Abstract
Background
We recently reported that normal aldosterone levels are associated with cardiovascular, renal, and metabolic disease in a sample of the US general community (Visit 1). For the current analyses we used the same cohort in a new 4‐year follow‐up study (Visit 2).
Methods and Results
We measured aldosterone at Visit 1 and analyzed its predictive role for new diseases at Visit 2 (n=1140). We measured aldosterone at Visit 2 and investigated its associations with disease at Visit 2 (n=1368). We analyzed aldosterone continuously and we also dichotomized the variable as whether subjects were in the third tertile versus second and first tertiles. As continuous variable at Visit 1, aldosterone predicted new onset hypertension (HTN) (OR=1.36, CI=1.13–1.63, P=0.001), central obesity (OR=1.36, CI=1.07–1.73, P=0.011), and use of lipid‐lowering drugs (OR=1.25, CI=1.05–1.48, P=0.012) at Visit 2, after adjustment for age, sex, and body mass index. When in the third tertile (8.5–88.6 ng/dL), aldosterone predicted type 2 diabetes (T2DM, OR=1.96, CI=1.03–3.70, P=0.039). At Visit 2, aldosterone remained associated with HTN, obesity, and chronic kidney disease (CKD), as reported for Visit 1. However, aldosterone was not associated with heart failure (HF) at Visit 1 and 2, nor was aldosterone a predictor of HF between visits.
Conclusions
Aldosterone predicts new HTN, central obesity, T2DM, and use of lipid‐lowering drugs in the general community and remains associated with HTN, obesity, and CKD over 4 years. Aldosterone is not associated nor predicts HF. Further studies are warranted to evaluate aldosterone as therapeutic target in the general community.
Keywords: aldosterone, diabetes mellitus, general community, heart failure, obesity
Subject Categories: Hypertension; Diabetes, Type 2; Biomarkers; Risk Factors; Primary Prevention
Introduction
Aldosterone and mineralocorticoid receptor (MR) activation have been shown to play an important role in the pathogenesis of cardiovascular, renal, and metabolic disease, such as hypertension (HTN), chronic kidney disease (CKD), metabolic syndrome (MetS), type 2 diabetes mellitus (T2DM), and obesity. Specifically, recent studies demonstrated that in the heart, aldosterone binds to the MR expressed in cardiomyocytes and induces inflammation and fibrosis.1, 2 In the kidney, aldosterone has been shown to mediate glomerular podocyte injury followed by proteinuria in a rat model of metabolic syndrome3 in addition to sodium retention, while in isolated vascular smooth muscle cells the mineralocorticoid hormone can induce insulin resistance.4 Beyond the cardiorenal axis, a strong link between adipose tissue and MR activation is supported by the recent findings of increased renal MR expression induced by a high‐fat diet in mice, and by the ability of adipocytes to produce aldosterone in vitro.5, 6 Importantly, in 3 key longitudinal investigations, aldosterone has been shown to predict future HTN,7 CKD,8 and MetS.9 We recently reported statistically significant associations between aldosterone levels, even within the normal range, and HTN, obesity, high triglycerides, central obesity, MetS, and CKD in a well‐characterized randomly selected sample of the adult general community.10 Additionally, plasma aldosterone levels above the normal range were strongly associated with an increased incidence of all‐cause mortality in a 12‐year follow‐up.
The current study addressed 3 objectives in a 4‐year follow‐up study from our Olmsted County population cohort.10 First, we sought to define the role of circulating aldosterone at Visit 1 in predicting new cardiovascular, renal, and metabolic disease at Visit 2 (after 4‐year follow‐up). We hypothesized that plasma aldosterone at Visit 1 predicts new onset cardiovascular, renal, and metabolic disease at Visit 2. Having performed aldosterone measurement at Visit 2, a second objective was to determine whether the associations between aldosterone levels and cardiovascular, renal, and metabolic disease that were present at Visit 1 were sustained at Visit 2. We hypothesized that the associations between aldosterone and cardiovascular, renal, and metabolic disease are sustained at Visit 2. Our third objective was to investigate whether aldosterone at Visit 1 correlated with impaired myocardial structure and function at Visit 1 and/or predicted new impaired myocardial structure and function, including chronic heart failure (HF), at Visit 2. Also, we sought to analyze whether aldosterone measured at Visit 2 correlated with impaired myocardial structure and function at Visit 2. We hypothesized that aldosterone at Visit 1 is associated with impaired myocardial structure and function at Visit 1; aldosterone at Visit 1 predicts impairment of myocardial structure and function at Visit 2; and aldosterone measured at Visit 2 is associated with impaired myocardial structure and function at Visit 2.
Methods
Study Population
This study was approved by the Mayo Clinic Institutional Review Board and the subjects gave informed consent. Using the resources of the Rochester Epidemiology Project11 at Mayo Clinic, we analyzed a random sample of the general population from Olmsted County, MN. The design, selection criteria, and characteristics of this cohort have been previously described.12, 13 For the current study, at Visit 1, a total of 1674 subjects had aldosterone levels measured.10 Subjects (n=1368) returned for a second visit after 4 years (Visit 2, 2001–2004) with similar assessment as Visit 1 and plasma aldosterone measured. In a subset of 1140 subjects, we had available aldosterone levels for both visits. See Statistical Analyses for further details.
We defined as lipid‐lowering therapy the use of one or more of the following drugs: statins, fibrates, niacin, ezetimibe, and cholestyramine. Further, we considered as antihypertensive therapy interfering with the renin‐angiotensin‐aldosterone system the following drugs: beta‐blockers (BBs), angiotensin II receptor blockers (ARBs), angiotensin‐converting enzyme inhibitors (ACEi) and all classes of diuretics such as thiazides, thiazides‐like, loop diuretics, potassium‐sparing, and aldosterone antagonists, as previously reported.10 We were not able to establish a diagnosis of primary aldosteronism in our cohort, because plasma renin activity was not available. Nevertheless, to reduce the impact that higher aldosterone levels can have in the associations observed, we performed our analyses also entirely with individuals who were not suspected of primary aldosteronism and with aldosterone levels within the normal range. For this sensitivity analysis we used subjects with aldosterone within the normal range at Visit 1 for the prediction of disease at Visit 2 and subjects with normal aldosterone levels at Visit 2 for the associations with diseases at Visit 2.
Body mass index (BMI), myocardial infarction, and coronary artery disease were defined with established criteria as previously described.13 Obesity was defined as a BMI ≥ 30 kg/m². Waist circumference, measured at the top of the umbilicus, was expressed in centimeters (cms) and central obesity was defined as waist circumference >102 cm in men and >88 cm in women. Hypertension was defined according to the use of Joint National Committee VII diagnosis criteria.10 Smoking status was defined as never, prior, or active. CKD was defined as a glomerular filtration rate (GFR) <60 and >30 mL/min per 1.73 m2 based on the Modification of Diet in Renal Disease formula. The clinical diagnosis of T2DM, stroke, and HF was abstracted from the subject's chart. For Visit 2, we were not able to define the MetS (in accordance with the National Cholesterol Education Program Adult Treatment Panel III, as previously described10), since lipidic profile and glucose levels were not available.
Plasma Collection
At Visit 1 and Visit 2, baseline blood samples were obtained from subjects in the sitting position and there was no discontinuation of any therapy nor change in salt intake prior to the blood collection. Blood was drawn in EDTA tubes and chilled until it was centrifuged at 4°C at 2500g for 10 minutes. 0.5 mL plasma was aliquoted into polystyrene tubes and stored at −80°C until assayed.
