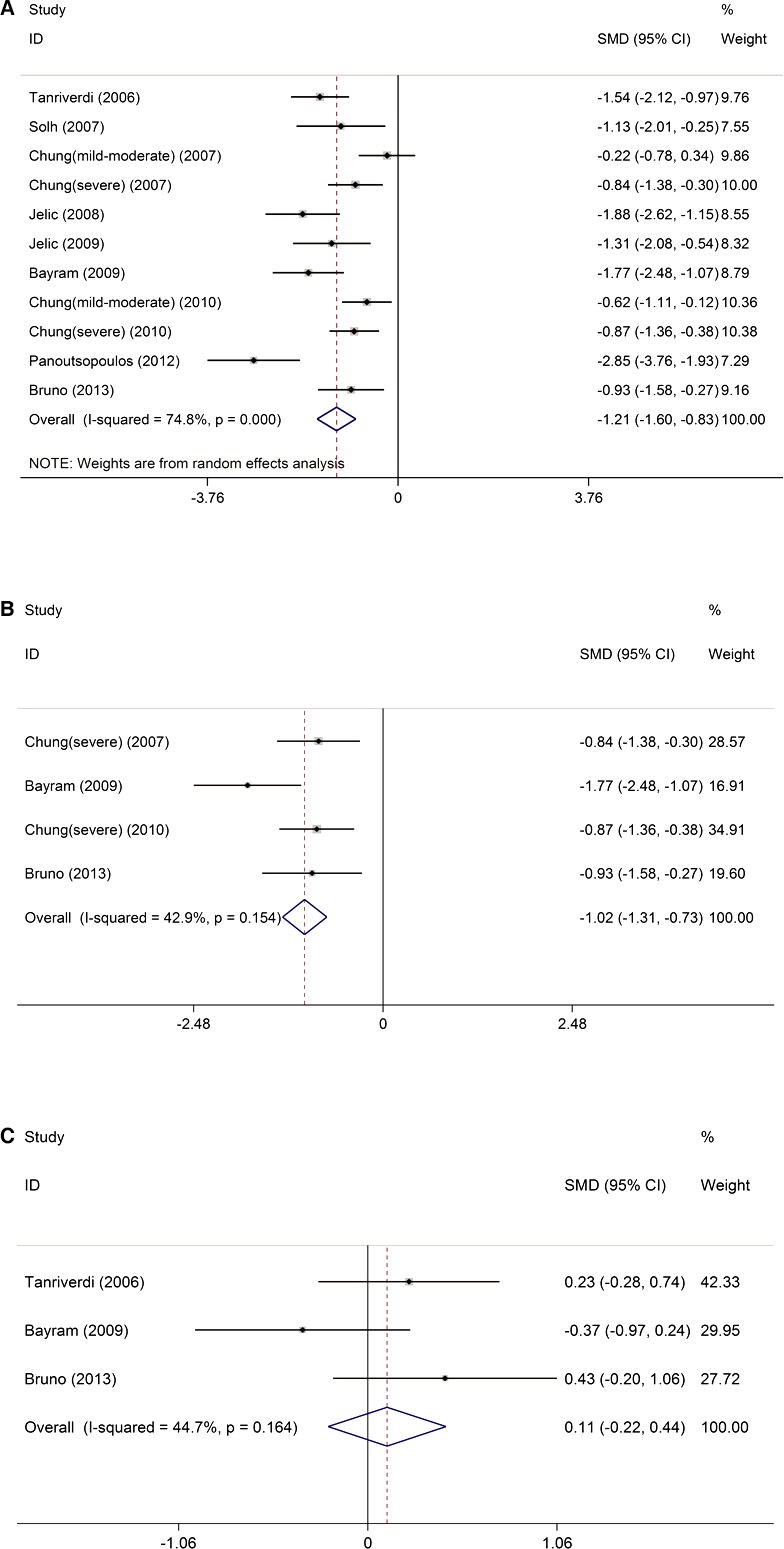
The impact of OSAS on FMD. A, FMD was lower in patients with OSAS than in controls. B, FMD was lower in patients with moderate–severe OSAS than in controls. C, Nitroglycerin‐induced dilation was similar in OSAS and control groups. FMD indicates flow‐mediated dilation; ID, identifier; OSAS, obstructive sleep apnea syndrome; SMD, standardized mean differences.
Patients With OSAS Showed Significantly Higher Carotid‐Femoral PWV and AIx Than Controls
Carotid‐femoral PWV was significantly higher in patients with OSAS than in controls (SMD 0.45, 95% CI 0.21–0.69, P<0.0001) (Figure 3A) without significant study heterogeneity (I2=47.3%, P=0.108) and publication bias (P=0.830). Similarly, subgroup analysis also demonstrated significantly higher carotid‐femoral PWV in patients with moderate–severe OSAS (SMD 0.55, 95% CI 0.27–0.84, P<0.0001) (Figure 3B) without significant study heterogeneity (I2=46.4%, P=0.133). Consistently, AIx, which also indicates arterial stiffness, was significantly higher in patients with OSAS than in controls (SMD 0.57, 95% CI 0.25–0.90, P<0.0001) (Figure 3C) without study heterogeneity (I2=0.0%, P=0.826).
Figure 3.
