Abstract
Background
Endothelial dysfunction plays a pivotal role in cardiovascular disease progression, and is associated with adverse events. The purpose of this systematic review and meta‐analysis was to investigate the prognostic magnitude of noninvasive peripheral endothelial function tests, brachial artery flow‐mediated dilation (FMD), and reactive hyperemia–‐peripheral arterial tonometry (RH‐PAT) for future cardiovascular events.
Methods and Results
Databases of MEDLINE, EMBASE, and the Cochrane Library were systematically searched. Clinical studies reporting the predictive value of FMD or RH‐PAT for cardiovascular events were identified. Two authors selected studies and extracted data independently. Pooled effects were calculated as risk ratio (RR) for continuous value of FMD and natural logarithm of RH‐PAT index (Ln_RHI) using random‐effects models. Thirty‐five FMD studies of 17 280 participants and 6 RH‐PAT studies of 1602 participants were included in the meta‐analysis. Both endothelial function tests significantly predicted cardiovascular events (adjusted relative risk [95% CI]: 1% increase in FMD 0.88 [0.84–0.91], P<0.001, 0.1 increase in Ln_RHI 0.79 [0.71–0.87], P<0.001). There was significant heterogeneity in the magnitude of the association across studies. The magnitude of the prognostic value in cardiovascular disease subjects was comparable between these 2 methods; a 1 SD worsening in endothelial function was associated with doubled cardiovascular risk.
Conclusions
Noninvasive peripheral endothelial function tests, FMD and RH‐PAT, significantly predicted cardiovascular events, with similar prognostic magnitude. Further research is required to determine whether the prognostic values of these 2 methods are independent of each other and whether an endothelial function–guided strategy can provide benefit in improving cardiovascular outcomes.
Keywords: cardiovascular diseases, endothelium, meta‐analysis, prognosis
Introduction
The vascular endothelium is a delicate monolayer of cells lining all blood vessels, which plays important structural and functional roles in initiation and development of cardiovascular diseases (CVD). A properly functioning endothelium is key for cardiovascular health, whereas endothelial dysfunction is associated with numerous disease states. Importantly, endothelial dysfunction is not only a marker but also a contributor to atherosclerotic diseases. Specifically, endothelial dysfunction has been reported to be associated with coronary plaque progression, anatomical complexity, and vulnerability.1 Furthermore, endothelial function has a substantial role in developing thrombotic complications.1 Thus, a strategy based on endothelial function assessment might provide a more tailored approach to prevent cardiovascular events. A number of methods to assess endothelial function have been investigated. Initially, endothelial function was assessed in coronary arteries using an invasive method during cardiac catheterization. More recently, several noninvasive methods for assessment of endothelial function have been developed. Studies of brachial artery flow‐mediated dilation (FMD) have been reported since 1992,2 and it is the most widely used method in clinical research. Reactive hyperemia–peripheral arterial tonometry (RH‐PAT) is a newly developed method. In 2002, RH‐PAT was reported to be the test for peripheral vascular endothelial function,3 and its use has been rapidly increasing. The RH‐PAT technique is less operator dependent and uses a contralateral arm as its internal control to correct for systemic changes during testing. Both methods are based on the same principle of reactive hyperemia phenomenon: that is, increased blood flow following a period of transient arterial occlusion, which serves as an index of endothelium‐dependent vasodilator function. FMD assesses the endothelial response to shear stress in the brachial artery as a result of hyperemia, whereas RH‐PAT measures the actual hyperemia. However, these methods differ in target vasculature: the brachial artery diameter in FMD versus a finger arterial pulse wave in RH‐PAT. The Framingham Heart Study reported no statistically significant relationship between signals obtained with RH‐PAT and FMD, suggesting that these reflect distinct aspects of vascular function.4 Although both tests have been reported to predict cardiovascular events,5, 6, 7 their relative value for predicting cardiovascular risk has not been directly compared, to date. Previously, 2 meta‐analyses on the prognostic value of FMD have been reported,5, 6 and Xu et al7 reported another meta‐analysis of the prognostic value of both FMD and RH‐PAT. However, only 3 RH‐PAT studies were included in their meta‐analysis, and 2 methods were not directly compared. Since then, several additional prospective studies have been published focusing on the prognostic value of these tests.
Therefore, in this systematic review with meta‐analysis, we aimed to update the evidence of FMD and RH‐PAT as predictors of cardiovascular events, and compare the prognostic magnitude on cardiovascular risk between these 2 methods.
Methods
This systematic review and meta‐analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement, and in accordance with the Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) guidelines.
Data Sources and Search Strategies
A comprehensive search of several databases from each database's earliest inception to September 24, 2014 was conducted and updated on December 4. The databases included Ovid Medline In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, and Ovid Cochrane Database of Systematic Reviews. The strategy to search potentially relevant prospective observational studies investigating FMD or Reactive hyperemia index (RHI) as assessed by RH‐PAT and cardiovascular event risk was designed and conducted by an experienced librarian with input from the study's principal investigator. Controlled vocabulary supplemented with keywords was used to search for studies of endothelial function tests for cardiovascular events. The actual strategy is available in Data S1. We also manually searched PubMed, Ovid Medline, and references in pertinent articles that were identified during the screening.
Study Selection
Two investigators (Y.M. and T.G.K.) independently reviewed all records identified by these search methods. The selection was performed in 2 steps; the first step was abstract review and the second step was full text review. Studies with discrepant decisions in screening of the abstract proceeded to full text review, and discrepancies in full text review were resolved through consensus. Studies were eligible for inclusion in this systematic review if they met the following criteria: (1) study provided original data, (2) prospective observational study with follow‐up time ≥6 months, (3) study reported risk estimates of endothelial function as assessed by brachial FMD or RH‐PAT for cardiovascular events or mortality, (4) study of human adults, and (5) study published in English.
Data Extraction
Data from included studies were extracted independently by 2 investigators (Y.M. and T.G.K.) using predetermined forms. Discrepancies found in the verification process were resolved by discussion with a third investigator (A.L.). The following data were extracted (where available): first author, year of publication, years of enrollment of the cohort, sex composition, average age, sample size, duration of follow‐up, characteristics of the population, method of endothelial function assessment, outcome characteristics (number of cardiovascular events and type of events [eg, cardiovascular death, myocardial infarction, and stroke]), unadjusted and adjusted hazard ratios (HRs) for continuous value of FMD or logarithmic value of RHI (Ln_RHI), and variables adjusted for. We adopted Ln_RHI rather than RHI because of its skewed distribution. When data were missing or results for continuous value of FMD or Ln_RHI were not reported, the original authors were contacted in an attempt to obtain these data. One study reported risk estimates for RHI.8 We contacted and asked the authors to transform RHI results to logarithmic value and obtained results with Ln_RHI. The studies were classified according to CVD or non‐CVD population. CVD population included patients with coronary artery disease, chest pain, heart failure, stroke, and peripheral arterial disease. Non‐CVD subjects included those without established CVD (general population, healthy subjects, elderly, postmenopausal women, and patients with coronary risk factors).
Risk Bias Assessment
We followed the recommendations for bias assessment of nonrandomized studies, as suggested by the Cochrane collaboration,9 and information on the methodological quality of each included study was recorded and quality assessment was performed using the Newcastle‐Ottawa Scale (NOS)10 by 2 independent investigators (Y.M. and T.G.K.). Disagreement was resolved by discussion with a third investigator (A.L.). The score assessed major classifications: selection (4 items), comparability (1 item), and outcome (3 items) (Figure 1). A maximum score of 1 was graded for each item, except that related to comparability, which allowed for 2. Total scores were calculated by adding each score for each item. For quality, total scores ranged from 0 (lowest) to 9 (highest), and studies with ≥7 points were considered as good quality. The presence of CVD was defined as the most important covariate that would define comparability. Studies that controlled for the presence of CVD received 1 score, whereas studies that controlled for another important confounder (age, sex, hypertension, diabetes, or dyslipidemia) received an additional score. Since the risk of patients with established coronary heart disease are at 4‐ to 6‐folds higher than those without CVD,11 we defined sufficient follow‐up duration separately. Studies of patients without CVD at enrollment with median follow‐up time >5 years were assigned a score of 1, whereas studies of patients with CVD at enrollment with follow‐up time >1 year were assigned a score of 1. Studies with a follow‐up rate >80% were assigned a score of 1.
Figure 1.