Aldosterone Assay
For both Visit 1 and Visit 2 aldosterone was measured using a competitive radioimmunoassay (RIA) kit (Coat‐a‐Count kit, Siemens, Los Angeles, CA). Samples or 200 μL of standards were pipetted into antibody‐coated tubes with 1 mL of I‐125 labeled aldosterone, and incubated overnight at room temperature. The standard curve range was 2.5 to 120 ng/dL, inter‐and intra‐assay variability was 16% and 5.8%, respectively. There was no cross‐reactivity with other related steroids.14 The normal range of aldosterone was from 2.5 to 16.2 ng/dL as previously defined.10
Natriuretic Peptide Assays
For Visit 1, plasma natriuretic peptides (NPs) were measured as previously described.10 For Visit 2, plasma atrial natriuretic peptide (ANP, n=144) was determined as previously described.10 Plasma B‐type natriuretic peptide (BNP, n=1314) was measured with a Beckman Coulter DXI 800 platform, using a 2‐site immunoenzymatic sandwich assay. Plasma N‐terminus pro‐ANP (NT‐proANP, n=143) was detected by a radioimmunoassay using anti‐human NT‐proANP antibody.15 Plasma N‐terminus proBNP (NT‐proBNP, n=1370) was measured with an automated, double‐incubation sandwich assay using monoclonal NT‐proBNP antibody (Roche Diagnostics Corporation, Indianapolis, IN). Plasma pro‐BNP concentrations (n=1320) were determined using an updated version of the Bio‐Rad proBNP assay automated 2‐step sandwich fluorescence immunoassay on the BioPlex™2200 analyzer (Bio‐Rad, Hercules, CA).
Echocardiography
Echocardiograms were performed as previously described.10 Left ventricular (LV) systolic function (reduced ejection fraction [EF] defined as <40%), LV diastolic function and relaxation, and LV hypertrophy (LVH) were examined as previously described.10, 13 Concentric LVH (cLVH) was defined as LV mass index >95 g/m2 in women and >115 g/m2 in men+relative wall thickness >0.42, as by standard methods.16
Statistical Analyses
To study the associations between aldosterone at Visit 1 and new cardiovascular, renal, and metabolic diseases and impaired myocardial structure and function at Visit 2, we used 1140 subjects who had aldosterone measured both at Visit 1 and Visit 2. To investigate the associations between aldosterone and cardiovascular, renal, and metabolic disease as well as cardiac structure and function, at Visit 2, 1368 subjects who had plasma aldosterone measured were used. For analyzing the associations between aldosterone and impaired cardiac structure and function at Visit 1, 1674 subjects who had aldosterone measured were utilized. Covariate and outcome variables were defined and summarized by mean and standard deviations or median and quartiles for variables that were not normally distributed. Distributional assumptions were examined for continuous variables and time points were compared using paired t test or signed rank sum test, as appropriate based on the distribution. Categorical variables were summarized as number as percentage and comparison between time points was done using McNemar's test. To evaluate new onset of each disease, logistic regression analyses was used after exclusion of subjects with the specific condition at Visit 1. These methods were used to test for association between aldosterone levels and outcomes, and results were summarized with odds ratios (OR) and 95% confidence intervals. As it was not known if aldosterone had a linear association with outcome measures, aldosterone was analyzed both as a continuous variable after log transformation, and in the highest tertile (versus the lowest and middle tertiles together). Analyses to confirm Visit 1 associations at Visit 2 again used logistic regression to evaluate outcomes, and aldosterone was evaluated as both continuous and categorical, in attempt to replicate previous analyses. Analyses were adjusted for covariates that were thought to be associated with particular outcomes. Continuous aldosterone results are given as OR per 1 standard deviation increase to make results comparable between different time points. All tests were 2 sided and P values <0.05 were considered to be statistically significant. SAS version 9.3 (Cary, NC) was used for all analyses.
Results
Characteristics of the Study Subjects
The characteristics of our entire cohort at Visit 2 (n=1368) are presented in Table 1. Plasma aldosterone ranged from 2.5 to 88.6 ng/dL while the median (Q1, Q3) and mean±SD values were 6.2 (3.8, 10.0) and 8.1±6.7 ng/dL, respectively (Figure–Panel A). There was no difference in aldosterone levels between men and women and age did not influence circulating concentrations. For analysis of the entire cohort, we also divided aldosterone into tertiles where the 1st tertile (T1: n=457) ranged from 2.5 to 4.6 ng/dL, the 2nd tertile (T2: n=457) ranged from 4.7 to 8.4 ng/dL, and the 3rd tertile (T3: n=454) ranged from 8.5 to 88.6 ng/dL (Figure–Panel B). Importantly, 115 subjects (≈8% of the cohort) had abnormal aldosterone levels (above 16.2 ng/dL). The distribution of aldosterone in the general population is reported in (Figure–Panel C). Characteristics of the 1140 subjects with aldosterone levels measured at Visit 1 and Visit 2 are reported in Table 2.
Table 1.
Characteristics of the Entire Cohort of the General Community at Visit 2
| Variable | Overall (N=1368) |
|---|---|
| Age at exam, y | 65.15±9.54 |
| Sex F, n (%) | 692 (51) |
| BMI of subjects, kg/m2 | 28.46±5.05 |
| Medication use, n (%) | 1295 (95) |
| BMI >30 kg/m2, n (%) | 433 (32) |
| Waist circumference, cm >102M, 88F, n (%) | 472 (35) |
| Aldosterone ng/dL, median (Q1, Q3) | 6.20 (3.80, 10.00) |
| proBNP pg/mL, median (Q1, Q3) | 17.00 (8.00, 37.00) |
| NT‐proBNP pg/mL, median (Q1, Q3) | 78.25 (39.75, 153.00) |
| BNP pg/mL, median (Q1, Q3) | 14.60 (6.90, 31.70) |
| NT‐proANP pg/mL, median (Q1, Q3) | 3201.0 (2045.0, 4667.0) |
| ANP pg/mL, median (Q1, Q3) | 17.60 (10.95, 26.55) |
| Current/former smoker, n (%) | 625 (48) |
| Type 2 diabetes, n (%) | 118 (10) |
| Hypertension, n (%) | 579 (42) |
| Atrial fibrillation/flutter, n (%) | 77 (6) |
| Coronary artery disease, n (%) | 205 (15) |
| Heart failure, n (%) | 30 (2) |
| Myocardial infarction, n (%) | 50 (4) |
| Stroke, n (%) | 39 (3) |
| Systolic blood pressure | 125.98±18.98 |
| Diastolic blood pressure | 69.51±10.39 |
| Lipid‐lowering therapy, n (%) | 420 (32) |
| Antihypertensive therapy, n (%) | 579 (45) |
| Creatinine, median (Q1, Q3) | 0.90 (0.80, 1.00) |
| Calculated GFR (MDRD) | 76.93±17.94 |
| GFR <60, n (%) | 186 (14) |
| Echocardiographic parameters | |
| Ejection fraction <40, n (%) | 19 (1) |
| Mild/moderate/severe diastolic dysfunction, n (%) | 565 (46) |
| Left ventricular hypertrophy, n (%) | 216 (21) |
| Concentric left ventricular hypertrophy, n (%) | 111 (11) |
ACEi indicates angiotensin converting enzyme inhibitors; ANP, atrial natriuretic peptide; ARBs, angiotensin receptor blockers; BBs, beta‐blockers; BMI, body mass index; BNP, B type natriuretic peptide; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease (formula); NT‐proANP, N‐terminus of pro‐atrial natriuretic peptide; NT‐proBNP, N‐terminus of pro‐B type natriuretic peptide; pro‐BNP, pro‐B type natriuretic peptide.
Figure 1.
