Abstract
Background
Although a myocardial bridge (MB) is often regarded as a benign coronary variant, recent studies have associated MB with focal myocardial ischemia. The physiological consequences of MB on ventricular function during stress have not been well established.
Methods and Results
We enrolled 58 patients with MB of the left anterior descending artery, diagnosed by intravascular ultrasound. Patients underwent invasive physiological evaluation of the MB by diastolic fractional flow reserve during dobutamine challenge and exercise echocardiography. Septal and lateral longitudinal strain (LS) were assessed at rest and immediately after exercise and compared with strain of matched controls. Absolute and relative changes in strain were also calculated. The mean age was 42.5±16.0 years. Fifty‐five patients had a diastolic fractional flow reserve ≤0.76. At rest, there was no significant difference between the 2 groups in septal LS (19.0±1.8% for patients with MB versus 19.2±1.5% for control, P=0.53) and lateral LS (20.1±2.0% versus 20.0±1.6%, P=0.83). With stress, compared with controls, patients with MB had a lower peak septal LS (18.9±2.6% versus 21.7±1.6%, P<0.001) and lower absolute (−0.1±2.1% versus 2.5±1.3%, P<0.001) and relative change (−0.6±11.2% versus 13.1±7.8%, P<0.001) in septal LS, whereas there was no significant difference in lateral LS. In multivariate analysis, diastolic fractional flow reserve and length were independent determinants of lower changes in septal LS.
Conclusions
Patients with a hemodynamically significant MB, determined by invasive diastolic fractional flow reserve, have significantly lower change in septal LS on exercise echocardiography, suggesting that septal LS may be useful for noninvasively assessing the hemodynamic significance of an MB.
Keywords: coronary physiology, deformation imaging, myocardial bridge, myocardial strain
Introduction
A myocardial bridge (MB) is a common coronary variant that occurs when one of the coronary arteries tunnels through the myocardium rather than resting on the epicardium. The prevalence of MB varies depending on the cohort studied, but invasive studies with intravascular ultrasound (IVUS) and computed tomographic angiography suggest a prevalence of ≈25%.1, 2 Although often regarded as benign, MBs have been associated with ischemia,3 acute coronary syndrome involving the proximal left anterior descending artery,4 coronary spasm,5 arrhythmia including both supraventricular and ventricular tachycardia,6 potentially early death after cardiac transplantation,7 and sudden cardiac death.8
Identifying an MB among patients with chest pain syndrome is challenging. The current gold standard for detecting an MB is IVUS, with much greater sensitivity than coronary angiogram by both systolic compression3 and a characteristic echolucent “half‐moon sign” within the MB.9 More recently, computed tomographic angiography has been shown to be valuable in diagnosing the presence of an MB.10 Functionally, Escaned et al highlighted the importance of diastolic fractional flow reserve (dFFR) during dobutamine challenge, as opposed to adenosine challenge and measurement of a mean fractional flow reserve, to assess the hemodynamic severity of an MB.11 In addition, abnormal septal motion and myocardial strain have been increasingly associated with the presence of an MB. Our group previously described a distinctive septal wall motion abnormality with apical sparing in patients with an MB.12 Jhi et al reported that reduced left ventricular (LV) strain has been identified during dobutamine challenge in patients with an MB,13 and Wang et al reported a decrease in resting strain in the MB perfusion territory.14
For this study, we hypothesized that patients with an MB would have a reduced septal longitudinal strain (LS) and comparable lateral LS during exercise compared with age‐ and sex‐matched controls. We further hypothesized that the degree of impairment would be associated with the hemodynamic severity of the MB based on dFFR.
Methods
We identified 65 patients with an MB in the left anterior descending artery, diagnosed by IVUS, who had undergone an evaluation of dFFR during dobutamine challenge and an exercise echocardiogram at our institution. Seven patients were excluded because they had previous cardiac surgery or a significant coronary artery stenosis (>50%) by coronary angiography. The patients had been referred for invasive testing for refractory unexplained exertional chest pain with noninvasive imaging suggestive of an MB (septal wall buckling with apical sparing on stress echocardiography).12 The patients with an MB were compared with 50 age‐ and sex matched controls who were also matched for work load and peak blood pressure during exercise. Controls had undergone stress echocardiography and had no history or echocardiographic findings of MB, coronary artery disease, valvular heart disease, LV dysfunction, or LV hypertrophy. All study participants reached target heart rate (85% of maximum predicted heart rate) during exercise. This study was approved by Stanford University's institutional review board, and informed written consent was obtained from all patients.