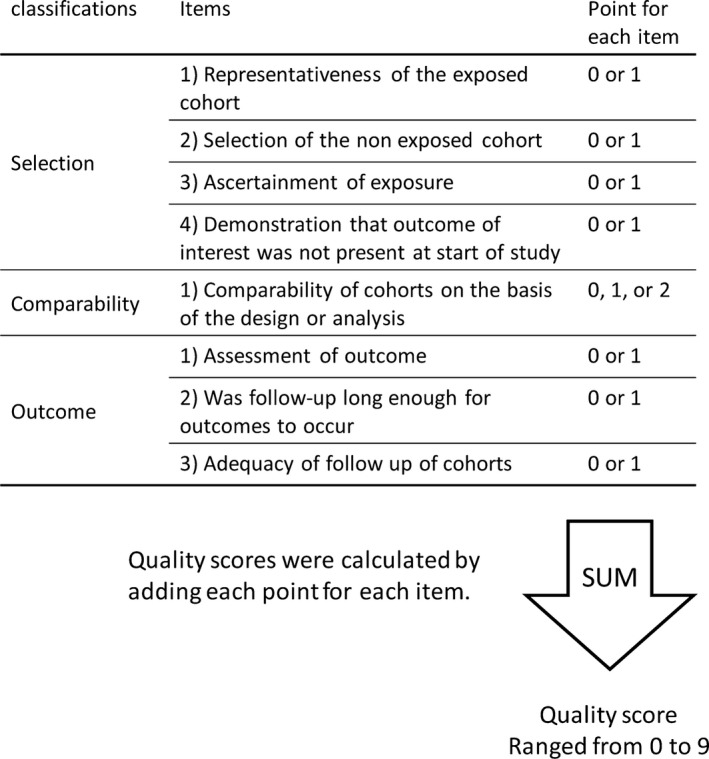
Scheme of risk bias assessment.
Statistical Methods
The risk estimates of each study were reported as HR or risk ratio (RR). We considered HRs as estimates of RRs. If, in addition to original HRs, the studies reported separate HRs for sex, population health status (CVD and non‐CVD), or outcome (overall cardiovascular events and hard cardiovascular events, which consist of cardiovascular death, myocardial infarction, and stroke), these separate HRs were also pooled for subsequent subgroup analyses. If the original author only provided results for categorical values of FMD or Ln_RHI, we converted it into 1 continuous RR using Greenland and Longnecker's covariance‐corrected generalized least‐square trend estimation method.12 In this meta‐analysis, RR represents the increase in risk per 1% increase in brachial FMD or per 0.1 increase in Ln_RHI. Standard errors, which were calculated from CIs, were used for weighing the studies. A random‐effects model was used for calculating the pooled overall risk estimate. The heterogeneity among studies was evaluated by the Cochran's Q‐statistic and the I2‐statistic. Values of 25%, 50%, and 75% value for I2‐statistic represented low, moderate, and high heterogeneity, respectively.13 To assess the robustness of our meta‐analysis, we examined the following study characteristics in subgroup analyses: study population (CVD population versus non‐CVD population, age, and sex), sample size, duration of follow‐up, annual event rate, FMD technique (forearm versus upper arm occlusion), study quality, and study outcome (cardiovascular mortality and hard cardiovascular events). Owing to the limited number of RH‐PAT studies, no subgroup analyses were performed. In order to assess the impact on cardiovascular outcomes between FMD and RH‐PAT, a pooled SD for each of FMD and Ln_RHI in all included studies of CVD population was calculated using following equation, and RRs for the pooled SD increase in FMD and Ln_RHI were compared.
κ, number of groups; ni, number of patients in each group; nt, total number of patients; Ui, unbiased estimate of population variance; , mean value of each group; and , pooled mean value.
Finally, publication bias was evaluated by examining the asymmetry of funnel plot. All P values are 2 tailed and P<0.05 was considered statistically significant. All analyses were performed using the Review Manager, version 5.3.5 (Cochrane Collaboration, Oxford, UK) and R software, version 3.2.0.
Results
Study Retrieval
The process of study selection is shown in Figure 2. According to our systematic search strategy, 2197 titles were identified from electronic databases, and 3 studies were retrieved via hand searching. After screening the title and abstract, 98 studies were eligible for full text review; of these, 57 were excluded, resulting in 35 FMD studies14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 and 6 RH‐PAT studies8, 49, 50, 51, 52, 53 being eligible for this meta‐analysis. Overall, these studies comprised data from a total of 17 280 participants with FMD, and 1602 with RH‐PAT.
Figure 2.
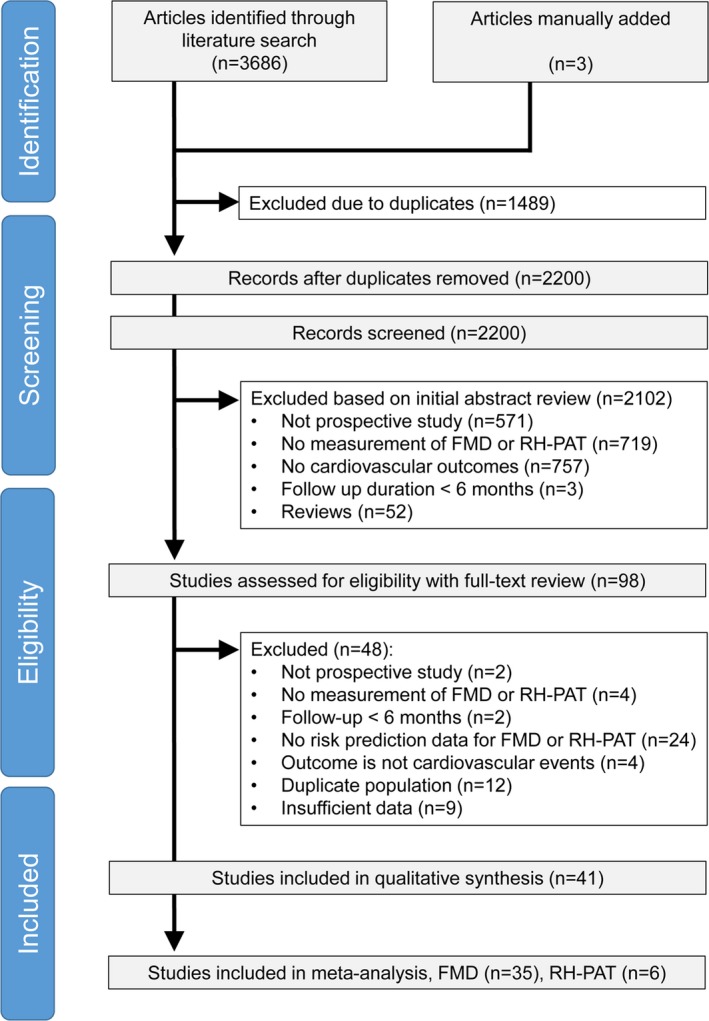
Flow chart of the study selection procedure. FMD indicates flow‐mediated dilation; RH‐PAT, reactive hyperemia–peripheral arterial tonometry.
Characteristics and Quality Assessment of the Included Studies
The characteristics of included FMD studies and RH‐PAT studies are shown in Tables 1 through 3, and abstracted in Table 4. A total of 16 studies took place in East Asia (China, South Korea, and Japan), 13 studies in Europe (Austria, Denmark, Greece, Italy, Serbia, Spain, and Sweden), and 8 in North America (Canada and United States). Among 35 FMD studies, 13 were derived from a non‐CVD population,14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 and 22 from a CVD population27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 (2 studies14, 29 reported results of non‐CVD and CVD samples separately). Contrarily, all RH‐PAT studies derived from a CVD population.8, 49, 50, 51, 52, 53 The years of publication ranged from 2007 to 2014, sample size from 60 to 3025, mean age from 46 to 79, and mean follow‐up duration from 6 to 115 months. FMD studies of CVD subjects had smaller sample sizes, higher male prevalence, shorter follow‐up duration, and higher annual event rate, compared with FMD studies from non‐CVD populations. In comparison with FMD studies, the number of RH‐PAT studies has been increasing recently. Although the overall quality of studies was good, 6 FMD studies16, 18, 23, 26, 45, 47 received a low quality score (≤6). Clinical heterogeneity, in particular differences in end points, had to be taken into consideration.
Table 1.
Characteristics of FMD Studies of Non‐CVD Subjects
| Study | Description of Study Subjects | Agea, y | Male | Follow‐upa | No. Event | No. Population | Annual Event Rate | End Point |
|---|---|---|---|---|---|---|---|---|
| Yeboah, 200714 | Elderly | 79 | 42% | 60 mo | 674 | 2791 | 4.8% | CV death, MI, coronary revascularization, stroke, CHF, PAD |
| Muiesan, 200815 | Hypertension | 56 | 59% | 95 mo | 32 | 172 | 2.4% | Sudden death, MI, UA, angina, coronary revascularization, arrhythmia, stroke, TIA, CHF, PAD |
| Rossi, 200816 | Postmenopausal women | 54 | 0% | 45 mo | 90 | 2264 | 1.1% | Cardiac death, MI, coronary revascularization, stroke, TIA |
| Suzuki, 200817 | General population | 67 | 43% | 81 mo | 84 | 819 | 1.5% | Vascular death, MI, stroke |
| Morimoto, 200918 | CKD with hemodialysis | 61 | 56% | 43 mo | 14 | 199 | 2.0% | CV death |
| Yeboah, 200919 | General population | 61 | 49% | 60 mo | 182 | 3025 | 1.2% | CV death, resuscitated cardiac arrest, MI, UA, angina, coronary revascularization, stroke |
| Akishita, 201020 | Men with CV risk factors | 48 | 100% | 77 mo | 20 | 171 | 1.8% | Cardiac death, CAD, stroke, PAD |
| Anderson, 201121 | Male firefighter | 49 | 100% | 86 mo | 71 | 1574 | 0.6% | CV death, resuscitated cardiac arrest, MI, coronary/carotid/peripheral artery revascularization, vascular disease, stroke, TIA |
| Lind, 201122 | General population of 70 y of age | 70 | 47% | 62 mo | 101 | 921 | 2.1% | All‐cause death, MI, stroke |
| Yilmaz, 201123 | CKD without dialysis | 46 | 52% | 41 mo | 89 | 304 | 8.6% | CV death, MI, stroke, PAD |
| Nagai, 201324 | Elderly | 71 | 42% | 41 mo | 42 | 274 | 4.5% | MI, UA, angina, stroke, TIA, CHF, renal failure, aortic disease, PAD |
| Shechter, 201425 | Healthy subjects | 54 | 63% | 55 mo | 48 | 618 | 1.7% | All‐cause death, MI, angina, coronary revascularization, stroke, CHF |
| Lee, 201426 | CKD with peritoneal dialysis | 50 | 48% | 42 mo | 25 | 143 | 4.9% | Fatal and nonfatal ACS, angina requiring coronary revascularization, stroke, TIA, CHF |
ACS indicates acute coronary syndrome; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; FMD, flow‐mediated dilation; MI, myocardial infarction; PAD, peripheral arterial disease; TIA, transient cerebral ischemic attack; UA, unstable angina pectoris.