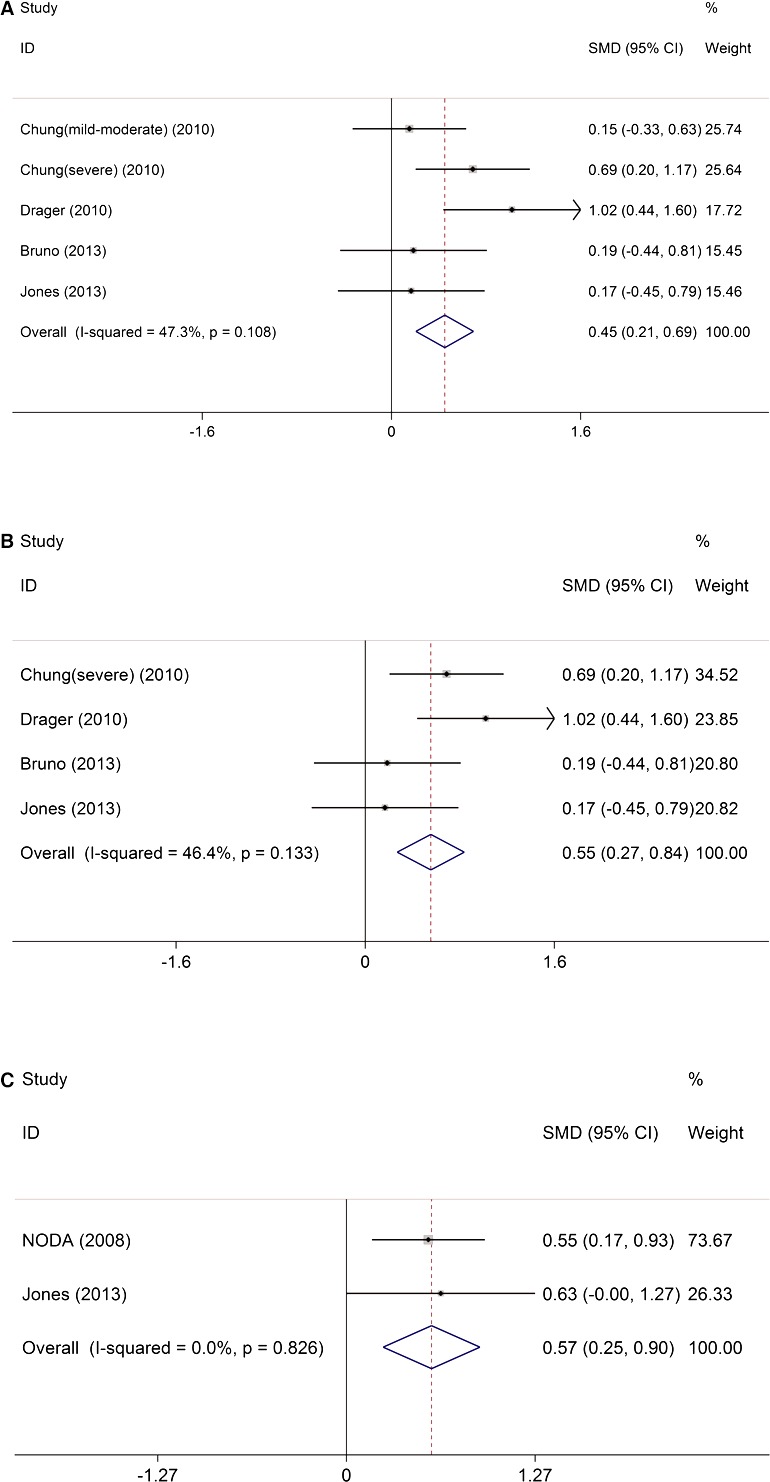
The impact of obstructive sleep apnea on carotid‐femoral PWV and augmentation index. A, Carotid‐femoral PWV was significantly higher in patients with OSAS than in controls. B, Carotid‐femoral PWV was significantly higher in patients with moderate–severe OSAS than in controls. C, Augmentation index was significantly higher in patients with moderate–severe OSAS than in controls. ID indicates identifier; OSAS, obstructive sleep apnea syndrome; PWV, pulse wave velocity; SMD, standardized mean differences.
Patients With OSAS Showed Significantly Higher Serum Levels of hsCRP/CRP and TNF‐α Than Controls
Serum levels of hsCRP/CRP were significantly higher in patients with OSAS than in controls (SMD 0.58, 95% CI 0.42–0.73, P<0.0001) (Figure 4A) without significant study heterogeneity (I2=28.6%, P=0.173) and publication bias (P=0.468). Subgroup analysis of isolated hsCRP (SMD 0.71, 95% CI 0.49–0.92, P<0.0001) (Figure 4A) and isolated CRP (SMD 0.43, 95% CI 0.20–0.66, Z=3.69, P<0.0001) (Figure 4A) consistently showed significantly higher levels of the inflammatory markers in patients with OSAS. In addition to hsCRP/CRP, serum levels of TNF‐α were also significantly higher in patients with OSAS than in controls (SMD 1.29, 95% CI 0.25–2.33, P<0.0001) (Figure 4B) without significant publication bias (P=0.906) but with significant study heterogeneity (I2=91.9%, P<0.0001). The source of heterogeneity might be associated with OSAS severity. Because of the small number of eligible studies, we were unable to perform subgroup analysis of TNF‐α in patients with moderate–severe OSAS.
Figure 4.