Coronary angiography was performed before and after 200 μg of intracoronary nitroglycerin. IVUS image acquisition was performed with a 40‐MHz mechanical transducer ultrasound catheter (Atlantis SR Pro2 or OptiCross; Boston Scientific, Marlborough, Massachusetts) in the left anterior descending artery with placement of the IVUS sensor as far distally as safely possible. The presence of an MB was defined by the identification of an echolucent half‐moon sign on IVUS. The sign was identified during live recording. MB length was measured by the distance from the first proximal appearance of the echolucent half‐moon sign to its distal end. ComboWire recordings were stored on the ComboMap console (Volcano, San Diego, California) for offline analysis. All patients then underwent hemodynamic testing of the MB using the invasive dobutamine challenge protocol described below. Hemodynamic measurements were made using the ComboWire XT Pressure and Flow Wire (Volcano). Pressure and flow velocity waveforms were recorded proximal to, within, and at least 1 cm distal to the MB, identified by IVUS, at baseline and peak dobutamine stress. Dobutamine was given intravenously in increments of 10 to 20 μg kg−1 min−1 every 3 minutes (a maximal dose of 50 μg kg−1 min−1 with up to 1.0 mg atropine) until the maximum predicted heart rate on previous exercise echocardiography was achieved. Using a digital electronic caliper, instantaneous dFFR (the fraction of diastolic coronary artery pressure divided by diastolic aortic pressure) at rest and with stress was calculated proximal to, within, and distal to the MB, as described previously12 (Figure 1). A dFFR ≤0.76 was considered to be hemodynamically significant in this study, as in the previous study by Escaned et al.11
Figure 1.
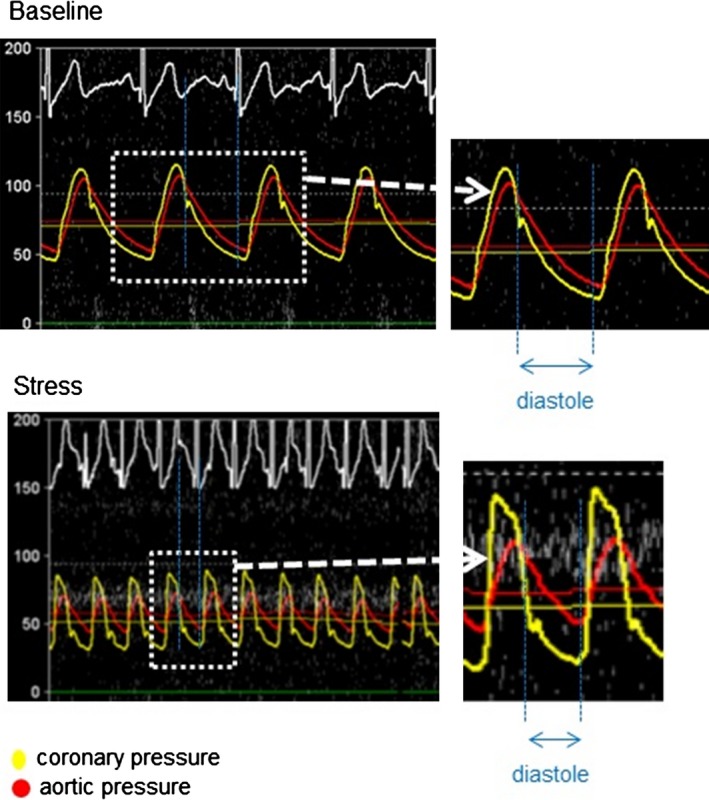
Pressure curves of a representative case of patients with a myocardial bridge (MB). The red curve shows the aortic pressure, and the yellow curve shows the coronary pressure within the MB. Both pressures are similar at baseline; however, coronary systolic pressure increases with stress, whereas diastolic pressure decreases compared with aortic pressure. Diastolic fractional flow reserve equals diastolic coronary pressure divided by diastolic aortic pressure.
All echocardiographic studies were performed using commercially available echo systems (Sonos 7500, iE33, and EPIQ 7C; Philips Medical Imaging, Eindhoven, the Netherlands). Standard echocardiographic views, including parasternal long‐ and short‐axis and apical 2‐, 3‐, and 4‐chamber views, were obtained in 2‐dimensional and color tissue Doppler modes. LV ejection fraction was derived from the modified Simpson method according to the American Society of Echocardiography (ASE) guidelines.15 Exercise echocardiography was performed according to the ASE recommendations.16 An average of 5 to 10 beats per loop was recorded at rest and immediately after stress. All participants performed the Bruce treadmill protocol test17 with a target heart rate of 85% of maximum predicted for age.