Either mean or median as reported.
Table 3.
Characteristics of RH‐PAT Studies
| Study | Description of Study Subjects | Agea, y | Male | Follow‐upa | No. Events | No. Population | Annual Event Rate | End Point |
|---|---|---|---|---|---|---|---|---|
| Rubinshtein, 201049 | Chest pain | 54 | 52% | 70 mo | 86 | 270 | 5.5% | CV death, MI, coronary revascularization, hospitalization for any cardiac cause |
| Akiyama, 201250 | HFPEF | 72 | 50% | 20 mo | 59 | 321 | 11.0% | CV death, MI, UA, coronary revascularization, stroke, CHF |
| Matsue, 201351 | HFPEF | 75 | 44% | 10 mo | 32 | 159 | 24.2% | Heart failure–related death, CHF |
| Matsuzawa, 201352 | Chest pain | 67 | 69% | 34 mo | 105 | 528 | 7.0% | CV death, MI, UA, coronary revascularization, stroke, HF, aortic disease, PAD |
| Ikonomidis, 20148 | CAD | 60 | 86% | 34 mo | 12 | 111 | 3.8% | All‐cause death, MI |
| Matsue, 201453 | CAD | 67 | 74% | 31 mo | 22 | 213 | 4.0% | Death due to CAD, MI, angina |
CAD indicates coronary artery disease; CHF, congestive heart failure; CV, cardiovascular; HFPEF, heart failure with preserved ejection fraction; MI, myocardial infarction; PAD, peripheral arterial disease; RH‐PAT, reactive hyperemia–peripheral arterial tonometry; UA, unstable angina pectoris.
Either mean or median as reported.
Table 4.
Summary of Study Characteristics
| FMD Studies of Non‐CVD Subjects N=13 | FMD Studies of CVD Subjects N=22 | RH‐PAT Studies N=6 | |
|---|---|---|---|
| Year of publication | |||
| Median | 2010 | 2010 | 2013a |
| IQR | 2008–2012 | 2007–2013 | 2012–2014 |
| Range | 2007–2014 | 2000–2014 | 2010–2014 |
| Sample size | |||
| Median | 618 | 124b | 242 |
| IQR | 186–1919 | 97–251 | 147–373 |
| Range | 143–3025 | 60–547 | 111–528 |
| Mean age, y | |||
| Median | 56 | 63 | 67 |
| IQR | 50–69 | 59–66 | 59–73 |
| Range | 46–79 | 51–73 | 54–75 |
| Male prevalence, % | |||
| Median | 50 | 72b | 60 |
| IQR | 42–61 | 61–84 | 48–77 |
| Range | 0–100 | 0–100 | 44–86 |
| Mean follow‐up duration, mo | |||
| Median | 60 | 29b | 33 |
| IQR | 43–79 | 16–50 | 18–43 |
| Range | 41–95 | 6–115 | 10–70 |
| Annual event rate, % | |||
| Median | 2.0 | 8.1b | 6.3 |
| IQR | 1.4–4.7 | 4.9–16.4 | 4.0–14.3 |
| Range | 0.6–8.6 | 2.3–45.0 | 3.8–24.2 |
| Quality score | |||
| Median | 7 | 8 | 8 |
| IQR | 6–8 | 7–9 | 7–9 |
| Range | 4–9 | 5–9 | 7–9 |
| Low quality score (≤6) | |||
| N (%) | 4 (31) | 2 (9) | 0 (0) |
CVD indicates cardiovascular disease; FMD, flow‐mediated dilation; IQR, interquartile range; RH‐PAT, reactive hyperemia–peripheral arterial tonometry.
P<0.05 compared with FMD studies of CVD subjects by Wilcoxon test.
P<0.05 compared with FMD studies of non‐CVD subjects by Wilcoxon test.
Table 2.
Characteristics of FMD Studies of CVD Subjects
| Study | Description of Study Subjects | Agea, y | Male | Follow‐upa | No. Events | No. Population | Annual Event Rate | End Point |
|---|---|---|---|---|---|---|---|---|
| Neunteufl, 200027 | Chest pain | 51 | 52% | 60 mo | 27 | 73 | 7.4% | All‐cause death, MI, coronary revascularization |
| Brevetti, 200328 | PAD | 64 | 90% | 23 mo | 39 | 131 | 15.5% | CV death, MI, UA, coronary revascularization, stroke, TIA, PAD |
| Fathi, 200429 |
CAD CKD with dialysis CV risk factors |
58 | 60% | 24 mo | 70 | 444 | 7.9% | All‐cause death, MI, UA, coronary revascularization, stroke |
| Katz, 200530 | Chronic HF with NYHA class II‐III | 54 | 84% | 28 mo | 17 | 149 | 4.9% | All‐cause death, heart transplantation |
| Karatzis, 200631 | NSTE‐ACS | 63 | 100% | 25 mo | 20 | 98 | 9.9% | CV death, ACS, stroke |
| Huang, 200732 | PAD | 66 | 74% | 10 mo | 50 | 267 | 22.5% | CV death, MI, UA, stroke, CHF |
| Hu, 200833 | Chest pain | 62 | 58% | 16 mo | 36 | 279 | 9.7% | CV death, MI, UA, stroke, CHF |
| Takase, 200834 | Chest pain | 62 | 77% | 50 mo | 15 | 103 | 3.5% | Cardiac death, MI, UA, CHF |
| Shechter, 200935 | Chronic HF with NYHA class IV | 64 | 92% | 14 mo | 30 | 82 | 31.4% | All‐cause death, MI, CHF |
| Ulriksen, 200936 | Chest pain | 54 | 76% | 50 mo | 90 | 223 | 9.7% | CV death, MI, UA, coronary revascularization |
| Wang, 200937 | STEMI | 62 | 66% | 12 mo | 29 | 101 | 28.5% | Cardiac death, MI, UA, coronary revascularization, stroke, CHF |
| Akamatsu, 201038 |
PAD, aortic aneurysm |
71 | 93% | 47 mo | 18 | 93 | 4.9% | CV death, MI, UA, coronary revascularization, stroke, aortic disease, PAD |
| Santos‐García, 201139 | Stroke | 73 | 58% | 48 mo | 32 | 120 | 6.7% | CV death, MI, coronary revascularization, stroke, PAD |
| Chan, 201240 | Stroke | 67 | 69% | 30 mo | 12 | 127 | 3.8% | CV death, ACS, coronary revascularization, stroke, CHF, PAD |
| Takishima, 201241 | Chronic HF | 66 | 68% | 33 mo | 33 | 245 | 4.9% | Cardiac death, CHF |
| Careri, 201342 | NSTE‐ACS | 62 | 73% | 32 mo | 14 | 60 | 8.8% | Cardiac death, ACS, angina |
| Nakamura, 201343 | CAD | 63 | 71% | 52 mo | 69 | 547 | 2.9% | Cardiac death, MI, UA, stroke |
| Savic‐Radojevic, 201344 | Chronic HF | 59 | 62% | 13 mo | 11 | 120 | 8.4% | All‐cause death |
| Sedlak, 201345 | Women with chest pain | 58 | 0% | 115 mo | 83 | 377 | 2.3% | All‐cause death, MI, stroke, CHF |
| Tarro Genta, 201346 | Chronic HF | 65 | 86% | 17 mo | 19 | 71 | 18.9% | Cardiac death, heart transplantation, LVAD implantation |
| Sawada, 201347 | CAD | 69 | 76% | 6 mo | 25 | 111 | 45.0% | All‐cause death, MI, target vessel revascularization |
| Hafner, 201448 | PAD | 67 | 67% | 50 mo | 49 | 184 | 6.4% | CV death |
ACS indicates acute coronary syndrome; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CV, cardiovascular; CVD, cardiovascular disease; FMD, flow‐mediated dilation; HF, heart failure; LVAD, left ventricular assist device; MI, myocardial infarction; NSTE‐ACS, non‐ST‐segment elevation acute coronary syndrome; NYHA, New York Heart Association; PAD, peripheral arterial disease; STEMI, ST‐segment elevation myocardial infarction; TIA, transient cerebral ischemic attack; UA, unstable angina pectoris.