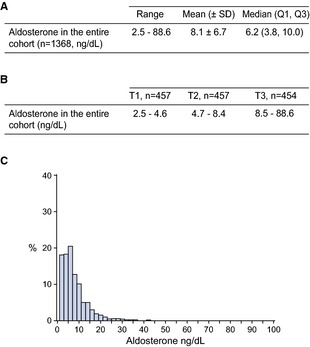
A, Aldosterone levels at Visit 2; B, Aldosterone according to tertiles at Visit 2; C, Distribution of aldosterone levels in the general community at Visit 2. Q indicates quartile; SD, standard deviation; T, tertile.
Table 2.
Characteristics of Subjects of the General Community With Aldosterone Levels at Visit 1 and Visit 2
| Variable | Visit 1 (N=1140) | Visit 2 (N=1140) | P Value |
|---|---|---|---|
| Age at exam, y | 61.50±9.66 | 65.52±9.68 | |
| Sex F, n (%) | 614 (54) | 614 (54) | |
| BMI of subjects, kg/m2 | 28.23±5.10 | 28.45±5.14 | <0.001 |
| Medication use, n (%) | 1051 (92) | 1080 (95) | <0.001 |
| BMI >30 kg/m2, n (%) | 354 (31) | 354 (31) | 1.0 |
| Waist circumference, cm >102 M, 88 F, n (%) | 360 (32) | 392 (34) | 0.017 |
| Aldosterone ng/dL, median (Q1, Q3) | 4.70 (2.50, 7.80) | 6.20 (3.80, 10.00) | <0.001 |
| NT‐proBNP pg/mL, median (Q1, Q3) | 63.85 (27.46, 129.20) | 80.90 (40.90, 156.00) | <0.001 |
| BNP pg/mL, median (Q1, Q3) | 13.75 (5.25, 28.35) | 15.20 (7.20, 33.20) | <0.001 |
| NT‐proANP pg/mL, median (Q1, Q3) | 2200.0 (1422.5, 3253.5) | 3201.0 (2045.0, 4704.0) | <0.001 |
| ANP pg/mL, median (Q1, Q3) | 11.30 (7.40, 15.80) | 17.50 (11.00, 26.20) | <0.001 |
| Current/Former Smoker, n (%) | 544 (48) | 510 (47) | 0.077 |
| Type 2 diabetes, n (%) | 74 (7) | 113 (11) | <0.001 |
| Hypertension, n (%) | 304 (27) | 492 (43) | <0.001 |
| Atrial fibrillation/flutter, n (%) | 38 (3) | 66 (6) | <0.001 |
| Coronary artery disease, n (%) | 121 (11) | 169 (15) | <0.001 |
| Heart failure, n (%) | 15 (1) | 27 (2) | <0.001 |
| Myocardial infarction, n (%) | 42 (4) | 44 (4) | 0.706 |
| Stroke, n (%) | 9 (1) | 35 (4) | <0.001 |
| Systolic blood pressure | 130.92±19.94 | 126.35±19.63 | <0.001 |
| Diastolic blood pressure | 73.13±10.01 | 69.33±10.52 | <0.001 |
| Lipid‐lowering therapy, n (%) | 208 (20) | 355 (33) | <0.001 |
| BBs, ACEi, Diuretics, ARBs, n (%) | 325 (31) | 489 (45) | <0.001 |
| Creatinine, median (Q1, Q3) | 0.80 (0.70, 0.90) | 0.90 (0.80, 1.00) | <0.001 |
| Calculated GFR (MDRD) | 81.30±16.71 | 76.46±17.81 | <0.001 |
| GFR < 60, n (%) | 137 (12) | 177 (16) | 0.002 |
| Echocardiographic parameters | |||
| EF<40, n (%) | 8 (1) | 17 (1) | 0.007 |
| Mild/mod/severe diastolic Dysfunction, n (%) | 280 (26) | 475 (46) | <0.001 |
| Left ventricular hypertrophy (LVH), n (%) | 291 (31) | 196 (23) | 0.001 |
| Concentric LVH, n (%) | 160 (17) | 96 (11) | 0.007 |
Time points compared using McNemar's test for categorical variables or paired t test/signed rank test for continuous variables. ACEi indicates angiotensin converting enzyme inhibitors; ANP, atrial natriuretic peptide; ARBs, angiotensin receptor blockers; BBs, beta‐blockers; BMI, body mass index; BNP, B type natriuretic peptide; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease (formula); NT‐proANP, N‐terminus of pro‐atrial natriuretic peptide; NT‐proBNP, N‐terminus of pro‐B type natriuretic peptide; pro‐BNP, pro‐B type natriuretic peptide.
Aldosterone Levels at Visit 1 Predict New Onset Cardiovascular and Metabolic Disease at Visit 2
When analyzed as continuous variable, aldosterone at Visit 1 was associated with new onset HTN after adjustment for age, sex, BMI, GFR, antihypertensives, central obesity, and smoking (Table 3). Also, aldosterone when analyzed as a continuous variable predicted new central obesity after adjustments for age, sex, BMI, GFR, antihypertensive therapy, and T2DM. In a logistic regression model adjusted for the same covariates, aldosterone in the third tertile predicted new HTN but this association was attenuated when adjusted for GFR and central obesity. Aldosterone levels in the third tertile were associated with new onset T2DM, and this association was attenuated after adjustment for central obesity. Given the strong correlation that we observed between aldosterone and cardiovascular, renal, and metabolic disease and having available the use of lipid‐lowering drugs in our cohort, we decided to also analyze the relationship between aldosterone and future use of lipid‐lowering therapy. These drugs, which can reduce plasma cholesterol and triglycerides levels, are effective in preventing cardiovascular events in high‐risk subjects17 and may be prescribed in patients with the MetS.18 Thus, lipid‐lowering drugs may be considered a further indirect measure of future cardiovascular, renal, and metabolic disease development. We found a statistically significant association between plasma aldosterone at Visit 1 and future use of lipid‐lowering drugs, when aldosterone was analyzed as continuous variable and when in the top tertile. This prediction remained statistically significant after considering age, sex, BMI, GFR, and numerous additional risk factors in the multilogistic regression model (Table 4). Importantly, after considering only subjects with plasma aldosterone within the normal range at Visit 1, we found that these associations with new onset disease (Table 5) and use of lipid‐lowering drugs (Table 6) were still present.
Table 3.
Logistic Regression Analysis for Aldosterone at Visit 1 and New Diseases at Visit 2
| Outcome (Total N/New Event N) | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| New hypertension (N=836/203) | ||||
| Age, sex, BMI (base) | 1.36 (1.13, 1.63) | 0.001 | 1.44 (1.00, 2.08) | 0.042 |
| Base+GFR | 1.36 (1.12, 1.64) | 0.001 | 1.36 (0.93, 1.99) | 0.108 |
| Base+antihypertensives | 1.38 (1.13, 1.68) | 0.001 | 1.52 (1.03, 2.25) | 0.036 |
| Base+central obesity | 1.30 (1.08, 1.57) | 0.006 | 1.36 (0.94, 1.97) | 0.106 |
| Base+smoking | 1.36 (1.13, 1.64) | 0.001 | 1.45 (1.00, 2.10) | 0.048 |
| New central obesity (N=780/106) | ||||
| Age, sex, BMI (base) | 1.36 (1.07, 1.73) | 0.011 | 1.53 (0.94, 2.48) | 0.087 |
| Base+GFR | 1.34 (1.05, 1.72) | 0.020 | 1.49 (0.91, 2.46) | 0.116 |
| Base+antihypertensives | 1.33 (1.03, 1.70) | 0.026 | 1.49 (0.90, 2.47) | 0.124 |
| Base+Type 2 diabetes | 1.38 (1.08, 1.75) | 0.009 | 1.54 (0.95, 2.50) | 0.082 |
| New Type 2 diabetes (N=1066/43) | ||||
| Age, sex, BMI (base) | 1.27 (0.94, 1.71) | 0.115 | 1.96 (1.03, 3.70) | 0.039 |
| Base+GFR | 1.39 (1.02, 1.90) | 0.038 | 2.43 (1.25, 4.74) | 0.009 |
| Base+antihypertensives | 1.25 (0.90, 1.73) | 0.185 | 2.00 (1.01, 3.96) | 0.047 |
| Base+central obesity | 1.17 (0.87, 1.57) | 0.310 | 1.72 (0.91, 3.27) | 0.097 |
Results of logistic regression analysis, covariates taken from Visit 1. BMI indicates body mass index; GFR, glomerular filtration rate; OR, odds ratio.