LV LS was assessed from the 4‐chamber view both at rest and with exercise, and the absolute change in strain (exercise LS minus rest LS) and the relative change in strain (exercise LS minus rest LS divided by rest LS) were evaluated. Strain values were obtained by manual tracing on an offline Xcelera workstation (Philips Medical Imaging), for which the frame rate was 50 to 52 Hz to ensure the best measurement quality; postprocessing analysis of peak exercise does not always yield optimal tracking. Initial length was obtained in end‐diastole (peak of QRS), and final length was obtained in end‐systole (the smallest LV volume). To assess more detailed LV strain, septal and lateral LS and global LS (GLS) were assessed, as shown in Figure 2. For quality control, the same mitral annular reference points were used; in addition, the apical reference point was determined to be the furthest point of the left ventricle from the mitral annulus, and it was kept stable to avoid overestimating strain measures due to foreshortening. Foreshortening was defined as being when the endocardial border of the apex was significantly displaced in systole. In fact, magnetic resonance–based studies have shown that the endocardial border of the apex usually displaces about 1.5 mm in systole.18 One representative beat with minimal respiratory or translational motion was chosen to trace in both end‐diastole and end‐systole. All strain values were calculated by the following formula in which L0 indicates initial length and L1 indicates final length: strain (%)=100×(L1−L0)/L0.19 Prior to performing the manual tracing, we compared strain values derived from manual tracing and the software analysis (TOMTEC Imaging System, Unterschleissheim, Germany) in 50 healthy persons and found a relative difference 4.8±4.3%. A more negative value represents better function. For the presentation of strain values in this study, the directional change is presented as an absolute value for better understanding.
Figure 2.
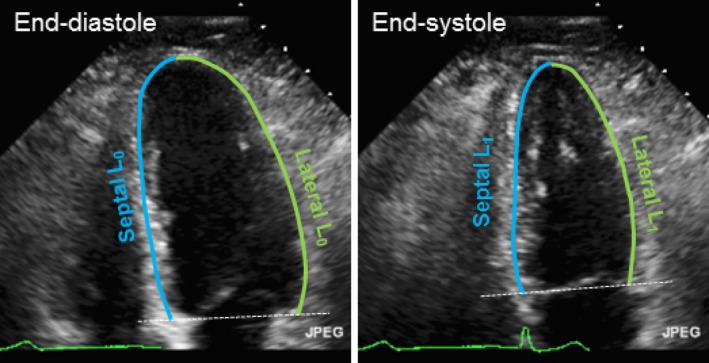
Schema of strain evaluation by manual tracing. Initial length (L0) was obtained in end‐diastole, and final length (L1) was obtained in end‐systole. To divide left ventricle longitudinal components into septal and lateral segments, the apex was standardized as midventricle from the middle of the transverse segment in both systole and diastole. Strain (%)=100×(L1−L0)/L0.
Variables are presented as count and percentage or mean and standard deviation (SD) for normally distributed variables. Variables were compared with (Student or Welch) t test or Mann–Whitney U test, as appropriate. Multivariate regression analysis was performed to identify the independent correlates of absolute and relative changes in strain. We used a regression model that included age, sex, MB length, systolic blood pressure, peak heart rate, and dFFR either within or distal to the MB. Comparisons of strain values between baseline and stress were analyzed by paired t test. For inter‐ and intraobserver variability, 15 patients with an MB and 15 controls were randomly selected and septal LS, lateral LS, and GLS were blindly reanalyzed 2 to 4 weeks after the first analysis without references to the initial tracings, and the absolute difference was assessed. A P<0.05 was considered significant. All analyses were performed using SPSS 21 software (IBM Corp, Armonk, NY).
Results
Table 1 shows the clinical and echocardiographic characteristics of the age‐, sex‐, and blood pressure–matched patients with an MB and controls.
Table 1.