Either mean or median as reported.
Pooled Overall Risk Estimate of FMD and RH‐PAT
Twenty‐six studies1 reported an unadjusted risk estimate of FMD, and 282 reported an adjusted value, whereas 5 RH‐PAT studies49, 50, 51, 52, 53 reported both, and one8 reported only an adjusted value. Both adjusted and unadjusted pooled RRs were significant for both FMD (per 1% increase: unadjusted RR 0.88, 95% CI 0.86–0.91, adjusted RR 0.88, 95% CI 0.84–0.91, Figures 3 and 4) and Ln_RHI (per 0.1 increase: unadjusted RR 0.76, 95% CI 0.65–0.88, adjusted RR 0.79, 95% CI 0.71–0.87, Figures 5 and 6). Except for the adjusted RR estimates for Ln_RHI, significant between‐study heterogeneity was observed.
Figure 3.
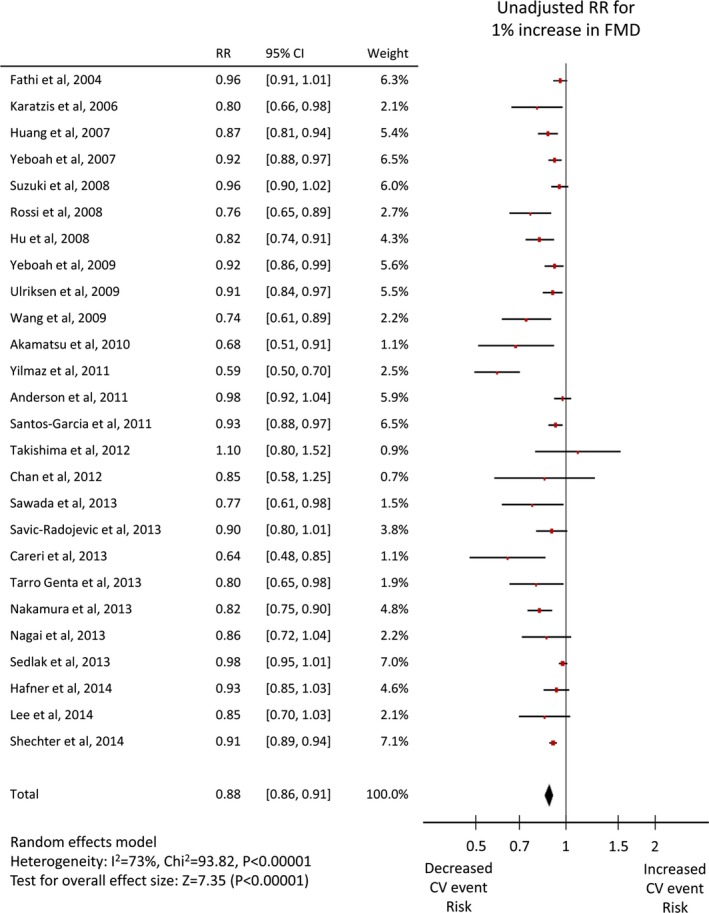
Forest plot of unadjusted risk ratio of FMD for cardiovascular events. CV indicates cardiovascular; FMD, flow‐mediated dilation; RR, risk ratio.
Figure 4.
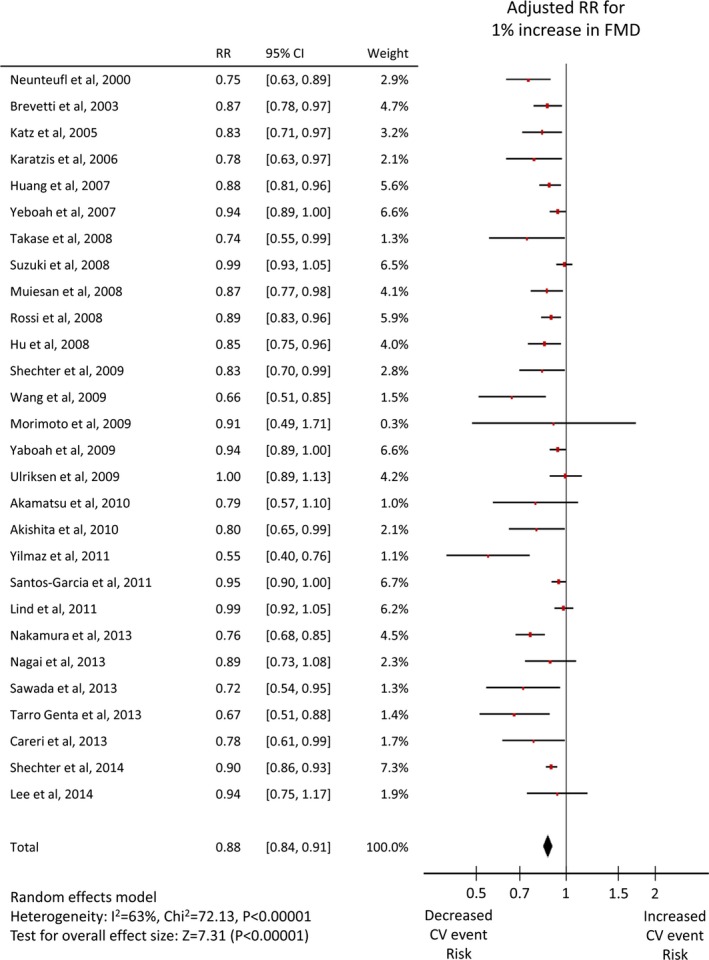
Forest plot of adjusted risk ratio of FMD for cardiovascular events. CV indicates cardiovascular; FMD, flow‐mediated dilation; RR, risk ratio.
Figure 5.
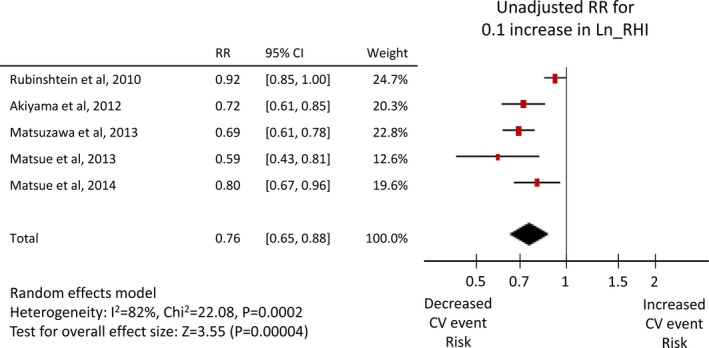
Forest plot of unadjusted risk ratio of Ln_RHI for cardiovascular events. CV indicates cardiovascular; Ln_RHI, logarithmic value of reactive hyperemia index; RR, risk ratio.
Figure 6.
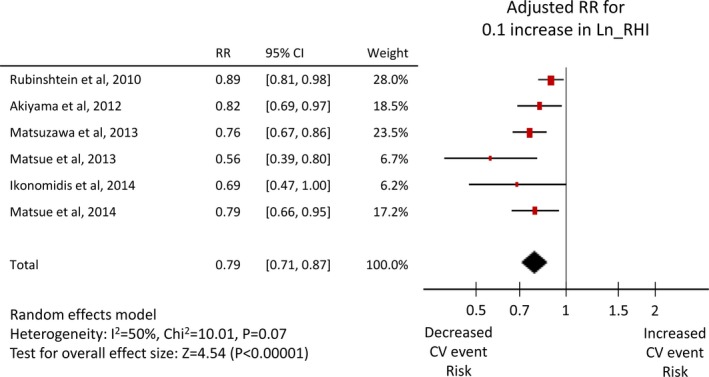
Forest plot of adjusted risk ratio of Ln_RHI for cardiovascular events. CV indicates cardiovascular; Ln_RHI, logarithmic value of reactive hyperemia index; RR, risk ratio.
Subgroup Analysis of FMD Studies
Subgroup analyses were performed only in FMD studies, but not in RH‐PAT studies due to the small number of studies (Table 5). Sensitivity analyses were restricted to the studies in which an end point included cardiovascular death, and the studies with hard cardiovascular events as end point showed similar results when compared with the full analyses. The prognostic value of FMD was consistently significant in each subgroup. However, there were significant between‐subgroup heterogeneities regarding baseline CVD status, sex, follow‐up duration, annual event rate, sample size, and study quality. In the CVD population, the prognostic value of FMD for cardiovascular events was higher when compared to the non‐CVD population (RR [95% CI] 0.84 [0.79–0.88] versus 0.92 [0.89–0.96], P=0.005). Additionally, in studies with male prevalence ≥half (versus <half), follow‐up duration <median (versus ≥median), annual event rate ≥median (versus <median), sample size <median (versus ≥median), and quality score <median (versus ≥median), the association between FMD value and cardiovascular outcomes was stronger.
Table 5.