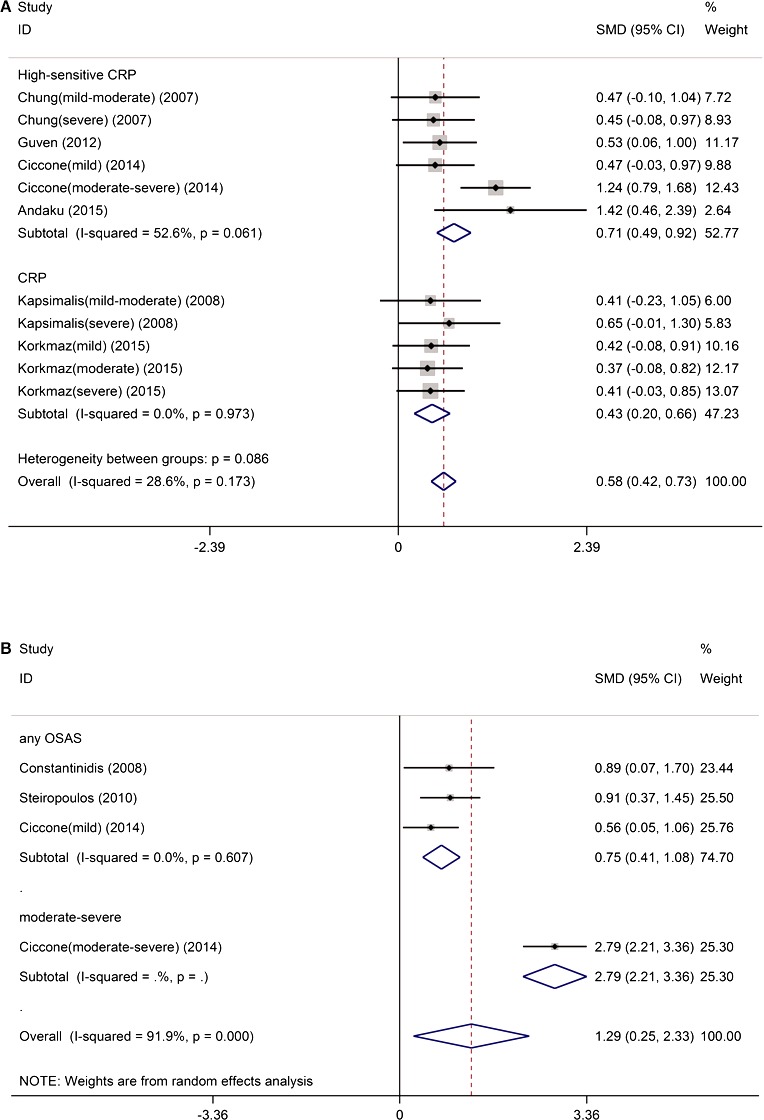
The impact of obstructive sleep apnea on serum inflammatory markers. A, The serum levels of hsCRP/CRP were significantly higher in patients with OSAS than in controls. B, The serum levels of TNF‐α were significantly higher in patients with OSAS than in controls. CRP indicates C‐reactive protein; hsCRP, high‐sensitivity C‐reactive protein; OSAS, obstructive sleep apnea syndrome; SMD, standardized mean differences; TNF‐α, tumor necrosis factor α.
Metaregression Analysis of the Potential Modifiers
We performed metaregression to examine the impact of potential modifiers on OSAS‐related changes in FMD, NTG‐induced dilation, carotid‐femoral PWV, CRP/hsCRP, and TNF‐α. A total of 14 covariates—mean age, proportion of men, systolic and diastolic blood pressures, smoking status, BMI, levels of total cholesterol, triglycerides (TG), fasting blood glucose, high‐ and low‐density lipoprotein, heart rate, Epworth sleepiness score, and publication year—were extracted from the included studies (Tables 4 and 5). When all OSAS patients were included, BMI and TG significantly modified the impact of OSAS on FMD (both P<0.05) (Table 4), suggesting that different BMI and TG in patients with OSAS may be a source of study heterogeneity for the meta‐analysis of FMD. The impact of OSAS on other markers, including NTG‐induced dilation, carotid‐femoral PWV, hsCRP/CRP, and TNF‐α, were not affected by the 14 potential modifiers (Table 4). Further examination of the relationship between the SMD of FMD and BMI and TG revealed that the difference in FMD between OSAS patients and controls increased as BMI increased (Figure 5A) and decreased as TG increased (Figure 5B). In the subgroup of patients with moderate–severe OSAS, none of the 14 covariates significantly modified the impact of OSAS on FMD and carotid‐femoral PWV (Table 5). Consequently, the result showing that patients with moderate–severe OSAS had significantly lower FMD than controls was not influenced by the potential modifiers. We were unable to perform metaregression to analyze the effects of potential modifiers on the study end point AIx because of the limited number of eligible studies.
Table 4.
Metaregression to Examine the Impact of Potential Modifiers on the Effects of Obstructive Sleep Apnea Syndrome on FMD, NTG‐Induced Dilation, cfPWV, CRP/hsCRP, and TNF‐α
| Metaregression Variables | FMD | NTG‐Induced Dilation | cfPWV | CRP/hsCRP | TNF‐α | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp (b) | P Value | Exp (b) | P Value | Exp (b) | P Value | Exp (b) | P Value | Exp (b) | P Value | |
| Mean age | 0.965 | 0.396 | 1.086 | 0.309 | 0.969 | 0.611 | 1.013 | 0.591 | 1.063 | 0.857 |
| BMI | 0.804 | 0.003a | 0.845 | 0.479 | 1.429 | 0.126 | 0.960 | 0.425 | 0.978 | 0.900 |
| Male sex | 1.024 | 0.250 | NE | NE | 1.018 | 0.241 | 1.012 | 0.189 | 1.058 | 0.220 |
| SBP | 1.062 | 0.315 | 1.191 | 0.370 | 0.931 | 0.197 | 1.029 | 0.870 | 1.039 | 0.934 |
| DBP | 1.081 | 0.065 | 0.708 | 0.346 | 1.026 | 0.669 | 0.970 | 0.678 | 1.423 | 0.643 |
| Smoke | 1.002 | 0.955 | 0.921 | 0.391 | 1.026 | 0.288 | No | No | NE | NE |
| TC | 1.004 | 0.685 | 1.019 | 0.321 | 1.016 | 0.700 | 0.984 | 0.391 | No | No |
| TG | 1.015 | 0.030a | NE | NE | 0.983 | 0.591 | 0.998 | 0.529 | No | No |
| FBG | 1.022 | 0.540 | 0.972 | 0.547 | 1.017 | 0.592 | 0.999 | 0.791 | No | No |
| HDL | 1.015 | 0.840 | NE | NE | 1.051 | 0.831 | 1.280 | 0.603 | No | No |
| LDL | NE | NE | NE | NE | NE | NE | NE | NE | No | No |
| HR | No | No | No | No | NE | NE | NE | NE | NE | NE |
| EES | NE | NE | No | No | No | No | 0.917 | 0.567 | 1.491 | 0.795 |
| Publication year | 0.934 | 0.529 | 1.035 | 0.810 | 0.870 | 0.325 | 1.017 | 0.606 | 1.154 | 0.582 |
Exp(b) is the value of the risk ratio of continuous variables. BMI indicates body mass index (kg/m2); cfPWV, carotid‐femoral pulse wave velocity (m/s); CRP, C‐reactive protein; DPB, diastolic blood pressure (mm Hg); EES, Epworth sleepiness score; FBG, fasting blood glucose (mg/dL); FMD, flow‐mediated dilatation (%); HDL, high‐density lipoprotein (mg/dL); HR, heart rate (beats per minute); hsCRP, high‐sensitivity C‐reactive protein (mg/dL); LDL, low‐density lipoprotein (mg/dL); NE, not enough studies; No, no study reported the covariate; NTG, nitroglycerin; OSAS, obstructive sleep apnea syndrome; SBP, systolic blood pressure (mm Hg); TC, total cholesterol (mg/dL); TG, triglycerides (mg/dL); TNF‐α, tumour necrosis factor‐α (pg/mL).