Patient Characteristics
| Patients With an MB (n=55) | Controls (n=50) | P Value | |
|---|---|---|---|
| Age, y | 42.5±16.0 | 46.3±13.8 | 0.14 |
| Male (%) | 20 (36) | 19 (38) | 0.86 |
| BSA, m2 | 1.8±0.2 | 1.8±0.2 | 0.82 |
| Hypertension, n (%) | 10 (18) | 8 (16) | 0.67 |
| Medication | |||
| Beta blocker, n (%) | 22 (40) | 0 (0) | <0.001 |
| Calcium blocker, n (%) | 5 (9) | 0 (0) | 0.03 |
| Nitroglycerin, n (%) | 11 (20) | 4 (8) | 0.003 |
| At rest | |||
| Hemodynamics | |||
| Heart rate, bpm | 67.0±10.4 | 71.2±11.6 | 0.05 |
| Systolic BP, mm Hg | 120.3±14.2 | 114.3±10.2 | 0.05 |
| Diastolic BP, mm Hg | 72.6±8.9 | 69.7±9.9 | 0.12 |
| Double product | 7675±2476 | 8130±1480 | 0.56 |
| Echocardiography | |||
| IVSd, mm | 8.8±1.8 | 9.1±1.3 | 0.34 |
| PWd, mm | 9.0±1.2 | 9.1±1.2 | 0.34 |
| LV dimension, cm | 4.4±0.3 | 4.3±0.5 | 0.06 |
| SVI, mL/m2 | 31.9±6.1 | 29.0±8.8 | 0.09 |
| LVEF, % | 62±4.8 | 63±3.6 | 0.21 |
| LA dimension, cm | 3.3±0.6 | 3.3±0.5 | 0.88 |
| At peak stress | |||
| Hemodynamics | |||
| Heart rate, bpm | 167.6±18.9 | 167.4±18.5 | 0.95 |
| Systolic BP, mm Hg | 164.1±21.5 | 156.3±14.2 | 0.13 |
| Diastolic BP, mm Hg | 77.8±13.4 | 72.7±13.5 | 0.60 |
| Double product | 27 051±6136 | 26 145±3640 | 0.19 |
| Echocardiography | |||
| SVI, mL/m2 | 27.7±6.2 | 22.1±5.7 | <0.001 |
| LVEF, % | 70±6.2 | 74±4.5 | 0.001 |
| Chest pain, n (%) | 33 (60) | 0 (0) | <0.001 |
| ST‐segment change in ECG, n (%) | 13 (24) | 0 (0) | <0.001 |
BP indicates blood pressure; bpm, beats per minutes; BSA, body surface area; IVSd, diastolic interventricular septum; LA, left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; MB, myocardial bridge; PWd, diastolic posterior wall; SVI, stroke volume index.
Coronary angiography found that no patient with an MB had significant epicardial coronary disease (≥50% stenosis). By IVUS, the mean length of an MB was 26.8±13.9 mm. Men had longer MBs than women (32.6±13.9 versus 23.1±12.8 mm, respectively; P=0.02). The dFFR was ≤0.76 in 55 patients either within or distal to the MB, whereas it was >0.76 in 3 patients at both sites. Because the number of patients with a dFFR >0.76 was extremely small, further analysis was performed only in patients with a dFFR ≤0.76. Assessment of hemodynamics both within and distal to an MB was possible in 40 patients; 13 patients had a dFFR ≤0.76 only within the MB, 4 had a dFFR ≤0.76 only distal to the MB, and 23 had a dFFR ≤0.76 both within and distal to the MB (Figure 3). Of the rest, 14 patients had a dFFR ≤0.76 within the MB, but no data were obtained distal to the MB because the length of the MB precluded safe placement of the ComboWire in the distal vessel. One patient had a dFFR ≤0.76 distal to the MB, but no data were obtained within the MB because the length of the MB was too short to accurately measure dFFR within it. MBs were significantly longer in patients whose distal dFFR was ≤0.76 compared with patients whose distal dFFR was >0.76 (25.6±13.0 versus 17.9±8.1 mm; P=0.03).
Figure 3.
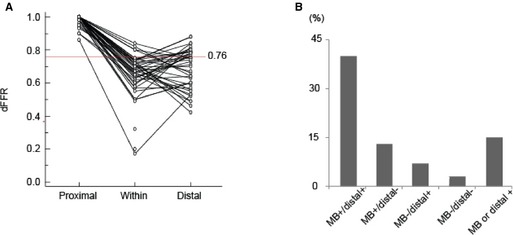
The dFFR values at stress. The values of dFFR proximal to the MB are ≈1. Most patients with an MB had a dFFR ≤0.76. Some patients with an MB showed full recovery of dFFR distal to the MB (A). B, The distribution of the patients according to the region of dFFR. – indicates dFFR >0.76; +, dFFR ≤0.76; dFFR, diastolic fractional flow reserve; MB, myocardial bridge.