Subgroup Analysis of FMD Studies
| Subgroup | Unadjusted RR | Adjusted RR | ||||
|---|---|---|---|---|---|---|
| No. Studies | Pooled RR (95% CI) | P Value Between Subgroups | No. Studies | Pooled RR (95% CI) | P Value Between Subgroups | |
| All studies | 26 | 0.88 (0.86, 0.91) | 28 | 0.88 (0.84, 0.91) | ||
| Non‐CVD subjects | 10 | 0.89 (0.84, 0.94) | 0.86 | 12 | 0.92 (0.89, 0.96) | 0.005 |
| CVD subjects | 18 | 0.88 (0.85, 0.92) | 17 | 0.84 (0.79, 0.88) | ||
| End point includes CV death | 20 | 0.86 (0.83, 0.90) | 21 | 0.87 (0.83, 0.91) | ||
| End point includes CV death, MI, and stroke | 15 | 0.86 (0.81, 0.90) | 16 | 0.88 (0.84, 0.92) | ||
| Mean age ≤62 y, median | 13 | 0.88 (0.83, 0.92) | 0.53 | 15 | 0.86 (0.82, 0.91) | 0.43 |
| Mean age >62 y | 13 | 0.89 (0.86, 0.93) | 13 | 0.89 (0.84, 0.93) | ||
| Male prevalence ≥half | 19 | 0.87 (0.83, 0.90) | 0.07 | 22 | 0.85 (0.81, 0.88) | <0.0001 |
| Male prevalence <half | 8 | 0.92 (0.88, 0.96) | 7 | 0.95 (0.92, 0.98) | ||
| Mean follow‐up ≥43 mo, median | 12 | 0.92 (0.89, 0.95) | 0.007 | 15 | 0.91 (0.88, 0.95) | 0.005 |
| Mean follow‐up <43 mo | 14 | 0.82 (0.76, 0.89) | 13 | 0.82 (0.77, 0.87) | ||
| Annual event rate ≥6.4 events per y, median | 13 | 0.85 (0.80, 0.90) | 0.030 | 15 | 0.82 (0.77, 0.88) | 0.010 |
| Annual event rate <6.4 events per y | 13 | 0.91 (0.88, 0.95) | 13 | 0.91 (0.87, 0.95) | ||
| Sample size ≥192, median | 15 | 0.90 (0.86, 0.93) | 0.11 | 13 | 0.91 (0.87, 0.95) | 0.010 |
| Sample size <192 | 11 | 0.84 (0.79, 0.90) | 15 | 0.82 (0.77, 0.88) | ||
| Forearm occlusion | 19 | 0.87 (0.84, 0.91) | 0.45 | 19 | 0.88 (0.83, 0.94) | 0.77 |
| Upper arm occlusion | 7 | 0.90 (0.85, 0.95) | 9 | 0.87 (0.83, 0.91) | ||
| Lowest tertile of mean FMD value | 9 | 0.87 (0.81, 0.94) | 0.17 | 8 | 0.90 (0.85, 0.95) | 0.21 |
| Middle tertile of mean FMD value | 6 | 0.93 (0.88, 0.97) | 8 | 0.91 (0.86, 0.96) | ||
| Highest tertile of mean FMD value | 9 | 0.87 (0.82, 0.92) | 9 | 0.84 (0.78, 0.90) | ||
| Quality score <8 (median) | 12 | 0.86 (0.80, 0.92) | 0.30 | 12 | 0.81 (0.76, 0.87) | 0.01 |
| Quality score ≥8 | 14 | 0.90 (0.87, 0.92) | 16 | 0.90 (0.87, 0.94) | ||
CV indicates cardiovascular; CVD, cardiovascular disease; FMD, flow‐mediated dilation; MI, myocardial infarction; RR, risk ratio.
Comparison Between FMD and PAT
In comparison to FMD studies of non‐CVD subjects, those of CVD subjects had higher male prevalence, smaller sample size, lower event rate, and shorter follow‐up duration (Table 4). Furthermore, all RHI studies were derived from CVD subjects and had good quality. Among studies of CVD population, characteristics of studies including male prevalence, sample size, follow‐up duration, and event rate were not significantly different between FMD and RHI. Therefore, in order to compare these 2 methods, we restricted FMD studies to those of CVD population and good quality. Although the risk ratios for 1% increase in distal occlusion FMD and proximal occlusion FMD were not different as shown in Table 5, the distribution of mean values between distal and proximal occlusion FMD were different (P=0.02), which would affect pooled SD. Thus, we divided studies into 3 groups: proximal occlusion FMD, distal occlusion FMD, and Ln_RHI. Pooled mean±SD of proximal occlusion FMD, distal occlusion FMD, and Ln_RHI, which was calculated from all studies of CVD population, were 6.4±5.2, 4.3±4.6, and 0.56±0.26, respectively. Table 6 shows the pooled risk estimates for a 1 SD increase in proximal occlusion FMD (unadjusted RR [95% CI] 0.60 [0.44–0.80], adjusted 0.61 [0.44–0.85]), distal occlusion FMD (unadjusted RR [95% CI] 0.47 [0.35–0.63], adjusted 0.47 [0.32–0.67]), and Ln_RHI (unadjusted RR [95% CI] 0.48 [0.33–0.72], adjusted 0.54 [0.42–0.71]). Pooled RRs for these 3 methods were not significantly different.
Table 6.
Comparison Between Proximal Occlusion FMD, Distal Occlusion FMD, and Ln_RHI
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Pooled RR for Pooled SD (95% CI) | P Value | Pooled RR for Pooled SD (95% CI) | P Value | |
|
Proximal occlusion FMD Mean=6.4%, SD=5.2% |
0.60 (0.44, 0.80) | 0.0005 | 0.61 (0.44, 0.85) | 0.004 |
|
Distal occlusion FMD Mean=4.3%, SD=4.6% |
0.47 (0.35, 0.63) | <0.0001 | 0.47 (0.32, 0.67) | <0.0001 |
|
Ln_RHI Mean=0.56, SD=0.26 |
0.48 (0.33, 0.72) | 0.0004 | 0.54 (0.42, 0.71) | <0.0001 |
FMD indicates flow‐mediated dilation; Ln_RHI, logarithmic value of reactive hyperemia index; RR, risk ratio.
The relation between FMD or Ln_RHI level and cardiovascular event risk is shown in Figure 7. An ≈1 SD increase in FMD or Ln_RHI was associated with reduced cardiovascular event risk by half, whereas a 1 SD decrease was associated with doubling of risk.
Figure 7.
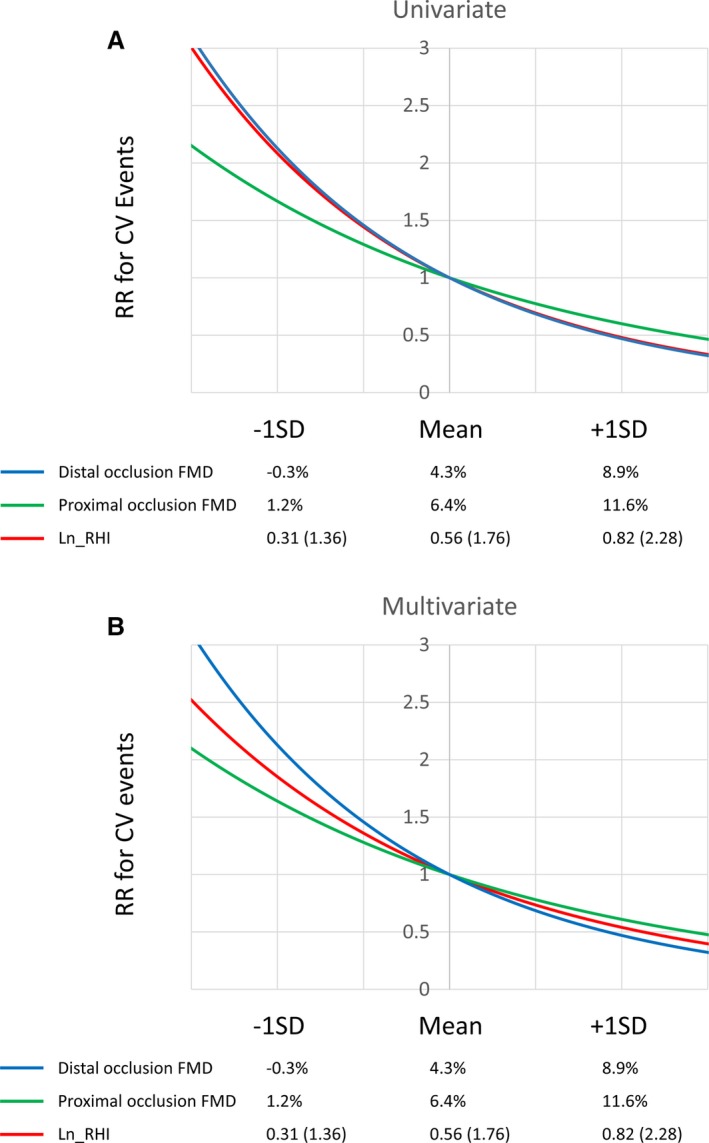
Relative risk for FMD and Ln_RHI values. (A) Univariate relative risk and (B) Multivariate relative risk. The relative risk for cardiovascular events in each FMD or Ln_RHI value is relative to the expected event rate with the median value of FMD or Ln_RHI. CV indicates cardiovascular; FMD, flow‐mediated dilation; Ln_RHI, logarithmic value of reactive hyperemia index; RR, risk ratio.