Table 4.
Logistic Regression Analysis for Aldosterone at Visit 1 and New Use of Lipid‐Lowering Therapy at Visit 2
| Outcome (Total N/New Event N) | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| New lipid‐lowering therapy (N=848/146) | ||||
| Age, sex, BMI (base) | 1.25 (1.05, 1.48) | 0.012 | 1.59 (1.09, 2.31) | 0.016 |
| Base+GFR | 1.26 (1.05, 1.51) | 0.013 | 1.65 (1.11, 2.44) | 0.012 |
| Base+antihypertensives | 1.14 (0.96, 1.37) | 0.143 | 1.36 (0.92, 2.01) | 0.121 |
| Base+smoking | 1.25 (1.05, 1.82) | 0.242 | 1.61 (1.10, 2.35) | 0.013 |
| Base+coronary artery disease | 1.30 (1.09, 1.54) | 0.004 | 1.72 (1.17, 2.53) | 0.005 |
| Base+heart failure | 1.24 (1.05, 1.48) | 0.013 | 1.58 (1.08, 2.30) | 0.018 |
| Base+myocardial infarction | 1.25 (1.05, 1.48) | 0.012 | 1.59 (1.09, 2.31) | 0.016 |
| Base+stroke | 1.25 (1.05, 1.48) | 0.012 | 1.59 (1.09, 2.31) | 0.016 |
| Base+Type 2 diabetes | 1.28 (1.07, 1.52) | 0.006 | 1.61 (1.10, 2.35) | 0.015 |
| Base+central obesity | 1.26 (1.06, 1.50) | 0.009 | 1.61 (1.10, 2.34) | 0.014 |
| Base+blood pressure | 1.27 (1.07, 1.51) | 0.007 | 1.63 (1.12, 2.38) | 0.012 |
Results of logistic regression analysis, covariates taken from Visit 1. BMI indicates body mass index; GFR, glomerular filtration rate; OR, odds ratio.
Table 5.
Logistic Regression Analysis for Aldosterone Within Normal Range at Visit 1 and New Diseases at Visit 2
| Outcome (Total N/New Event N) | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| New hypertension (N=816/199) | ||||
| Age, sex, BMI (base) | 1.42 (1.19, 1.69) | <0.001 | 1.60 (1.12, 2.30) | 0.011 |
| Base+GFR | 1.41 (1.18, 1.70) | <0.001 | 1.54 (1.06, 2.23) | 0.024 |
| Base+Antihypertensives | 1.46 (1.20, 1.76) | <0.001 | 1.68 (1.14, 2.48) | 0.008 |
| Base+central obesity | 1.36 (1.13, 1.63) | <0.001 | 1.48 (1.03, 2.15) | 0.036 |
| Base+smoking | 1.42 (1.19, 1.70) | <0.001 | 1.61 (1.12, 2.32) | 0.010 |
| New central obesity (N=756/103) | ||||
| Age, sex, BMI (base) | 1.36 (1.07, 1.74) | 0.012 | 1.52 (0.92, 2.49) | 0.099 |
| Base+GFR | 1.34 (1.05, 1.73) | 0.021 | 1.42 (0.85, 2.37) | 0.178 |
| Base+antihypertensives | 1.36 (1.06, 1.76) | 0.018 | 1.51 (0.90, 2.53) | 0.115 |
| Base+Type 2 diabetes | 1.40 (1.09, 1.79) | 0.008 | 1.56 (0.95, 2.56) | 0.080 |
| New Type 2 diabetes (N=1019/40) | ||||
| Age, sex, BMI (base) | 1.32 (0.96, 1.82) | 0.090 | 2.11 (1.09, 4.11) | 0.028 |
| Base+GFR | 1.42 (1.02, 1.98) | 0.039 | 2.60 (1.30, 5.20) | 0.007 |
| Base+antihypertensives | 1.28 (0.92, 1.77) | 0.171 | 2.15 (1.06, 4.38) | 0.034 |
| Base+central obesity | 1.22 (0.88, 1.69) | 0.225 | 1.83 (0.94, 3.58) | 0.076 |
Results of logistic regression analysis, covariates taken from Visit 1. BMI indicates body mass index; GFR, glomerular filtration rate; OR, odds ratio.
Table 6.
Logistic Regression Analysis for Aldosterone Within Normal Range at Visit 1 and New Use of Lipid‐Lowering Therapy at Visit 2
| Outcome (Total N/New Event N) | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| New lipid‐lowering therapy (N=808/136) | ||||
| Age, sex, BMI (base) | 1.24 (1.03, 1.50) | 0.022 | 1.61 (1.09, 2.37) | 0.017 |
| Base+GFR | 1.26 (1.04, 1.53) | 0.017 | 1.67 (1.12, 2.49) | 0.013 |
| Base+antihypertensives | 1.17 (0.97, 1.41) | 0.103 | 1.45 (0.97, 2.16) | 0.069 |
| Base+smoking | 1.24 (1.03, 1.50) | 0.022 | 1.61 (1.09, 2.38) | 0.016 |
| Base+coronary artery disease | 1.28 (1.06, 1.55) | 0.010 | 1.73 (1.16, 2.57) | 0.007 |
| Base+heart failure | 1.24 (1.03, 1.50) | 0.022 | 1.61 (1.09, 2.38) | 0.017 |
| Base+myocardial infarction | 1.24 (1.03, 1.49) | 0.023 | 1.60 (1.09, 2.37) | 0.018 |
| Base+stroke | 1.24 (1.03, 1.50) | 0.021 | 1.61 (1.09, 2.37) | 0.017 |
| Base+Type 2 diabetes | 1.27 (1.05, 1.53) | 0.013 | 1.66 (1.11, 2.64) | 0.013 |
| Base+central obesity | 1.26 (1.04, 1.52) | 0.016 | 1.64 (1.11, 2.43) | 0.013 |
| Base+blood pressure | 1.26 (1.04, 1.52) | 0.016 | 1.64 (1.11, 2.42) | 0.014 |
Results of logistic regression analysis, covariates taken from Visit 2. BMI indicates body mass index; GFR, glomerular filtration rate; OR, odds ratio.
In addition to aldosterone levels at baseline, we investigated whether an increase in aldosterone levels between Visit 1 and Visit 2 was associated with any of the new outcomes analyzed at Visit 2. Notably, we found that from 142 subjects with aldosterone level in the first or second tertile at Visit 1 who moved into the third tertile at Visit 2, 53 developed new HTN (OR=2.6, 95% CI=1.6–4.0, P<0.001).
We further used receiver‐operating characteristic (ROC) curves to assess the role of aldosterone as a marker for cardiovascular, renal, and metabolic disease. Aldosterone at Visit 1, analyzed as continuous variable as well as in the top tertile did not add statistical significance to the area under the curve (AUC) of the baseline model consisting of age, sex, and BMI (Figure S1A through S1C).