P<0.05.
Table 5.
Metaregression to Examine the Impact of Potential Modifiers on the Effects of Moderate–Severe Obstructive Sleep Apnea Syndrome on FMD and cfPWV
| Metaregression Variables | FMD | cfPWV | ||
|---|---|---|---|---|
| Exp (b) | P Value | Exp (b) | P Value | |
| Mean age | 1.017 | 0.812 | 0.957 | 0.531 |
| BMI | 0.801 | 0.185 | 1.378 | 0.305 |
| Male sex | 1.003 | 0.890 | 1.023 | 0.162 |
| SBP | 1.039 | 0.804 | 0.925 | 0.174 |
| DBP | 1.059 | 0.463 | 1.025 | 0.718 |
| Smoking | 1.010 | 0.858 | 1.027 | 0.378 |
| TC | 1.020 | 0.159 | 0.988 | 0.860 |
| TG | 1.015 | 0.265 | NE | NE |
| FBG | 0.998 | 0.965 | 1.030 | 0.413 |
| HDL | 1.077 | 0.291 | NE | NE |
| Publication year | 1.015 | 0.912 | 0.806 | 0.158 |
| LDL | No | No | No | No |
| EES | No | No | No | No |
| HR | No | No | No | No |
BMI indicates body mass index (kg/m2); cfPWV, carotid‐femoral pulse wave velocity (m/s); DPB, diastolic blood pressure (mm Hg); EES, Epworth sleepiness score; FBG, fasting blood glucose (mg/dL); FMD, flow‐mediated dilatation (%); HDL, high‐density lipoprotein (mg/dL); HR, heart rate (beats per minute); LDL, low‐density lipoprotein (mg/dL); NE, not enough studies; No, no study reported the covariate; SBP, systolic blood pressure (mm Hg); TC, total cholesterol (mg/dL); TG, triglycerides (mg/dL).
Figure 5.
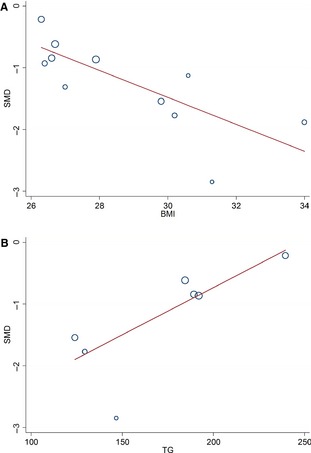
The effect of covariates on the impact of obstructive sleep apnea syndrome on flow‐mediated dilation. A, Regression of BMI on SMD. B, Regression of TG on SMD. BMI indicates body mass index (kg/m2); SMD, standardized mean differences; TG, triglycerides (mg/dL).
Discussion
This updated meta‐analysis demonstrated that patients with OSAS, particularly patients with moderate–severe OSAS, had significantly lower FMD and higher carotid‐femoral PWV, AIx, and serum levels of hsCRP/CRP and TNF‐α than controls. These findings suggest that OSAS might be associated with impaired endothelial function, arterial stiffening, and chronic systemic inflammation.
To the best of our knowledge, this meta‐analysis is the first to investigate the impact of OSAS, per se, on endothelial function and arterial stiffening. All included studies were conducted in the past decade, reflecting the use of more recent guidelines. This meta‐analysis eliminated studies that included patients with OSAS and concomitant traditionally known diseases, such as CVD and diabetes. To further examine whether the 14 potential modifiers could affect the study end points, we conducted metaregression. The metaregression results showed that BMI and TG significantly modified OSAS‐related reduction in FMD but did not affect the impact of moderate–severe OSAS on FMD, suggesting that the association between moderate–severe OSAS and endothelial dysfunction appears to be independent of BMI; however, the influence of mild OSAS on endothelial function may be complicated by other factors, such as obesity and high TG. Nevertheless, metaregression also showed that BMI and the other 13 potential modifiers did not modify OSAS‐related changes in markers representing arterial stiffening and inflammation. Because arterial stiffening and inflammation are also associated with endothelial dysfunction, OSAS may still contribute to vascular dysfunction, although obesity may play a role in endothelial dysfunction in patients with OSAS.