Publication Bias
Based on a visual inspection of the funnel plots, there may be publication bias among the included studies. The funnel plot showed asymmetrical distribution of FMD studies, indicating that publication bias may exist (Figure 8A and 8B). The small number of RH‐PAT studies limits inference from the funnel plots (Figure 9A and9B).
Figure 8.
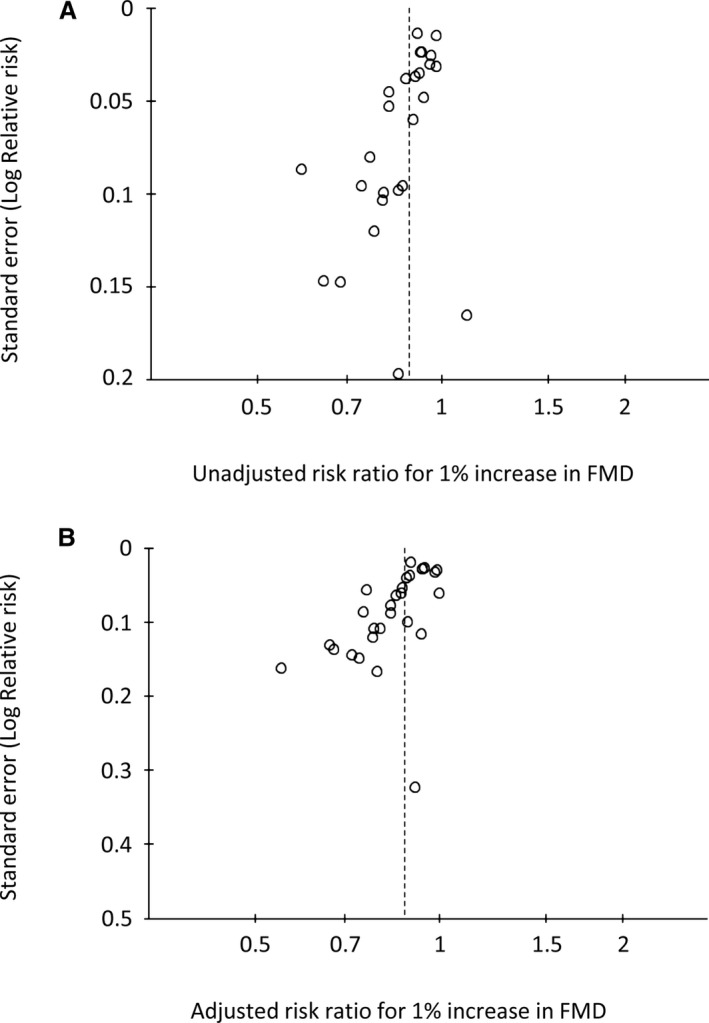
Funnel plot of flow‐mediated vasodilation (FMD) studies. Funnel plot of univariate (A) and multivariate (B) risk ratio of FMD.
Figure 9.
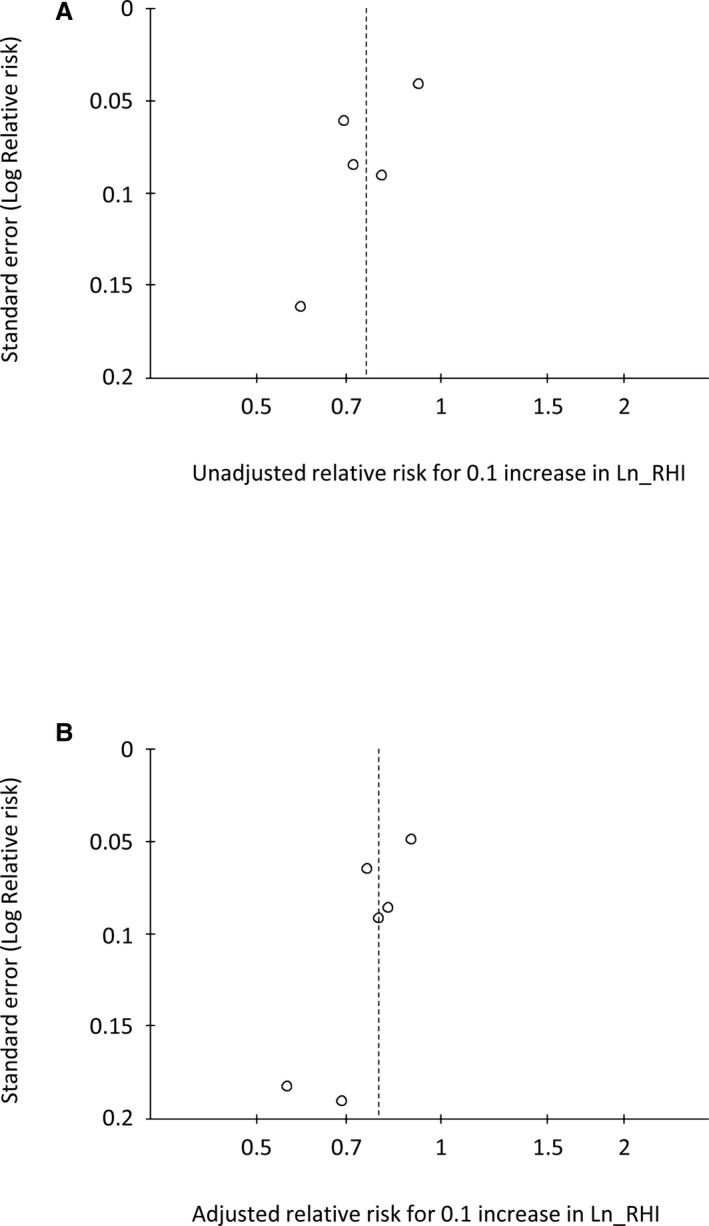
Funnel plot of RH‐PAT studies. Funnel plot of univariate (A) and multivariate (B) risk ratio of Ln_RHI. Ln_RHI indicates logarithmic value of reactive hyperemia index; RH‐PAT, reactive hyperemia–peripheral arterial tonometry.
Discussion
In this systematic review and meta‐analysis, we included 35 FMD and 6 RH‐PAT papers reporting the prognostic utility of peripheral endothelial function. We confirmed that peripheral endothelial function as assessed by FMD or RH‐PAT is a significant predictor of future cardiovascular events. According to the subgroup analysis of FMD studies, this prognostic utility was consistent across diverse population subgroups, although between‐study and between‐subgroup heterogeneity were found. The prognostic magnitudes of these 2 methods in CVD population were similar. A 1 SD deterioration in endothelial function could double the risk of cardiovascular events; conversely, a 1 SD improvement could halve it.
Our findings are in line with previous meta‐analyses that reported a significant association between brachial FMD or finger‐tip RH‐PAT and cardiovascular event risk.5, 6, 7 The only meta‐analysis of RH‐PAT studies was reported by Xu et al in 2014,7 which included 3 studies and 865 patients. Since then, 3 more RH‐PAT studies have been published, and as a result almost twice as many (1602) subjects with RH‐PAT assessment were included in our meta‐analysis. Recent studies showed that the results of RH‐PAT are better evaluated as a logarithmic value rather than RHI itself due to its abnormal distribution. Thus, while the meta‐analysis by Xu et al was done for 1 increase in RHI (pooled unadjusted RR 0.82 [0.76–0.89], and pooled adjusted RR 0.85 [0.78–0.93]), the present study reported for 0.1 increase in Ln_RHI (pooled unadjusted RR 0.76 [0.65–0.88], and pooled adjusted RR 0.79 [0.71–0.87]). In our study, the pooled SD was calculated for Ln_RHI as well.
In clinical research, brachial FMD has been a widely used noninvasive method that used reactive hyperemia after artery occlusion as a trigger for endothelium‐dependent vasodilation. The RH‐PAT technique is semi‐automatic and much simpler than FMD, and can potentially provide better interobserver reproducibility. Test–retest reliability of RH‐PAT has been reported to be very good.54 Brachial arterial diameter before and after reactive hyperemia‐induced vasodilation is measured by ultrasound in FMD, whereas a finger pulse amplitude is recorded by a hard‐shell‐covered tonometry cuff in RH‐PAT. Therefore, FMD is a measure of vasodilation in a conduit artery, whereas RH‐PAT samples smaller resistance arteries. Although nitric oxide bioavailability plays a substantial role in the both methodologies,55, 56 other substances, such as prostaglandin, adenosine, and hydrogen peroxide, can also influence vasodilation in response to shear stress and ischemia in different manners.57 Vasodilatory responses result from a complex interaction between a variety of these vasoactive substances and vascular smooth muscle, and can differ between conduit arteries and microvessels. Endothelium‐derived nitric oxide might have a more important role in FMD technique than in RH‐PAT. Although it has been reported that RH‐PAT mainly reflects endothelium‐derived nitric oxide,55 further studies are needed to elucidate the detailed mechanism of RH‐PAT signals. In a study of the Framingham Heart Study cohort, FMD (n=7031) and RH‐PAT (n=4352) were measured. Abnormal FMD was related to advancing age, hypertension, and obesity, whereas abnormal RH‐PAT was associated with obesity, increasing total/high‐density lipoprotein cholesterol ratio, diabetes, and smoking. Lower systolic blood pressure was also associated with abnormal RH‐PAT. Interestingly, after adjustment for risk factors and underlying CVD, RH‐PAT was not significantly associated with FMD. Thus, brachial FMD and digital RH‐PAT had differing relations with cardiovascular risk factors and provide distinct information regarding vascular function in conduit versus smaller digital vessels. Nevertheless, our results demonstrated that both methods provide significant predictive value for cardiovascular events and that their prognostic magnitudes in CVD population are similar. Future studies are needed to explore whether the prognostic values of these 2 for cardiovascular events are synergistic or independent of each other.