In terms of stress echocardiographic assessment, all participants were analyzed in this study. At baseline, LV segmental wall motion was normal in all patients, and LV ejection fraction and stroke volume index at rest were not significantly different between the 2 groups. With exercise, abnormal septal motion was found in 50 patients with an MB, whereas no wall motion abnormality was found in controls. LV ejection fraction and stroke volume index were significantly lower in patients with an MB compared with controls. In the MB group, 33 patients (60%) exhibited chest pain, 13 (24%) presented with ST‐segment depression (defined as ST‐segment depression ≥1 mm) on ECG, and one had a 3‐beat run of ventricular tachycardia during exercise. In the control group, nobody presented with ST‐segment depression on ECG or chest pain (Table 1).
Regarding strain assessment, at baseline, there was no significant difference between the 2 groups for all strain values: Septal LS was 19.0±1.8% for patients with an MB versus 19.2±1.5% for controls (P=0.58); lateral LS was 20.1±2.0% versus 20.0±1.6% (P=0.53), respectively; and GLS was 19.5±1.8% versus 19.7±1.4% (P=0.68), respectively. With exercise, in the MB groups, septal LS did not change significantly (from 19.0±1.8% to 18.9±2.6%; P=0.83), whereas lateral LS increased significantly (from 20.1±2.0% to 22.8±2.9%; P<0.001). In contrast, in the control group, both septal and lateral LS increased significantly (from 19.2±1.5% to 21.7±1.6% and from 20.0±1.6% to 22.7±1.8%; P<0.001 for both) (Figure 4A and 4C). The absolute and relative changes in septal LS were significantly smaller in patients with an MB than in controls (−0.1±2.1% versus 2.5±1.3% for absolute change, and −0.6±11.2% versus 13.1±7.8% for relative change; P<0.001 for both). In contrast, the absolute and relative changes in lateral LS were similar between the 2 groups (P=0.97 and P=0.98, respectively) (Figure 4B and 4D).
Figure 4.
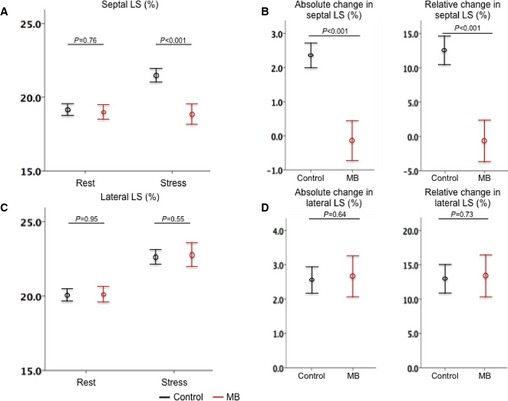
Strain changes at rest and with stress. In septal segments, there was a significant difference between the 2 groups in strain with stress (A) and in absolute and relative changes (B). In contrast, in lateral segments, there was no significant difference in strain with stress (C) and absolute and relative changes (D). The bar range represents the 95% CI of the mean. LS indicates longitudinal strain.
In patients with an MB, the absolute and relative changes in septal LS had a better positive correlation with the dFFR distal to the MB (r=0.52, P<0.001 for septal absolute change and r=0.52, P=0.001 for septal relative change) than the dFFR within the MB (Table 2). The absolute and relative changes in septal LS also had a moderate negative correlation with the length of the MB (r=−0.32, P=0.02 for absolute change and r=−0.32, P=0.02 for relative change). In multivariate analysis, a lower dFFR distal to the MB and male sex were independent correlates of lower absolute and relative changes in septal LS. When the dFFR within the MB was included instead of the dFFR distal to the MB, a lower dFFR within the MB and male sex were independent correlates of a lower absolute change in septal LS; however, male sex was the only independent correlate of a lower relative change in septal LS (Table 2). Although the length of the MB was not left in the multivariate analysis when included as a continuous variable, it was left as an independent correlate of absolute change in septal LS instead of male sex when it was categorized using the median value.
Table 2.
Correlation Between Absolute and Relative Changes in Septal LS and dFFR Values
| Absolute Change in Septal LS | Relative Change in Septal LS | |
|---|---|---|
| Univariate analysis | ||
| dFFR within MB | r=0.34, P=0.01 | r=0.33, P=0.02 |
| dFFR distal to MB | r=0.52, P=0.01 | r=0.52, P=0.001 |
| Length | r=−0.33, P=0.02 | r=−0.34, P=0.02 |
| Male sex | P=0.03 | P=0.03 |
| Multivariate analysis | ||
| Pattern 1 | R=0.60 | R=0.61 |
| dFFR distal to MB | β=0.51, P=0.001 | β=0.52, P=0.001 |
| Male sexa | β=−0.37, P=0.01 | β=−0.35, P=0.02 |
| Pattern 2 | R=0.456 | R=0.38 |
| dFFR within MB | β=0.27, P=0.047 | — |
| Male sexa | β=−0.34, P=0.01 | β=−0.38, P=0.01 |
Pattern 1 includes dFFR distal to the MB. Pattern 2 includes dFFR within the MB instead of distal to the MB. dFFR indicates diastolic fractional flow reserve; LS, longitudinal strain; MB, myocardial bridge.