Associations Between Aldosterone at Visit 2 and Cardiovascular, Renal, and Metabolic Diseases at Visit 2
Plasma aldosterone at Visit 2, analyzed as continuous variable and when in the highest tertile, was associated with HTN, obesity, and CKD at Visit 2, after adjusting for multiple covariates, as shown in Table 7. Notably, when we investigated subjects with plasma aldosterone within the normal range only, we found that these associations remained statistically significant (Table 8). In addition, we investigated whether there was an association between aldosterone and blood pressure or GFR. We found that aldosterone at Visit 2, when analyzed as continuous variable, was associated with systolic blood pressure (SBP) at Visit 2, after adjusting for age, sex, BMI, and Visit 1 SBP (OR=1.48, 95% CI=0.53–2.43, P=0.002). Aldosterone in the top tertile also had a statistically significant correlation with SBP at Visit 2 after same adjustments (OR=3.24, 95% CI=1.22–5.27, P=0.002). We found that diastolic blood pressure (DBP) at Visit 2 was only associated with aldosterone in the top tertile at Visit 2 after adjusting for age, sex, BMI, and Visit 1 DBP (OR=1.48, 95% CI=0.53–2.43, P=0.002). We did not find a correlation between GFR and aldosterone at Visit 2.
Table 7.
Logistic Regression Analysis for Aldosterone at Visit 2 and Diseases at Visit 2 (N=1368)
| Outcome | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| Hypertension (N=579) | ||||
| Age, sex, BMI (base) | 1.65 (1.46, 1.87) | <0.001 | 1.99 (1.56, 2.54) | <0.001 |
| Base+GFR | 1.64 (1.45, 1.87) | <0.001 | 1.94 (1.51, 2.49) | <0.001 |
| Base+antihypertensives | 1.55 (1.31, 1.84) | <0.001 | 1.89 (1.34, 2.66) | <0.001 |
| Base+central obesity | 1.65 (1.45, 1.86) | <0.001 | 1.97 (1.54, 2.52) | <0.001 |
| Base+smoking | 1.67 (1.47, 1.90) | <0.001 | 1.98 (1.53, 2.55) | <0.001 |
| Obesity (N=433) | ||||
| Age, sex (base) | 1.36 (1.21, 1.52) | <0.001 | 1.66 (1.31, 2.11) | <0.001 |
| Base+GFR | 1.35 (1.20, 1.52) | <0.001 | 1.62 (1.27, 2.07) | 0.001 |
| Base+antihypertensives | 1.25 (1.11, 1.42) | <0.001 | 1.49 (1.15, 1.92) | 0.002 |
| Base+Type 2 diabetes | 1.38 (1.22, 1.57) | <0.001 | 1.82 (1.40, 2.38) | <0.001 |
| Chronic kidney disease (N=186) | ||||
| Age, sex, BMI (base) | 1.61 (1.37, 1.90) | <0.001 | 2.01 (1.43, 2.81) | <0.001 |
| Base+GFR | NA | |||
| Base+antihypertensives | 1.53 (1.29, 1.82) | <0.001 | 1.89 (1.34, 2.68) | <0.001 |
| Base+blood pressure | 1.62 (1.37, 1.91) | <0.001 | 1.98 (1.41, 2.78) | <0.001 |
| Base+Type 2 diabetes | 1.67 (1.38, 1.98) | <0.001 | 2.22 (1.53, 3.21) | <0.001 |
| Base+central obesity | 1.61 (1.37, 1.90) | <0.001 | 2.00 (1.43, 2.80) | <0.001 |
| Base+smoking | 1.58 (1.33, 1.87) | <0.001 | 1.84 (1.30, 2.61) | <0.001 |
Results of logistic regression analysis, covariates taken from Visit 2. BMI indicates body mass index; GFR, glomerular filtration rate; NA, not applicable; OR, odds ratio.
Table 8.
Logistic Regression Analysis for Aldosterone Within Normal Range at Visit 2 and Diseases at Visit 2 (N=1253)
| Outcome | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| Hypertension (N=505) | ||||
| Age, sex, BMI (base) | 1.54 (1.36, 1.75) | <0.001 | 1.94 (1.50, 2.51) | <0.001 |
| Base+GFR | 1.54 (1.35, 1.75) | <0.001 | 1.93 (1.49, 2.50) | <0.001 |
| Base+antihypertensives | 1.61 (1.35, 1.91) | <0.001 | 2.05 (1.44, 2.93) | <0.001 |
| Base+central obesity | 1.53 (1.35, 1.74) | <0.001 | 1.93 (1.49, 2.49) | <0.001 |
| Base+smoking | 1.56 (1.37, 1.78) | <0.001 | 1.96 (1.51, 2.55) | <0.001 |
| Obesity (N=380) | ||||
| Age, sex (base) | 1.29 (1.14, 1.46) | <0.001 | 1.71 (1.33, 2.20) | <0.001 |
| Base+GFR | 1.28 (1.13, 1.45) | <0.001 | 1.69 (1.31, 2.18) | <0.001 |
| Base+antihypertensives | 1.22 (1.07, 1.39) | 0.003 | 1.52 (1.16, 1.98) | 0.002 |
| Base+Type 2 diabetes | 1.31 (1.14, 1.50) | <0.001 | 1.79 (1.35, 2.37) | <0.001 |
| Chronic kidney disease (N=153) | ||||
| Age, sex, BMI (base) | 1.40 (1.16, 1.68) | <0.001 | 1.93 (1.34, 2.78) | <0.001 |
| Base+GFR | NA | |||
| Base+antihypertensives | 1.38 (1.15, 1.67) | <0.001 | 1.84 (1.27, 2.68) | 0.001 |
| Base+blood pressure | 1.40 (1.16, 1.68) | <0.001 | 1.92 (1.33, 2.77) | <0.001 |
| Base+Type 2 diabetes | 1.42 (1.16, 1.73) | <0.001 | 1.89 (1.27, 2.82) | 0.002 |
| Base+central obesity | 1.39 (1.16, 1.67) | <0.001 | 1.92 (1.33, 2.77) | <0.001 |
| Base+smoking | 1.33 (1.10, 1.61) | 0.003 | 1.71 (1.17, 2.60) | 0.005 |
Results of logistic regression analysis, covariates taken from Visit 2. BMI indicates body mass index; GFR, glomerular filtration rate; NA, not applicable; OR, odds ratio.
To study the associations between aldosterone and cardiovascular, renal, and metabolic disease at Visit 2, we also used ROC curves. In this case, plasma aldosterone at Visit 2 adds statistical significance to the ability of the baseline model, consisting of age, sex, and BMI, to discriminate disease and control (Figure S2A through S2C), supporting the role of aldosterone as mediator of disease.
Aldosterone and Cardiac Function in the General Community at Visit 1 and Visit 2
We also defined the relationship between aldosterone and HF, diastolic dysfunction (DDF), reduced LVEF, and cLVH. At Visit 1, aldosterone analyzed as continuous variable as well as in the top tertile was not associated with mild/moderate/severe DDF at Visit 1, after adjustment for age, sex, and BMI (when aldosterone continuous variable OR=0.99, 95% CI=0.87–1.13, P=0.932; when aldosterone top tertile OR=1.11, 95% CI=0.85–1.46, P=0.441, Table 9). As reported in our previous study10 and reconfirmed here, aldosterone at Visit 1 was associated with cLVH but this correlation was attenuated after adjustment for antihypertensives (Table 9). Limited to the relatively small number of subjects affected by these conditions, aldosterone at Visit 1 was not positively associated with HF (n=41) and reduced LVEF (n=24) at Visit 1, as shown in Table 9. Further, aldosterone analyzed as continuous variable and in the top tertile at Visit 1 did not predict future HF, DDF, LVEF <40%, or cLVH at Visit 2 (Table 10). At Visit 2, we did not find aldosterone correlated with any of these cardiac conditions or abnormalities at Visit 2, but a positive trend between aldosterone and cLVH was present at Visit 2 when aldosterone analyzed as continuous variable (OR=1.22, 95% CI=1.00 to 1.50, P=0.053 after adjustment for age, sex and BMI, Table 11).