Results of brachial FMD vary across institutions, and thus, it is difficult to compare between institutions.58 In this meta‐analysis, mean values of proximal and distal FMD varied from 2.1% to 6.5% (median 3.7, interquartile range 2.5–5.4) and 4.7% to 9.1% (median 5.8, interquartile range 4.8–8.7) even when limited to CVD population, while mean values of Ln_RHI ranged from 0.28 to 0.59 (median 0.54, interquartile range 0.45–0.57) (excluding the 0.28 value results in a range of 0.50–0.59). It might be partly explained by operator dependency, technical factors, and methodological varieties of FMD measurement; therefore it is challenging to conduct a review of brachial artery FMD. On the other hand, the RH‐PAT technique is less operator dependent and well standardized. We showed that cardiovascular risk change associated with a 1 SD change in test value is comparable between FMD and Ln_RHI. Specifically, a 1 SD decrease in distal occlusion FMD from the mean value corresponds to decrease from 4.3% to −0.3%, and in Ln_RHI (RHI) from 0.56 (1.76) to 0.31 (1.36). The brachial artery must not respond or constrict in order to achieve a 1 SD change.
In current clinical practice, CVD risk is estimated based on identifying and quantifying the established risk factors, while there is a notable interindividual heterogeneity in response to risk factors and therapies.1 Furthermore, nontraditional and unknown risk factors may also have a substantial role in atherosclerosis. By measuring endothelial function, we can directly assess the functional significance of atherogenesis. Thus, noninvasive peripheral endothelial function tests seem to be feasible and effective in cardiovascular risk stratification. However, further evidence is needed, especially on RH‐PAT.
Limitations
The limitations of this study must be considered. First, study subjects, sample size, follow‐up duration, end points, and included covariates in multivariable analyses differed among studies. We did not have access to individual subject data to enable consistent adjustments for confounding factors. Second, only papers published in the English language were included. Third, publication bias was suspected from the funnel plots implying probable overestimation of the observed association with important practical implications for the use of endothelial function assessments. Fourth, the number of RH‐PAT studies is small and no studies on non‐CVD subjects with RH‐PAT measures were included.
Conclusions
The current systematic review and meta‐analysis found that both brachial FMD and digital RH‐PAT have significant predictive value for future cardiovascular events after adjustment for other risk factors. The prognostic magnitudes of these 2 methods in CVD population were similar, and a 1 SD increase or decrease was associated with 50% lower risk or doubled risk of cardiovascular events. Future studies should explore whether the prognostic values of these 2 are independent of each other and whether endothelial function‐guided therapies provide benefits in improving cardiovascular outcomes.
Sources of Funding
This work was supported by the NIH (NIH Grants HL‐92954 and AG‐31750), and the Mayo Foundation. Role of the Sponsors: The NIH and Mayo Foundation had no role in the process of designing, implementing, and reporting of the study apart from their financial contribution.
Disclosures
Lerman declared consulting for Itamar Medical.
Supporting information
Data S1. Search strategies.
(J Am Heart Assoc. 2015;4:e002270 doi: 10.1161/JAHA.115.002270)
An accompanying Data S1 is available at http://jaha.ahajournals.org/content/4/11/e002270/suppl/DC1
Notes
References
- 1. Matsuzawa Y, Guddeti RR, Kwon TG, Lerman LO, Lerman A. Secondary prevention strategy of cardiovascular disease using endothelial function testing. Circ J. 2015;79:685–694. [DOI] [PubMed] [Google Scholar]
- 2. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 3. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. [DOI] [PubMed] [Google Scholar]
- 4. Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, Levy D, Mitchell GF, Vita JA, Benjamin EJ. Relation of brachial and digital measures of vascular function in the community: the Framingham Heart Study. Hypertension. 2011;57:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging. 2010;26:631–640. [DOI] [PubMed] [Google Scholar]
- 6. Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 7. Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, Rigatto C, Komenda P, Sood MM, Tangri N. Non‐invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736–746. [DOI] [PubMed] [Google Scholar]
- 8. Ikonomidis I, Kadoglou NN, Tritakis V, Paraskevaidis I, Dimas K, Trivilou P, Papadakis I, Tzortzis S, Triantafyllidi H, Parissis J, Anastasiou‐Nana M, Lekakis J. Association of Lp‐PLA2 with digital reactive hyperemia, coronary flow reserve, carotid atherosclerosis and arterial stiffness in coronary artery disease. Atherosclerosis. 2014;234:34–41. [DOI] [PubMed] [Google Scholar]
- 9. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collaborations. 2011. [Google Scholar]
- 10. Wells GA, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2014. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 30, 2015.
- 11. Briffa TG, Hobbs MS, Tonkin A, Sanfilippo FM, Hickling S, Ridout SC, Knuiman M. Population trends of recurrent coronary heart disease event rates remain high. Circ Cardiovasc Qual Outcomes. 2011;4:107–113. [DOI] [PubMed] [Google Scholar]
- 12. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeboah J, Crouse JR, Hsu F‐C, Burke GL, Herrington DM. Brachial flow‐mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. [DOI] [PubMed] [Google Scholar]
- 15. Muiesan ML, Salvetti M, Paini A, Monteduro C, Galbassini G, Poisa P, Porteri E, Agabiti‐Rosei C, Paderno V, Belotti E, Rizzoni D, Castellano M, Agabiti‐Rosei E. Prognostic role of flow‐mediated dilatation of the brachial artery in hypertensive patients. J Hypertens. 2008;26:1612–1618. [DOI] [PubMed] [Google Scholar]
- 16. Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow‐mediated dilation and cardiac risk factors in post‐menopausal women. J Am Coll Cardiol. 2008;51:997–1002. [DOI] [PubMed] [Google Scholar]
- 17. Suzuki T, Hirata K, Elkind MSV, Jin Z, Rundek T, Miyake Y, Boden‐Albala B, Di Tullio MR, Sacco R, Homma S. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS). Am Heart J. 2008;156:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morimoto S, Yurugi T, Aota Y, Sakuma T, Jo F, Nishikawa M, Iwasaka T, Maki K. Prognostic significance of ankle‐brachial index, brachial‐ankle pulse wave velocity, flow‐mediated dilation, and nitroglycerin‐mediated dilation in end‐stage renal disease. Am J Nephrol. 2009;30:55–63. [DOI] [PubMed] [Google Scholar]
- 19. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: the Multi‐Ethnic Study of Atherosclerosis. Circulation. 2009;120:502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Low testosterone level as a predictor of cardiovascular events in Japanese men with coronary risk factors. Atherosclerosis. 2010;210:232–236. [DOI] [PubMed] [Google Scholar]
- 21. Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long‐term results from the firefighters and their endothelium (FATE) study. Circulation. 2011;123:163–169. [DOI] [PubMed] [Google Scholar]
- 22. Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5‐year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. [DOI] [PubMed] [Google Scholar]
- 23. Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Cakar M, Altun B, Yenicesu M, Carrero JJ. Vascular health, systemic inflammation and progressive reduction in kidney function; clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant. 2011;26:3537–3543. [DOI] [PubMed] [Google Scholar]
- 24. Nagai K, Shibata S, Akishita M, Sudoh N, Obara T, Toba K, Kozaki K. Efficacy of combined use of three non‐invasive atherosclerosis tests to predict vascular events in the elderly; carotid intima‐media thickness, flow‐mediated dilation of brachial artery and pulse wave velocity. Atherosclerosis. 2013;231:365–370. [DOI] [PubMed] [Google Scholar]
- 25. Shechter M, Shechter A, Koren‐Morag N, Feinberg MS, Hiersch L. Usefulness of brachial artery flow‐mediated dilation to predict long‐term cardiovascular events in subjects without heart disease. Am J Cardiol. 2014;113:162–167. [DOI] [PubMed] [Google Scholar]