MB length was left as an independent correlate of absolute change in septal LS instead of male sex when it was categorized using the median value.
In terms of the inter‐ and intraobserver variability of this study, the absolute differences between the 2 investigators were 1.9±1.4% for septal LS, 2.2±1.2% for lateral LS, and 1.9±1.0% for GLS at rest and 2.6±1.6% for septal LS, 2.8±1.9% for lateral LS, and 2.2±1.6% for GLS immediately after stress. The absolute differences within the observer were 1.6±1.1% for septal LS, 1.4±0.7% for lateral LS, and 1.1±0.8% for GLS at rest and 1.7±1.1% for septal LS, 1.5±1.1% for lateral LS, and 1.2±0.9% for GLS immediately after stress.
Discussion
The main finding of our study is that patients with hemodynamically significant MBs have no increase or have a blunted increase in septal LS with exercise compared with controls. Moreover, the lack of significant increase in septal LS appears to be associated with the length of the MB and the hemodynamic significance assessed by dFFR. These functional characteristics may be helpful in the assessment of patients referred for unexplained exertional chest pain.
Fractional flow reserve is the invasive reference standard to assess the hemodynamic significance of a fixed coronary artery stenosis. In a recent study, Johnson et al demonstrated that fractional flow reserve during adenosine infusion is an independent and strong correlate of outcome: Patients with lower fractional flow reserve have a higher likelihood of adverse events.20 Assessing the hemodynamic significance of an MB, however, may be more challenging. In patients with an MB, dFFR may be more reliable than conventional mean fractional flow reserve to assess hemodynamic significance because the increased systolic pressure during compression and the decreased diastolic pressure may offset each other (Figure 2).11 In addition, the use of dobutamine rather than adenosine has been shown to be important in evaluating an MB. Adenosine results in maximal hyperemia through vasodilation of microcirculation, which is effective for a fixed stenosis, but the chronotropic and inotropic effects of dobutamine are necessary to evaluate the hemodynamic significance of the dynamic stenosis caused by an MB.21 Although the cutoff value to determine hemodynamically significance has been argued, a dFFR ≤0.76 was considered to be hemodynamically significant in this study, as in the previous study by Escaned et al.11
From a pathophysiological perspective, Lin et al previously proposed a model of ischemia associated with an MB involving the Venturi effect.12 Lumen area within the MB decreases during end‐systole to early diastole (Figure 5A), and the compression resembles the narrowed section of a pipe. When fluid passes through this “pipe,” the fluid velocity must increase (Figure 5B) to satisfy the principle of conservation of energy by the continuity equation. At the same time, the pressure must decrease to satisfy the principle of the Bernoulli equation, thus the pressure is decreased in the tunneled section. With this model, pressure should recover distally, provided the diameter of the distal arterial segment is larger than in the tunneled segment.22
Figure 5.
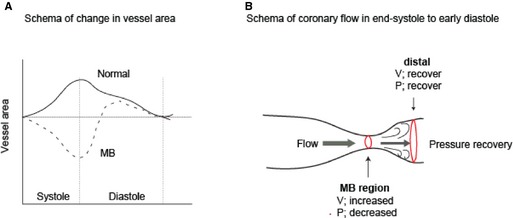
Proposed schematic of ischemia from MB. A, Normal vessels show the pattern in which an increase in area is seen throughout systole with the rise in aortic pressure (solid line). In contrast, MB segments undergo a marked decrease in area, particularly in the second half of systole. Importantly, this vessel compression persists into early diastole (dotted line).3 B, Venturi effect. When fluid is passing the narrow lesion, its velocity increases by the principle of energy conservation, and pressure decreases by the principle of the Bernoulli equation. Distally, the velocity decreases and the pressure increases to recover by the same principles. MB indicates myocardial bridge; P, pressure; V, velocity.