Table 9.
Logistic Regression Analysis for Aldosterone at Visit 1 and Cardiac Structure and Function at Visit 1 (N=1674)
| Outcome | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| HF (N=41) | ||||
| Age, sex, BMI (base) | 1.26 (0.95, 1.67) | 0.115 | 0.94 (0.48, 1.85) | 0.859 |
| Base+NT‐proANP | 1.29 (0.97, 1.72) | 0.085 | 1.07 (0.53, 2.17) | 0.847 |
| Base+ANP | 1.38 (1.03, 1.85) | 0.034 | 1.17 (0.58, 2.36) | 0.660 |
| Base+NT‐proBNP | 1.05 (0.76, 1.45) | 0.781 | 0.84 (0.39, 1.84) | 0.665 |
| Base+BNP | 1.37 (1.02, 1.85) | 0.038 | 1.21 (0.60, 2.45) | 0.588 |
| Base+GFR | 1.20 (0.90, 1.61) | 0.220 | 0.85 (0.43, 1.71) | 0.653 |
| c‐LVH (N=251)a | ||||
| Age, sex, BMI (base) | 1.18 (1.02, 1.36) | 0.022 | 1.31 (0.96, 1.77) | 0.086 |
| Base+NT‐proANP | 1.19 (1.03, 1.38) | 0.016 | 1.33 (0.98, 1.81) | 0.070 |
| Base+ANP | 1.20 (1.04, 1.38) | 0.014 | 1.33 (0.98, 1.81) | 0.068 |
| Base+NT‐proBNP | 1.19 (1.03, 1.37) | 0.020 | 1.29 (0.94, 1.75) | 0.110 |
| Base+BNP | 1.19 (1.03, 1.37) | 0.017 | 1.33 (0.98, 1.81) | 0.067 |
| Base+GFR | 1.23 (1.06, 1.42) | 0.007 | 1.38 (1.01, 1.89) | 0.044 |
| Base+antihypertensives | 1.13 (0.98, 1.32) | 0.097 | 1.25 (0.91, 1.73) | 0.164 |
| DDF (mild/moderate/severe) (N=454) | ||||
| Age, sex, BMI (base) | 0.99 (0.87, 1.13) | 0.932 | 1.11 (0.85, 1.46) | 0.441 |
| Base+NT‐proANP | 0.98 (0.86, 1.13) | 0.824 | 1.07 (0.81, 1.41) | 0.633 |
| Base+ANP | 1.00 (0.87, 1.14) | 0.999 | 1.12 (0.85, 1.48) | 0.413 |
| Base+NT‐proBNP | 1.02 (0.90, 1.17) | 0.727 | 1.18 (0.90, 1.56) | 0.231 |
| Base+BNP | 1.00 (0.88, 1.15) | 0.963 | 1.13 (0.86, 1.49) | 0.377 |
| Base+GFR | 1.01 (0.88, 1.16) | 0.867 | 1.15 (0.87, 1.52) | 0.331 |
| Base+antihypertensives | 0.92 (0.80, 1.06) | 0.230 | 0.97 (0.73, 1.29) | 0.828 |
| EF <40% (N=24) | ||||
| Age, sex, BMI (base) | 1.18 (0.80, 1.74) | 0.394 | 1.00 (0.42, 2.41) | 0.992 |
| Base+NT‐proANP | 1.20 (0.81, 1.77) | 0.358 | 1.15 (0.46, 2.89) | 0.765 |
| Base+ANP | 1.39 (0.93, 2.10) | 0.113 | 1.48 (0.58, 3.75) | 0.408 |
| Base+NT‐proBNP | 1.02 (0.65, 1.58) | 0.947 | 1.01 (0.36, 2.83) | 0.992 |
| Base+BNP | 1.34 (0.89, 2.02) | 0.168 | 1.57 (0.61, 4.03) | 0.347 |
| Base+GFR | 1.13 (0.76, 1.67) | 0.556 | 0.93 (0.38, 2.27) | 0.865 |
| Base+antihypertensives | 1.04 (0.71, 1.53) | 0.835 | 0.80 (0.33, 1.96) | 0.630 |
Results of logistic regression analysis, covariates taken from Visit 1. ANP indicates atrial natriuretic peptide; BMI, body mass index; BNP, B type natriuretic peptide; c‐LVH, concentric left ventricular hypertrophy; DDF, diastolic dysfunction; EF, ejection fraction; GFR, glomerular filtration rate; HF, heart failure; NT‐proANP, N‐terminus of pro‐atrial natriuretic peptide; NT‐proBNP, N‐terminus of pro‐B type natriuretic peptide; OR, odds ratio.
Data from our previous study.10
Table 10.
Logistic Regression Analysis for Aldosterone at Visit 1 and Cardiac Structure and Function at Visit 2
| Outcome (Total N/New Event N) | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| New HF (N=1125/12) | ||||
| Age, sex, BMI (base) | 1.03 (0.60, 1.77) | 0.920 | 1.83 (0.57, 5.84) | 0.308 |
| Base+NT‐proANP | 1.13 (0.65, 1.98) | 0.658 | 2.40 (0.69, 8.32) | 0.168 |
| Base+ANP | 1.12 (0.64, 1.96) | 0.698 | 2.33 (0.67, 8.03) | 0.182 |
| Base+NT‐proBNP | 1.07 (0.59, 1.93) | 0.830 | 2.53 (0.71, 8.95) | 0.150 |
| Base+BNP | 1.22 (0.68, 2.18) | 0.512 | 2.91 (0.85, 9.99) | 0.090 |
| Base+GFR | 1.05 (0.60, 1.82) | 0.866 | 1.90 (0.59, 6.09) | 0.282 |
| New c‐LVH (N=775/46) | ||||
| Age, sex, BMI (base) | 1.10 (0.81, 1.49) | 0.540 | 1.05 (0.54, 2.02) | 0.892 |
| Base+NT‐proANP | 1.13 (0.83, 1.54) | 0.433 | 1.12 (0.58, 2.18) | 0.740 |
| Base+ANP | 1.10 (0.81, 1.50) | 0.530 | 1.03 (0.54, 2.00) | 0.920 |
| Base+NT‐proBNP | 1.09 (0.80, 1.49) | 0.578 | 1.08 (0.55, 2.10) | 0.830 |
| Base+BNP | 1.15 (0.85, 1.57) | 0.368 | 1.15 (0.59, 2.22) | 0.684 |
| Base+antihypertensives | 1.04 (0.76, 1.42) | 0.796 | 0.95 (0.48, 1.87) | 0.876 |
| Base+GFR | 1.14 (0.83, 1.55) | 0.415 | 1.09 (0.56, 2.11) | 0.804 |
| New DDF (mild/moderate/severe) (N=790/262) | ||||
| Age, sex, BMI (base) | 1.15 (0.95, 1.39) | 0.545 | 1.25 (0.84, 1.86) | 0.781 |
| Base+NT‐proANP | 1.09 (0.90, 1.33) | 0.550 | 1.12 (0.75, 1.68) | 0.969 |
| Base+ANP | 1.15 (0.94, 1.39) | 0.384 | 1.22 (0.82, 1.83) | 0.654 |
| Base+NT‐proBNP | 1.13 (0.93, 1.38) | 0.569 | 1.28 (0.85, 1.92) | 0.745 |
| Base+BNP | 1.15 (0.95, 1.39) | 0.537 | 1.25 (0.84, 1.86) | 0.775 |
| Base+antihypertensives | 1.12 (0.91, 1.38) | 0.630 | 1.20 (0.78, 1.84) | 0.553 |
| Base+GFR | 1.10 (0.90, 1.34) | 0.542 | 1.17 (0.78, 1.76) | 0.834 |
| New EF <40% (N=1132/10) | ||||
| Age, sex, BMI (base) | 1.13 (0.63, 2.01) | 0.691 | 1.77 (0.50, 6.25) | 0.374 |
| Base+NT‐proANP | 1.13 (0.63, 2.02) | 0.683 | 1.80 (0.51, 6.39) | 0.361 |
| Base+ANP | 1.14 (0.64, 2.06) | 0.654 | 1.88 (0.53, 6.71) | 0.332 |
| Base+NT‐proBNP | 1.18 (0.66, 2.13) | 0.581 | 1.94 (0.54, 6.98) | 0.308 |
| Base+BNP | 1.21 (0.67, 2.20) | 0.524 | 2.06 (0.57, 7.39) | 0.268 |
| Base+antihypertensives | 0.97 (0.54, 1.74) | 0.908 | 1.39 (0.38, 5.09) | 0.618 |
| Base+GFR | 1.13 (0.63, 2.03) | 0.678 | 1.80 (0.51, 6.37) | 0.364 |
Results of logistic regression analysis, covariates taken from Visit 1. ANP indicates atrial natriuretic peptide; BMI, body mass index; BNP, B type natriuretic peptide; c‐LVH, concentric left ventricular hypertrophy; DDF, diastolic dysfunction; EF, ejection fraction; GFR, glomerular filtration rate; HF, chronic heart failure; NT‐proANP, N‐terminus of pro‐atrial natriuretic peptide; NT‐proBNP, N‐terminus of pro‐B type natriuretic peptide; OR, odds ratio.