- 26. Lee MJ, Han SH, Lee JE, Choi HY, Yoon CY, Kim EJ, Han JH, Han JS, Oh HJ, Park JT, Kang SW, Yoo TH. Endothelial dysfunction is associated with major adverse cardiovascular events in peritoneal dialysis patients. Medicine. 2014;93:e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neunteufl T, Heher S, Katzenschlager R, Wolfl G, Kostner K, Maurer G, Weidinger F. Late prognostic value of flow‐mediated dilation in the brachial artery of patients with chest pain. Am J Cardiol. 2000;86:207–210. [DOI] [PubMed] [Google Scholar]
- 28. Brevetti G, Silvestro A, Schiano V, Chiariello M. Endothelial dysfunction and cardiovascular risk prediction in peripheral arterial disease: additive value of flow‐mediated dilation to ankle‐brachial pressure index. Circulation. 2003;108:2093–2098. [DOI] [PubMed] [Google Scholar]
- 29. Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol. 2004;43:616–623. [DOI] [PubMed] [Google Scholar]
- 30. Katz SD, Hryniewicz K, Hriljac I, Balidemaj K, Dimayuga C, Hudaihed A, Yasskiy A. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111:310–314. [DOI] [PubMed] [Google Scholar]
- 31. Karatzis EN, Ikonomidis I, Vamvakou GD, Papaioannou TG, Protogerou AD, Andreadou I, Voidonikola PT, Karatzi KN, Papamichael CM, Lekakis JP. Long‐term prognostic role of flow‐mediated dilatation of the brachial artery after acute coronary syndromes without ST elevation. Am J Cardiol. 2006;98:1424–1428. [DOI] [PubMed] [Google Scholar]
- 32. Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu R, Wang W‐Q, Lau C‐P, Tse H‐F. Gender differences on brachial flow‐mediated dilation and carotid intima‐media thickness for prediction of spontaneous cardiovascular events. Clin Cardiol. 2008;31:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takase B, Matsushima Y, Uehata A, Ishihara M, Kurita A. Endothelial dysfunction, carotid artery plaque burden, and conventional exercise‐induced myocardial ischemia as predictors of coronary artery disease prognosis. Cardiovasc Ultrasound. 2008;6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shechter M, Matetzky S, Arad M, Feinberg MS, Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur J Heart Fail. 2009;11:588–593. [DOI] [PubMed] [Google Scholar]
- 36. Ulriksen LS, Malmqvist BB, Hansen A, Friberg J, Jensen GB. Flow‐mediated dilatation has no independent prognostic effect in patients with chest pain with or without ischaemic heart disease. Scand J Clin Lab Invest. 2009;69:475–480. [DOI] [PubMed] [Google Scholar]
- 37. Wang X, Guo F, Li G, Cao Y, Fu H. Prognostic role of brachial reactivity in patients with ST myocardial infarction after percutaneous coronary intervention. Coron Artery Dis. 2009;20:467–472. [DOI] [PubMed] [Google Scholar]
- 38. Akamatsu D, Sato A, Goto H, Watanabe T, Hashimoto M, Shimizu T, Sugawara H, Sato H, Nakano Y, Miura T, Zukeran T, Serizawa F, Hamada Y, Tsuchida K, Tsuji I, Satomi S. Nitroglycerin‐mediated vasodilatation of the brachial artery may predict long‐term cardiovascular events irrespective of the presence of atherosclerotic disease. J Atheroscler Thromb. 2010;17:1266–1274. [DOI] [PubMed] [Google Scholar]
- 39. Santos‐Garcia D, Blanco M, Serena J, Rodriguez‐Yanez M, Leira R, Castillo J. Impaired brachial flow‐mediated dilation is a predictor of a new‐onset vascular event after stroke. Cerebrovasc Dis. 2011;32:155–162. [DOI] [PubMed] [Google Scholar]
- 40. Chan YH, Lau KK, Yiu KH, Siu CW, Chan HT, Li SW, Tam S, Lam TH, Lau CP, Tse HF. Prospective observational study of isoflavone and the risk of stroke recurrence: potential clinical implications beyond vascular function. J Nutr Health Aging. 2012;16:383–388. [DOI] [PubMed] [Google Scholar]
- 41. Takishima I, Nakamura T, Hirano M, Kitta Y, Kobayashi T, Fujioka D, Saito Y, Watanabe K, Watanabe Y, Mishina H, Obata J‐E, Kawabata K‐I, Tamaru S, Kugiyama K. Predictive value of serial assessment of endothelial function in chronic heart failure. Int J Cardiol. 2012;158:417–422. [DOI] [PubMed] [Google Scholar]
- 42. Careri G, Nerla R, Di Monaco A, Russo G, Stazi A, Villano A, Sestito A, Lanza GA, Crea F. Clinical correlates and prognostic value of flow mediated dilation in patients with non‐ST segment elevation acute coronary syndromes [erratum appears in Am J Cardiol. 2013 Apr 1;111(7):1080]. Am J Cardiol. 2013;111:51–57. [DOI] [PubMed] [Google Scholar]
- 43. Nakamura T, Kitta Y, Uematsu M, Sugamata W, Hirano M, Fujioka D, Sano K, Saito Y, Kawabata K‐I, Obata J‐E, Kugiyama K. Ultrasound assessment of brachial endothelial vasomotor function in addition to carotid plaque echolucency for predicting cardiovascular events in patients with coronary artery disease. Int J Cardiol. 2013;167:555–560. [DOI] [PubMed] [Google Scholar]
- 44. Savic‐Radojevic A, Radovanovic S, Pekmezovic T, Pljesa‐Ercegovac M, Simic D, Djukic T, Matic M, Simic T. The role of serum VCAM‐1 and TNF‐alpha as predictors of mortality and morbidity in patients with chronic heart failure. J Clin Lab Anal. 2013;27:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sedlak TL, Johnson BD, Pepine CJ, Reis SE, Bairey Merz CN. Brachial artery constriction during brachial artery reactivity testing predicts major adverse clinical outcomes in women with suspected myocardial ischemia: results from the NHLBI‐sponsored women's ischemia syndrome evaluation (WISE) study. PLoS One. 2013;8:e74585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tarro Genta F, Eleuteri E, Temporelli PL, Comazzi F, Tidu M, Bouslenko Z, Bertolin F, Vigorito C, Giannuzzi P, Giallauria F. Flow‐mediated dilation normalization predicts outcome in chronic heart failure patients. J Cardiac Fail. 2013;19:260–267. [DOI] [PubMed] [Google Scholar]
- 47. Sawada T, Emoto T, Motoji Y, Hashimoto M, Kageyama H, Terashita D, Mizoguchi T, Mizuguchi T, Iwasaki M, Taira K, Okamoto H, Matsuo Y, Kim SK, Takarada A, Yokoyama M. Possible association between non‐invasive parameter of flow‐mediated dilatation in brachial artery and whole coronary plaque vulnerability in patients with coronary artery disease. Int J Cardiol. 2013;166:613–620. [DOI] [PubMed] [Google Scholar]
- 48. Hafner F, Kieninger A, Meinitzer A, Gary T, Froehlich H, Haas E, Hackl G, Eller P, Brodmann M, Seinost G. Endothelial dysfunction and brachial intima‐media thickness: long term cardiovascular risk with claudication related to peripheral arterial disease: a prospective analysis. PLoS One. 2014;9:e93357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non‐invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. [DOI] [PubMed] [Google Scholar]
- 50. Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1778–1786. [DOI] [PubMed] [Google Scholar]
- 51. Matsue Y, Suzuki M, Nagahori W, Ohno M, Matsumura A, Hashimoto Y, Yoshida K, Yoshida M. Endothelial dysfunction measured by peripheral arterial tonometry predicts prognosis in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2013;168:36–40. [DOI] [PubMed] [Google Scholar]
- 52. Matsuzawa Y, Sugiyama S, Sumida H, Sugamura K, Nozaki T, Ohba K, Matsubara J, Kurokawa H, Fujisue K, Konishi M, Akiyama E, Suzuki H, Nagayoshi Y, Yamamuro M, Sakamoto K, Iwashita S, Jinnouchi H, Taguri M, Morita S, Matsui K, Kimura K, Umemura S, Ogawa H. Peripheral endothelial function and cardiovascular events in high‐risk patients. J Am Heart Assoc. 2013;2:e000426 doi: 10.1161/JAHA.113.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matsue Y, Yoshida K, Nagahori W, Ohno M, Suzuki M, Matsumura A, Hashimoto Y, Yoshida M. Peripheral microvascular dysfunction predicts residual risk in coronary artery disease patients on statin therapy. Atherosclerosis. 2014;232:186–190. [DOI] [PubMed] [Google Scholar]
- 54. McCrea CE, Skulas‐Ray AC, Chow M, West SG. Test‐retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nohria A, Gerhard‐Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. [DOI] [PubMed] [Google Scholar]
- 56. Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium‐derived nitric oxide. Am J Physiol. 1996;270:H1435–H1440. [DOI] [PubMed] [Google Scholar]
- 57. Loscalzo J, Vita JA. Ischemia, hyperemia, exercise, and nitric oxide. Complex physiology and complex molecular adaptations. Circulation. 1994;90:2556–2559. [DOI] [PubMed] [Google Scholar]
- 58. Hijmering ML, Stroes ES, Pasterkamp G, Sierevogel M, Banga JD, Rabelink TJ. Variability of flow mediated dilation: consequences for clinical application. Atherosclerosis. 2001;157:369–373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search strategies.