Hemodynamic significance of an MB has been assessed invasively, and few studies have evaluated noninvasively. The key findings of our study are that change in strain with stress may be useful in identifying the hemodynamic significance of an MB and that the more hemodynamically significant it is (the lower the dFFR), the more impaired is the change in septal LS with stress. In our study, change in septal LS showed a stronger correlation with the dFFR distal to the MB than with the dFFR within the MB. Most of our patients with a significant dFFR distally also had a significant dFFR within the bridge, and we suspect that MBs with a significant dFFR distally may be exposing an even more extensive area of myocardium to ischemia. The primary reason for this phenomenon may be a longer MB delaying the pressure recovery, as shown in the relationship between septal LS and MB length. Likewise, male sex was an independent correlate of a diminished increase in septal strain with stress. A possible explanation may be the interrelationship between male sex and the length of MB, as suggested in the multivariate analysis. Along with the possible reasons for preventing full recovery of the pressure distally may be a small caliber vessel beyond the bridge, a short segment of vessel beyond the bridge, inability of the ComboWire to be placed far enough distally to detect recovery, or the undetected presence of further MB distal to the investigated area.
Wang et al recently published a study demonstrating resting LS of the left anterior descending artery territory by 3‐dimensional echocardiography being decreased.14 In contrast, we did not find that LS at rest was useful in identifying hemodynamically significant MBs, but it may be because 3 dimensions are more able to detect subtle changes in strain than 2 dimensions. Our study also consisted of a younger population and exposed patients to a shorter duration of ischemia compared with the population in the study by Wang et al.
Clinically, our study may be of value in the assessment of patients with exertional chest pain of undetermined etiology. If an MB is detected by exercise echocardiography (by the presence of focal septal buckling with apical sparing) and/or confirmed by multisliced computed tomography or IVUS during coronary angiography, determination of impaired strain increase on exercise echocardiography may suggest that the MB is hemodynamically significant. Furthermore, if the distinctive septal wall motion pattern on exercise echocardiography is proven to be accurate enough for the diagnosis of an MB,12 change in strain may be useful in stratifying the patients with an MB according to hemodynamic significance, possibly offering a totally noninvasive diagnosis of MB by exercise echocardiography. Another consideration to enhance image quality and feasibility of speckle tracking is to perform dobutamine stress echocardiography, although further validation study is needed.
Limitations
This study has several limitations. First, the number of patients enrolled was small; however, the study is the largest of patients with an MB who underwent both invasive physiological evaluation and exercise echocardiography. Second, the strain values were obtained by manual tracings because of the difficulty in obtaining reliable speckle tracking images during exercise; however, we showed good inter‐ and intraobserver variability, even for stress‐based images. Third, because we cannot yet provide long‐term follow‐up data for our cohort, we cannot determine how change in septal strain is useful in predicting cardiovascular outcomes in patients with an MB. Fourth, all patients with an MB evaluated in this study had a dFFR ≤0.76. Further study is needed to assess the change in septal LS during stress in patients with an MB who have a dFFR >0.76. Moreover, a dFFR threshold of 0.76 was used in this study, as in previous studies11; however, the cutoff value has not been well discussed, and whether or not a dFFR ≤0.76 truly represents hemodynamic significance is unknown. In addition, we still cannot say with certainty whether the strain pattern is due to ischemia or to an anatomic variation from having a tethered portion of left anterior descending artery. More studies are needed to establish the appropriate value.
Conclusions
Patients with a hemodynamically significant MB have a reduced change in septal strain on exercise echocardiography. The degree of reduction is associated with the severity of hemodynamic significance, as determined by invasive dFFR. Assessment of change in septal strain may be clinically useful in the noninvasive evaluation of patients with chest pain.
Disclosures
Tremmel received honoraria from Boston Scientific, Medtronic, Terumo, and Recor. Amsallem received a research fellowship from Federation Francaise de Cardiologie.