Table 11.
Logistic Regression Analysis for Aldosterone at Visit 2 and Cardiac Structure and Function at Visit 2 (N=1368)
| Outcome | OR (95% CI) Per 1 SD Increase in log Aldo | P Value | OR (95% CI) Top Tertile vs Bottom 2 Tertiles of Aldo | P Value |
|---|---|---|---|---|
| HF (N=30) | ||||
| Age, sex, BMI (base) | 1.11 (0.78, 1.59) | 0.559 | 1.15 (0.53, 2.48) | 0.722 |
| Base+NT‐proBNP | 1.15 (0.78, 1.69) | 0.490 | 1.00 (0.43, 2.35) | 0.997 |
| Base+BNP | 1.23 (0.83, 1.82) | 0.311 | 1.11 (0.47, 2.61) | 0.807 |
| Base+antihypertensives | NA | |||
| Base+GFR | 1.01 (0.70, 1.48) | 0.939 | 0.97 (0.44, 2.16) | 0.947 |
| c‐LVH (N=111) | ||||
| Age, sex, BMI (base) | 1.22 (1.00, 1.50) | 0.053 | 1.24 (0.81, 1.91) | 0.327 |
| Base+NT‐proBNP | 1.22 (0.99, 1.50) | 0.059 | 1.22 (0.80, 1.88) | 0.358 |
| Base+BNP | 1.27 (1.03, 1.57) | 0.029 | 1.35 (0.87, 2.10) | 0.180 |
| Base+antihypertensives | 1.16 (0.94, 1.43) | 0.171 | 1.18 (0.76, 1.83) | 0.466 |
| Base+GFR | 1.20 (0.97, 1.49) | 0.095 | 1.16 (0.74, 1.81) | 0.510 |
| DDF (mild/moderate/severe) (N=565) | ||||
| Age, sex, BMI (base) | 1.05 (0.93, 1.19) | 0.439 | 1.12 (0.87, 1.45) | 0.380 |
| Base+NT‐proBNP | 1.06 (0.94, 1.20) | 0.359 | 1.13 (0.87, 1.47) | 0.350 |
| Base+BNP | 1.05 (0.92, 1.19) | 0.469 | 1.12 (0.86, 1.45) | 0.410 |
| Base+antihypertensives | 1.01 (0.89, 1.15) | 0.896 | 1.07 (0.82, 1.40) | 0.621 |
| Base+GFR | 1.05 (0.93, 1.19) | 0.435 | 1.13 (0.87, 1.46) | 0.377 |
| EF <40% (N=19) | ||||
| Age, sex, BMI (base) | 1.18 (0.75, 1.83) | 0.478 | 1.60 (0.63, 4.06) | 0.325 |
| Base+NT‐proBNP | 1.19 (0.73, 1.95) | 0.486 | 1.34 (0.47, 3.86) | 0.587 |
| Base+BNP | 1.39 (0.85, 2.28) | 0.190 | 1.92 (0.68, 5.42) | 0.217 |
| Base+antihypertensives | 1.04 (0.67, 1.63) | 0.847 | 1.35 (0.52, 3.45) | 0.537 |
| Base+GFR | 0.97 (0.61, 1.54) | 0.896 | 1.23 (0.47, 3.19) | 0.674 |
Results of logistic regression analysis, covariates taken from Visit 2. BMI indicates body mass index; BNP, B type natriuretic peptide; c‐LVH, concentric left ventricular hypertrophy; DDF, diastolic dysfunction; EF, ejection fraction; GFR, glomerular filtration rate; HF, chronic heart failure; NA, not applicable; NT‐proBNP, N‐terminus of pro‐B type natriuretic peptide; OR, odds ratio.
Discussion
In the current study, we confirm and extend previous reports that plasma aldosterone is a predictor of future HTN, central obesity, T2DM, and use of lipid‐lowering drugs, as demonstrated in our 4‐year follow‐up. Further, after considering only those subjects with aldosterone levels within the normal range, the prediction for new onset disease and use of lipid‐lowering drugs was preserved. Finally, consistent with our previous report,10 we replicated and confirmed the strong association between plasma aldosterone measured at Visit 2, 4 years after the first visit, and HTN, obesity, and CKD. However, contrary to our hypothesis, aldosterone was not associated with nor aldosterone predicted HF, cLVH, and functional myocardial abnormalities in our sample of the general community either at Visit 1 or 4 years later at Visit 2.
Although it has been well established that aldosterone can contribute to cardiovascular, renal, and metabolic disease, only recently has circulating aldosterone been strongly associated with CKD and the MetS in addition to HTN in the general population, even within normal values.10 The predictive value of plasma aldosterone for HTN is supported by the role that this mineralocorticoid hormone plays through its key renal receptor mediating both water and sodium retention. However, beyond salt and water regulation, aldosterone may directly affect the vasculature inducing vasoconstriction and remodeling through the MR expressed in endothelial and vascular smooth muscle cells.19 Importantly, not only aldosterone at baseline, but also its increase between Visits was associated with new onset HTN.
Our findings that plasma aldosterone levels were associated with new HTN, central obesity as well as the use of lipid‐lowering drugs are consistent with previous studies that reported higher aldosterone levels in the MetS and obesity.20, 21 Key studies have reported that visceral white adipose tissue possesses its own renin‐angiotensin‐aldosterone system (RAAS) allowing for a local production of aldosterone.22 In addition, aldosterone may induce adipocyte activation and lipogenesis increasing the amount of adipose tissue.23, 24 Importantly, a high dietary fat intake has been associated with an up‐regulation of the MR expression in the kidney,5 and spironolactone, a MR antagonist, reduced white fat inflammation, and induced a transformation towards brown adipose tissue in high‐fat‐diet fed mice.25 Thus, increased adipose tissue is associated with higher aldosterone levels and MR expression, which in turn can contribute to the maintenance of dysfunctional adipose tissue.