The vascular endothelium, an active monolayer of cells, is involved in the regulation of vasodilation.46 Endothelial cells can produce nitric oxide to protect vessels from atherosclerosis by promoting local vasodilatation and inhibiting platelet aggregation, monocyte adhesion, and vascular smooth muscle proliferation.47 Consequently, endothelial dysfunction is often considered one of the earliest detectable and possibly reversible abnormalities during the development of atherosclerosis. The metaregression results of this current study and of previous studies suggest that the correlation of OSAS with endothelial dysfunction may depend on the severity of OSAS.
In a cross‐sectional study of a cohort of men with OSAS (AHI 15–50) and CVD, Seif et al found that patients with an oxygen desaturation index ≥24.6 showed declining endothelial function, whereas for patients with an oxygen desaturation index between 13.9 and 24.6, there was a positive correlation between increasing oxygen desaturation index and endothelial function.48 This finding suggests that mild to moderate OSAS may trigger some protective mechanisms against CVD, but moderate to severe OSAS appears to be associated with endothelial dysfunction.49 The Sleep Heart Health Study consistently demonstrated that increased incidence of mortality, stroke, coronary artery disease, and heart failure was associated with moderate–severe OSAS.5, 6, 7 Other cross‐sectional data of population‐based studies also showed an association between severe OSAS and impaired endothelial function. Chung et al found that reduced FMD was significantly associated with severe OSAS.31 Our results also showed that patients with moderate–severe OSAS had significantly lower FMD than the controls, indicating endothelial dysfunction in patients with moderate–severe OSAS. These findings suggest that moderate–severe OSAS appears to independently contribute to endothelial dysfunction, leading to OSAS‐mediated CVD; however, whether mild OSAS can damage endothelial function remains to be determined.
In addition, this current study showed that patients with OSAS, particularly patients with moderate–severe OSAS, had significantly lower FMD but similar NTG‐induced dilation compared with controls. NTG‐induced dilation has been used to evaluate vascular smooth muscle function.50 After entering vascular smooth muscle cells, NTG can trigger a series of events, including conversion of nitrate to nitric oxide, activation of soluble guanylate cyclase, synthesis of cyclic guanosine monophosphate, and reduction of cytosolic calcium levels, ultimately leading to smooth muscle relaxation.51 Reduced FMD and similar NTG‐induced dilation in patients with OSAS compared with controls indicate that patients with OSAS have endothelial dysfunction, although their vascular smooth muscle function is normal. The effects of OSAS on vascular smooth muscle function need to be investigated further.
Arterial stiffness, which can be measured by AIx and carotid‐femoral PWV, has been recognized as a cumulative indicator of vascular health.15 Compared with AIx (representing wave reflection), carotid‐femoral PWV (measuring arterial stiffness in the large, elastic, central arteries) is more often used to predict cardiovascular events in general and morbid populations.2 Reports by others showed inconsistent results regarding the association of OSAS with the changes in carotid‐femoral PWV. Bruno et al found that significantly increased carotid‐femoral PWV was observed only in patients with OSAS and concomitant traditional cardiovascular risk factors, whereas patients with OSAS only had levels of carotid‐femoral PWV similar to healthy persons.39 In contrast, in a prospective controlled study, Buchner et al found that OSAS was independently associated with increased arterial stiffening and treatment with continuous positive airway pressure significantly reduced arterial stiffening.52 Chung et al demonstrated that carotid‐femoral PWV was significantly higher in patients with severe OSAS than in patients with mild to moderate OSAS or in healthy controls.36 In this current study, we found that both carotid‐femoral PWV and AIx were significantly increased in patients with OSAS, indicating that patients with OSAS may suffer a higher extent of arterial stiffening. Arterial stiffening has been indicated to play a role in the development of systemic hypertension, cardiac systolic and diastolic dysfunction, pulmonary hypertension, and cardiac ischemia, leading to cardiovascular morbidity and mortality in patients with OSAS.27, 53, 54
This current meta‐analysis also showed that the serum levels of inflammatory markers, including hsCRP/CRP and TNF‐α, were increased in patients with OSAS. Numerous studies have demonstrated that serum level of hsCRP/CRP is a strong predictor of future cardiovascular events.18, 20, 55 Ciccone et al found a significant correlation between carotid intima‐media thickness and the inflammatory markers, hsCRP, IL‐6, TNF‐α, and pentraxin‐3 in patients with OSAS.45 They also showed that hsCRP and TNF‐α levels were independent predictors of carotid intima‐media thickness values.45 Increased carotid intima‐media thickness is a risk factor for early atherosclerosis development and CVD.56, 57 Pathophysiological mechanisms underlying the association of hsCRP/CRP and cardiovascular disturbances, such as atherosclerosis, have been investigated.45 HsCRP, which is found in atherosclerotic plaque,58 can induce the secretion of inflammatory factors from vascular endothelium,59 stimulate the expression of adhesion molecules,60 and opsonize low‐density lipoproteins for macrophage uptake to facilitate low‐density lipoprotein deposition on vessel walls.61
Whether OSAS, per se, can increase serum CRP levels remains inconclusive and requires further investigation. Sharma et al found that in patients with OSAS, obesity but not OSAS was associated with elevated serum levels of CRP, and there was no independent correlation between the severity of OSAS and serum levels of CRP.62 In contrast, Guven et al showed that OSAS was a potential factor for increased CRP levels regardless of BMI.18 The metaregression of this current study showed that BMI did not modify the impact of OSAS or moderate–severe OSAS on serum levels of hsCRP/CRP. Furthermore, this current meta‐analysis demonstrated that serum level of TNF‐α was elevated in patients with OSAS; however, we were limited by the small number of eligible studies and were unable to further examine the correlation between OSAS severity and serum TNF‐α level. A possible correlation between the levels of inflammatory markers and CVD severity in patients with OSAS has been proposed.17
The pathophysiological mechanisms underlying OSAS‐mediated endothelial dysfunction, arterial stiffening, and elevated serum levels of inflammatory markers may be related to OSAS‐induced intermittent hypoxia. Intermittent hypoxia has been found to increase the production of reactive oxygen species, consequently increasing oxidative stress, and prolonged oxidative stress can reduce the enzymatic activity of endothelial nitric oxide synthase by blocking endothelial nitric oxide synthase phosphorylation at S1179.63, 64, 65 Oxidative stress also reduces available substrates for nitric oxide synthesis.66, 67 Reduced nitric oxide production results in endothelial dysfunction and arterial stiffening, ultimately increasing cardiovascular complications such as atherosclerosis in patients with OSAS.32, 63, 68 Furthermore, intermittent hypoxia and sleep disturbances may cause chronic inflammation, increasing serum levels of CRP or other inflammatory markers and contributing to the development of atherosclerosis in patients with OSAS.4, 19, 32
This study has some limitations. Although 14 potential modifiers that could affect the study end points were analyzed in the metaregression, untested OSAS‐associated modifiers are complex and may still inevitably affect the results. The included studies were typical cross‐sectional studies with small sample sizes. Other indicators for endothelial function, such as the levels of circulating endothelial progenitor cells, could not be used for the meta‐analysis because of insufficient data and limited eligible studies. Moreover, different techniques and instruments were used for the measurement of FMD, PWV, and inflammatory markers, which could be another source of study heterogeneity.