(J Am Heart Assoc. 2015;4:e002496 doi: 10.1161/JAHA.115.002496)
References
- 1. Angelini P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–2454. [DOI] [PubMed] [Google Scholar]
- 2. Kneale BJ, Stewart AJ, Coltart DJ. A case of myocardial bridging: evaluation using intracoronary ultrasound, Doppler flow measurement, and quantitative coronary angiography. Heart. 1996;76:374–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Gorge G, Haude M, Meyer J. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994;89:1725–1732. [DOI] [PubMed] [Google Scholar]
- 4. Bauters C, Chmait A, Tricot O, Lamblin N, Van Belle E, Lablanche JM. Images in cardiovascular medicine. Coronary thrombosis and myocardial bridging. Circulation. 2002;105:130. [DOI] [PubMed] [Google Scholar]
- 5. Berry JF, von Mering GO, Schmalfuss C, Hill JA, Kerensky RA. Systolic compression of the left anterior descending coronary artery: a case series, review of the literature, and therapeutic options including stenting. Catheter Cardiovasc Interv. 2002;56:58–63. [DOI] [PubMed] [Google Scholar]
- 6. Feld H, Guadanino V, Hollander G, Greengart A, Lichstein E, Shani J. Exercise‐induced ventricular tachycardia in association with a myocardial bridge. Chest. 1991;99:1295–1296. [DOI] [PubMed] [Google Scholar]
- 7. Pittaluga J, de Marchena E, Posada JD, Romanelli R, Morales A. Left anterior descending coronary artery bridge. A cause of early death after cardiac transplantation. Chest. 1997;111:511–513. [DOI] [PubMed] [Google Scholar]
- 8. Tio RA, Van Gelder IC, Boonstra PW, Crijns HJ. Myocardial bridging in a survivor of sudden cardiac near‐death: role of intracoronary Doppler flow measurements and angiography during dobutamine stress in the clinical evaluation. Heart. 1997;77:280–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ge J, Jeremias A, Rupp A, Abels M, Baumgart D, Liu F, Haude M, Gorge G, von Birgelen C, Sack S, Erbel R. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–1716. [DOI] [PubMed] [Google Scholar]
- 10. Kim PJ, Hur G, Kim SY, Namgung J, Hong SW, Kim YH, Lee WR. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: a comparison between computed tomography and invasive coronary angiography. Circulation. 2009;119:1408–1416. [DOI] [PubMed] [Google Scholar]
- 11. Escaned J, Cortes J, Flores A, Goicolea J, Alfonso F, Hernandez R, Fernandez‐Ortiz A, Sabate M, Banuelos C, Macaya C. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42:226–233. [DOI] [PubMed] [Google Scholar]
- 12. Lin S, Tremmel JA, Yamada R, Rogers IS, Yong CM, Turcott R, McConnell MV, Dash R, Schnittger I. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:e000097 doi: 10.1161/JAHA.113.000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jhi JH, Cho KI, Ha JK, Jung CW, Kim BJ, Park SO, Jo AR, Kim SM, Lee HG, Kim TI. Alteration of left ventricular function with dobutamine challenge in patients with myocardial bridge. Korean J Intern Med. 2011;26:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang D, Sun JP, Lee AP, Ma GS, Yang XS, Yu CM, Ding JD, Liu NF. Evaluation of left ventricular function by three‐dimensional speckle‐tracking echocardiography in patients with myocardial bridging of the left anterior descending coronary artery. J Am Soc Echocardiogr. 2015;28:674–682. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 16. Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of E . American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. [DOI] [PubMed] [Google Scholar]
- 17. Bruce RA, Lovejoy FW Jr, Pearson R, Yu PNG, Brothers GB, Velasquez T. Normal respiratory and circulatory pathways of adaptation in exercise. J Clin Invest. 1949;28:1423–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rogers WJ Jr, Shapiro EP, Weiss JL, Buchalter MB, Rademakers FE, Weisfeldt ML, Zerhouni EA. Quantification of and correction for left ventricular systolic long‐axis shortening by magnetic resonance tissue tagging and slice isolation. Circulation. 1991;84:721–731. [DOI] [PubMed] [Google Scholar]
- 19. Dumesnil JG, Shoucri RM, Laurenceau JL, Turcot J. A mathematical model of the dynamic geometry of the intact left ventricle and its application to clinical data. Circulation. 1979;59:1024–1034. [DOI] [PubMed] [Google Scholar]
- 20. Johnson NP, Toth GG, Lai D, Zhu H, Acar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, Dominguez‐Franco AJ, Dupouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jimenez‐Navarro MF, Katritsis DG, Kocaman SA, Koo BK, Lopez‐Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodes‐Cabau J, Sedlis SP, Takeishi Y, Tonino PA, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne B, Gould KL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–1654. [DOI] [PubMed] [Google Scholar]
- 21. Bartunek J, Wijns W, Heyndrickx GR, de Bruyne B. Effects of dobutamine on coronary stenosis physiology and morphology: comparison with intracoronary adenosine. Circulation. 1999;100:243–249. [DOI] [PubMed] [Google Scholar]
- 22. Klues HG, Schwarz ER, vom Dahl J, Reffelmann T, Reul H, Potthast K, Schmitz C, Minartz J, Krebs W, Hanrath P. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997;96:2905–2913. [DOI] [PubMed] [Google Scholar]