Conclusions
Our findings suggest that OSAS, particularly moderate–severe OSAS, was significantly associated with impaired endothelial function, increased arterial stiffness, and elevated serum levels of inflammatory markers. The metaregression data suggest that the adverse effect of moderate–severe OSAS on endothelial function is not modified by potential covariates, such as BMI, and OSAS‐associated arterial stiffening and systemic inflammation are not modified by potential covariates, including BMI and blood lipid profile. Consequently, OSAS—particularly moderate–severe OSAS—may contribute to CVD development by causing endothelial dysfunction, arterial stiffening, and systemic chronic inflammation.
Sources of Funding
This study was supported by the International Science & Technology Cooperation Program of China (No. 2015DFA30160) and Beijing Municipal Science & Technology Commission (No. Z141100006014057).
Disclosures
None.
Acknowledgments
Author Contributions: Wang and W. Yu independently extracted the information from the eligible studies. Besides, Wang and Zhang participated in the design of the study and performed the statistical analysis. Gao participated in the quality assessment. Gu and Wei conceived of the study, and participated in its design. Y. Yu helped to draft the manuscript. All authors read and approved the final manuscript.
(J Am Heart Assoc. 2015;4:e002454 doi: 10.1161/JAHA.115.002454)
References
- 1. Dong JY, Zhang YH, Qin LQ. Obstructive sleep apnea and cardiovascular risk: meta‐analysis of prospective cohort studies. Atherosclerosis. 2013;229:489–495. [DOI] [PubMed] [Google Scholar]
- 2. Vlachantoni IT, Dikaiakou E, Antonopoulos CN, Stefanadis C, Daskalopoulou SS, Petridou ET. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta‐analysis. Sleep Med Rev. 2013;17:19–28. [DOI] [PubMed] [Google Scholar]
- 3. Young T, Finn L, Peppard PE, Szklo‐Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen‐year follow‐up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 4. Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all‐cause mortality: the Busselton Health Study. Sleep. 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 5. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep‐disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, Diener‐West M, Sanders MH, Wolf PA, Geraghty EM, Ali T, Lebowitz M, Punjabi NM. Obstructive sleep apnea‐hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener‐West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all‐cause mortality: a meta‐analysis of prospective cohort studies. Int J Cardiol. 2013;169:207–214. [DOI] [PubMed] [Google Scholar]
- 9. Butt M, Khair OA, Dwivedi G, Shantsila A, Shantsila E, Lip GY. Myocardial perfusion by myocardial contrast echocardiography and endothelial dysfunction in obstructive sleep apnea. Hypertension. 2011;58:417–424. [DOI] [PubMed] [Google Scholar]
- 10. Butt M, Dwivedi G, Khair O, Lip GY. Obstructive sleep apnea and cardiovascular disease. Int J Cardiol. 2010;139:7–16. [DOI] [PubMed] [Google Scholar]
- 11. Napoli C, Ignarro LJ. Nitric oxide and atherosclerosis. Nitric Oxide. 2001;5:88–97. [DOI] [PubMed] [Google Scholar]
- 12. Wada T, Kodaira K, Fujishiro K, Maie K, Tsukiyama E, Fukumoto T, Uchida T, Yamazaki S. Correlation of ultrasound‐measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14:479–482. [DOI] [PubMed] [Google Scholar]
- 13. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 14. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, Ducimetiere P, Benetos A. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 16. Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–2667. [DOI] [PubMed] [Google Scholar]
- 17. Arias MA, Garcia‐Rio F, Alonso‐Fernandez A, Hernanz A, Hidalgo R, Martinez‐Mateo V, Bartolome S, Rodriguez‐Padial L. Cpap decreases plasma levels of soluble tumour necrosis factor‐ receptor 1 in obstructive sleep apnoea. Eur Respir J. 2008;32:1009–1015. [DOI] [PubMed] [Google Scholar]
- 18. Guven SF, Turkkani MH, Ciftci B, Ciftci TU, Erdogan Y. The relationship between high‐sensitivity C‐reactive protein levels and the severity of obstructive sleep apnea. Sleep Breath. 2012;16:217–221. [DOI] [PubMed] [Google Scholar]
- 19. Kokturk O, Ciftci TU, Mollarecep E, Ciftci B. Elevated C‐reactive protein levels and increased cardiovascular risk in patients with obstructive sleep apnea syndrome. Int Heart J. 2005;46:801–809. [DOI] [PubMed] [Google Scholar]
- 20. Sanders MH. Elevated plasma C‐reactive protein and increased cardiovascular/cerebrovascular risk in sleep apnea patients. Article reviewed: elevated C‐reactive protein in patients with obstructive sleep apnea (a brief rapid communication). Sleep Med. 2002;3:449–450. [DOI] [PubMed] [Google Scholar]
- 21. Wilson AM, Ryan MC, Boyle AJ. The novel role of C‐reactive protein in cardiovascular disease: risk marker or pathogen. Int J Cardiol. 2006;106:291–297. [DOI] [PubMed] [Google Scholar]
- 22. Aderka D. The potential biological and clinical significance of the soluble tumor necrosis factor receptors. Cytokine Growth Factor Rev. 1996;7:231–240. [DOI] [PubMed] [Google Scholar]
- 23. Valgimigli M, Ceconi C, Malagutti P, Merli E, Soukhomovskaia O, Francolini G, Cicchitelli G, Olivares A, Parrinello G, Percoco G, Guardigli G, Mele D, Pirani R, Ferrari R. Tumor necrosis factor‐alpha receptor 1 is a major predictor of mortality and new‐onset heart failure in patients with acute myocardial infarction: the cytokine‐activation and long‐term prognosis in myocardial infarction (C‐ALPHA) study. Circulation. 2005;111:863–870. [DOI] [PubMed] [Google Scholar]
- 24. Nadeem R, Molnar J, Madbouly EM, Nida M, Aggarwal S, Sajid H, Naseem J, Loomba R. Serum inflammatory markers in obstructive sleep apnea: a meta‐analysis. J Clin Sleep Med. 2013;9:1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manabe S, Okura T, Watanabe S, Higaki J. Association between carotid haemodynamics and inflammation in patients with essential hypertension. J Hum Hypertens. 2005;19:787–791. [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 27. Doonan RJ, Scheffler P, Lalli M, Kimoff RJ, Petridou ET, Daskalopoulos ME, Daskalopoulou SS. Increased arterial stiffness in obstructive sleep apnea: a systematic review. Hypertens Res. 2011;34:23–32. [DOI] [PubMed] [Google Scholar]
- 28. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta‐analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- 29. Tanriverdi H, Evrengul H, Kara CO, Kuru O, Tanriverdi S, Ozkurt S, Kaftan A, Kilic M. Aortic stiffness, flow‐mediated dilatation and carotid intima‐media thickness in obstructive sleep apnea: non‐invasive indicators of atherosclerosis. Respiration. 2006;73:741–750. [DOI] [PubMed] [Google Scholar]
- 30. El Solh AA, Akinnusi ME, Baddoura FH, Mankowski CR. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med. 2007;175:1186–1191. [DOI] [PubMed] [Google Scholar]
- 31. Chung S, Yoon IY, Shin YK, Lee CH, Kim JW, Lee T, Choi DJ, Ahn HJ. Endothelial dysfunction and C‐reactive protein in relation with the severity of obstructive sleep apnea syndrome. Sleep. 2007;30:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jelic S, Padeletti M, Kawut SM, Higgins C, Canfield SM, Onat D, Colombo PC, Basner RC, Factor P, LeJemtel TH. Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation. 2008;117:2270–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kapsimalis F, Varouchakis G, Manousaki A, Daskas S, Nikita D, Kryger M, Gourgoulianis K. Association of sleep apnea severity and obesity with insulin resistance, C‐reactive protein, and leptin levels in male patients with obstructive sleep apnea. Lung. 2008;186:209–217. [DOI] [PubMed] [Google Scholar]
- 34. Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor P, Le Jemtel TH. Endothelial repair capacity and apoptosis are inversely related in obstructive sleep apnea. Vasc Health Risk Manag. 2009;5:909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bayram NA, Ciftci B, Keles T, Durmaz T, Turhan S, Bozkurt E, Peker Y. Endothelial function in normotensive men with obstructive sleep apnea before and 6 months after CPAP treatment. Sleep. 2009;32:1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung S, Yoon IY, Lee CH, Kim JW. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration. 2010;79:363–369. [DOI] [PubMed] [Google Scholar]
- 37. Drager LF, Diegues‐Silva L, Diniz PM, Bortolotto LA, Pedrosa RP, Couto RB, Marcondes B, Giorgi DM, Lorenzi‐Filho G, Krieger EM. Obstructive sleep apnea, masked hypertension, and arterial stiffness in men. Am J Hypertens. 2010;23:249–254. [DOI] [PubMed] [Google Scholar]
- 38. Panoutsopoulos A, Kallianos A, Kostopoulos K, Seretis C, Koufogiorga E, Protogerou A, Trakada G, Kostopoulos C, Zakopoulos N, Nikolopoulos I. Effect of CPAP treatment on endothelial function and plasma CRP levels in patients with sleep apnea. Med Sci Monit. 2012;18:CR747–CR751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bruno RM, Rossi L, Fabbrini M, Duranti E, Di Coscio E, Maestri M, Guidi P, Frenzilli G, Salvetti A, Taddei S, Bonanni E, Ghiadoni L. Renal vasodilating capacity and endothelial function are impaired in patients with obstructive sleep apnea syndrome and no traditional cardiovascular risk factors. J Hypertens. 2013;31:1456–1464. [DOI] [PubMed] [Google Scholar]
- 40. Noda A, Nakata S, Fukatsu H, Yasuda Y, Miyao E, Miyata S, Yasuma F, Murohara T, Yokota M, Koike Y. Aortic pressure augmentation as a marker of cardiovascular risk in obstructive sleep apnea syndrome. Hypertens Res. 2008;31:1109–1114. [DOI] [PubMed] [Google Scholar]
- 41. Constantinidis J, Ereliadis S, Angouridakis N, Konstantinidis I, Vital V, Angouridaki C. Cytokine changes after surgical treatment of obstructive sleep apnoea syndrome. Eur Arch Otorhinolaryngol. 2008;265:1275–1279. [DOI] [PubMed] [Google Scholar]
- 42. Jones A, Vennelle M, Connell M, McKillop G, Newby DE, Douglas NJ, Riha RL. Arterial stiffness and endothelial function in obstructive sleep apnoea/hypopnoea syndrome. Sleep Med. 2013;14:428–432. [DOI] [PubMed] [Google Scholar]
- 43. Korkmaz M, Korkmaz H, Kucuker F, Ayyildiz SN, Cankaya S. Evaluation of the association of sleep apnea‐related systemic inflammation with CRP, ESR, and neutrophil‐to‐lymphocyte ratio. Med Sci Monit. 2015;21:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andaku DK, D'Almeida V, Carneiro G, Hix S, Tufik S, Togeiro SM. Sleepiness, inflammation and oxidative stress markers in middle‐aged males with obstructive sleep apnea without metabolic syndrome: a cross‐sectional study. Respir Res. 2015;16:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ciccone MM, Scicchitano P, Zito A, Cortese F, Boninfante B, Falcone VA, Quaranta VN, Ventura VA, Zucano A, Di Serio F, Damiani MF, Resta O. Correlation between inflammatory markers of atherosclerosis and carotid intima‐media thickness in obstructive sleep apnea. Molecules. 2014;19:1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoyos CM, Melehan KL, Liu PY, Grunstein RR, Phillips CL. Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature. Sleep Med Rev. 2015;20:15–26. [DOI] [PubMed] [Google Scholar]
- 47. Celermajer DS. Reliable endothelial function testing: at our fingertips? Circulation. 2008;117:2428–2430. [DOI] [PubMed] [Google Scholar]
- 48. Seif F, Patel SR, Walia H, Rueschman M, Bhatt DL, Gottlieb DJ, Lewis EF, Patil SP, Punjabi NM, Babineau DC, Redline S, Mehra R. Association between obstructive sleep apnea severity and endothelial dysfunction in an increased background of cardiovascular burden. J Sleep Res. 2013;22:443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lavie L, Lavie P. Coronary collateral circulation in sleep apnea: a cardioprotective mechanism? Chest. 2010;137:511–512. [DOI] [PubMed] [Google Scholar]
- 50. Montero D, Walther G, Perez‐Martin A, Vicente‐Salar N, Roche E, Vinet A. Vascular smooth muscle function in type 2 diabetes mellitus: a systematic review and meta‐analysis. Diabetologia. 2013;56:2122–2133. [DOI] [PubMed] [Google Scholar]
- 51. Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S‐nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 52. Buchner NJ, Quack I, Stegbauer J, Woznowski M, Kaufmann A, Rump LC. Treatment of obstructive sleep apnea reduces arterial stiffness. Sleep Breath. 2012;16:123–133. [DOI] [PubMed] [Google Scholar]
- 53. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 54. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the sleep heart health study. Am J Respir Crit Care Med. 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 55. Clearfield MB. C‐reactive protein: a new risk assessment tool for cardiovascular disease. J Am Osteopath Assoc. 2005;105:409–416. [PubMed] [Google Scholar]
- 56. O'Leary DH, Polak JF. Intima‐media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol. 2002;90:18L–21L. [DOI] [PubMed] [Google Scholar]
- 57. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. [DOI] [PubMed] [Google Scholar]
- 58. Torzewski M, Rist C, Mortensen RF, Zwaka TP, Bienek M, Waltenberger J, Koenig W, Schmitz G, Hombach V, Torzewski J. C‐reactive protein in the arterial intima: role of C‐reactive protein receptor‐dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20:2094–2099. [DOI] [PubMed] [Google Scholar]
- 59. Lagrand WK, Visser CA, Hermens WT, Niessen HW, Verheugt FW, Wolbink GJ, Hack CE. C‐reactive protein as a cardiovascular risk factor: more than an epiphenomenon? Circulation. 1999;100:96–102. [DOI] [PubMed] [Google Scholar]
- 60. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C‐reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. [DOI] [PubMed] [Google Scholar]
- 61. Zwaka TP, Hombach V, Torzewski J. C‐reactive protein‐mediated low density lipoprotein uptake by macrophages: implications for atherosclerosis. Circulation. 2001;103:1194–1197. [DOI] [PubMed] [Google Scholar]
- 62. Sharma SK, Mishra HK, Sharma H, Goel A, Sreenivas V, Gulati V, Tahir M. Obesity, and not obstructive sleep apnea, is responsible for increased serum hs‐CRP levels in patients with sleep‐disordered breathing in Delhi. Sleep Med. 2008;9:149–156. [DOI] [PubMed] [Google Scholar]
- 63. Lavie L, Lavie P. Molecular mechanisms of cardiovascular disease in OSAHS: the oxidative stress link. Eur Respir J. 2009;33:1467–1484. [DOI] [PubMed] [Google Scholar]
- 64. Thomas SR, Chen K, Keaney JF Jr. Hydrogen peroxide activates endothelial nitric‐oxide synthase through coordinated phosphorylation and dephosphorylation via a phosphoinositide 3‐kinase‐dependent signaling pathway. J Biol Chem. 2002;277:6017–6024. [DOI] [PubMed] [Google Scholar]
- 65. Tanaka T, Nakamura H, Yodoi J, Bloom ET. Redox regulation of the signaling pathways leading to eNOS phosphorylation. Free Radic Biol Med. 2005;38:1231–1242. [DOI] [PubMed] [Google Scholar]
- 66. Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE‐deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. [DOI] [PubMed] [Google Scholar]
- 67. Vasquez‐Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci USA. 1998;95:9220–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lavie L. Oxidative stress–a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303–312. [DOI] [PubMed] [Google Scholar]