We also report for the first time that aldosterone, even within the normal range, can predict new onset T2DM in the general community. It should be noted that hypertensive subjects with primary aldosteronism have a higher prevalence of diabetes mellitus compared to essential hypertensive patients,26 which strengthens our study in the general population. Further, it is known that MR activation can affect insulin sensitivity at the peripheral tissue level by targeting different intracellular pathways such as the serum/glucocorticoid regulated kinase 1 (SGK1) pathway, or the mitogen‐activated protein kinase (MAPK)‐mediated pathway.27 Aldosterone may also directly induce inflammation at the pancreatic β‐cell level, compromising insulin secretion.28 Notably, treatment with MR antagonism‐induced renoprotection in diabetic patients.29 Given that T2DM is one of the major causes of morbidity and mortality worldwide,30 and to date, no biomarkers have been found to be routinely applicable and have a clinical impact in the prediction of diabetes in addition to classical risk factors,31 further studies are warranted to investigate the potential role of aldosterone in contributing to the pathogenesis and prediction of T2DM in the general community.
Importantly, we confirmed at Visit 2 our previous associations10 between aldosterone and cardiovascular, renal, and metabolic disease in the general community. These associations remained statistically significant even after considering in the analysis only subjects with plasma aldosterone within the normal range, thus reinforcing the concept of aldosterone as a mediator of cardiometabolic disease. To date there has been no such population study of a second aldosterone determination and if key associations are sustained.
In our sample of the general community, we did not find that aldosterone was associated with or predictive of HF. This lack of relationship may, however, be limited by the low number of subjects affected by this condition as well as the relatively short time interval between visits. Further, with the use of echocardiography, we did not find a relation between aldosterone and reduced EF, cLVH, and DDF. The last finding is in agreement with the recent study by Catena et al, which reported that plasma aldosterone has no independent relationship with LV diastolic function in treatment‐naïve hypertensive subjects free of comorbidities.32 Despite the absence of statistically significant associations at Visit 1 and Visit 2, the positive trend present between aldosterone and HF as well as cLVH supports further investigations in other cohorts including larger ones, particularly given the clinical impact that MR antagonists have in the treatment of cardiovascular diseases such as HF and HTN.
The current study has several strengths. First, our cohort consisted of a large number of randomly selected subjects and not volunteers (ie, over 1300). Secondly, our subjects were well characterized with a second visit after 4 years, which included a second measurement of aldosterone level and extensive phenotyping also with echocardiography. Third, our cohort consisted of 45 years old and older subjects, constituting a sample of individuals at high‐risk for developing cardiovascular, renal, and metabolic disease in the general community. This study also has limitations. Specifically, we did not measure plasma renin activity or cortisol levels. We do not have available the individual sodium intake in our population, but recent studies have reported that the average dietary sodium intake in Minnesotans is unchanged over the past 2 decades and still exceeds the recommended upper limit of 2300 mg/day.33 Further, about 90% of subjects in our cohort were Caucasian, 4% were Asian, 3% African‐American, and 3% Hispanic.34 Thus our findings may not be completely relevant to other ethnic groups other than Caucasians, although Musani et al have recently reported that higher aldosterone level predicts incident MetS in African‐Americans.35 Finally, we were limited in some of the discussed inferences due to small number of events.
In conclusion, we confirm and extend previous findings that support the concept that aldosterone, even within normal range, may be a mediator of cardiovascular, renal, and metabolic disease in the general community. The MR activation may be a pathway to be targeted before pathological conditions occur, raising clinically significant implications. These clinical implications relate to the measurement of plasma aldosterone in the general population and to the role that antagonizing this hormone may have as a therapeutic strategy for the prevention of cardiovascular, renal, and metabolic disease.36 Reducing MR activation through MR antagonists as well as the use of drugs able to suppress aldosterone synthesis, such as particulate guanylyl cyclase activators and related novel analogues,37, 38 may help in preventing new onset cardiovascular, renal, and metabolic disease in high‐risk subjects.
Sources of Funding
This study was supported by 3 grants from the National Institutes of Health: PO1HL76611, RO1AG034676 and NHLBI‐RO1‐55502.
Disclosures
None.
Supporting information
Figure S1. ROC curves for aldosterone at Visit 1 predictor of disease at Visit 2.
Figure S2. ROC curves for the associations between aldosterone at Visit 2 and disease at Visit 2.
(J Am Heart Assoc. 2015;4:e002505 doi: 10.1161/JAHA.115.002505)
Accompanying Figures S1 and S2 are available at http://jaha.ahajournals.org/content/4/12/e002505/suppl/DC1
References
- 1. Messaoudi S, Gravez B, Tarjus A, Pelloux V, Ouvrard‐Pascaud A, Delcayre C, Samuel J, Launay JM, Sierra‐Ramos C, Alvarez de la Rosa D, Clement K, Farman N, Jaisser F. Aldosterone‐specific activation of cardiomyocyte mineralocorticoid receptor in vivo. Hypertension. 2013;61:361–367. [DOI] [PubMed] [Google Scholar]
- 2. Rocha R, Stier CT, Kifor I, Ochoa‐Maya Margarita R, Rennke HG, Williams GH, Adler GK. Aldosterone: a mediator of myocardial necrosis and renal arteriopathy. Endocrinology. 2000;141:3871–3878. [DOI] [PubMed] [Google Scholar]
- 3. Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat‐derived factors. J Am Soc Nephrol. 2006;17:3438–3446. [DOI] [PubMed] [Google Scholar]
- 4. Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, Ihara G, Fujita Y, Ugawa T, Kohno M. Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate‐1 in vascular smooth muscle cells. Hypertension. 2007;50:750–755. [DOI] [PubMed] [Google Scholar]
- 5. Tokuyama H, Wakino S, Hara Y, Washida N, Fujimura K, Hosoya K, Yoshioka K, Hasegawa K, Minakuchi H, Homma K, Hayashi K, Itoh H. Role of mineralocorticoid receptor/Rho/Rho‐kinase pathway in obesity‐related renal injury. Int J Obes (Lond). 2012;36:1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y, Correa JW, Gagnon AM, Gomez‐Sanchez CE, Gomez‐Sanchez EP, Sorisky A, Ooi TC, Ruzicka M, Burns KD, Touyz RM. Adipocytes produce aldosterone through calcineurin‐dependent signaling pathways: implications in diabetes mellitus‐associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. [DOI] [PubMed] [Google Scholar]
- 7. Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. [DOI] [PubMed] [Google Scholar]
- 8. Fox CS, Gona P, Larson MG, Selhub J, Tofler G, Hwang SJ, Meigs JB, Levy D, Wang TJ, Jacques PF, Benjamin EJ, Vasan RS. A multi‐marker approach to predict incident CKD and microalbuminuria. J Am Soc Nephrol. 2010;21:2143–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ingelsson E, Pencina MJ, Tofler GH, Benjamin EJ, Lanier KJ, Jacques PF, Fox CS, Meigs JB, Levy D, Larson MG, Selhub J, D'Agostino RB Sr, Wang TJ, Vasan RS. Multimarker approach to evaluate the incidence of the metabolic syndrome and longitudinal changes in metabolic risk factors: the Framingham Offspring Study. Circulation. 2007;116:984–992. [DOI] [PubMed] [Google Scholar]
- 10. Buglioni A, Cannone V, Cataliotti A, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, Rodeheffer RJ, Dessi‐Fulgheri P, Sarzani R, Burnett JC Jr. Circulating aldosterone and natriuretic peptides in the general community: relationship to cardiorenal and metabolic disease. Hypertension. 2015;65:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKie PM, Cataliotti A, Lahr BD, Martin FL, Redfield MM, Bailey KR, Rodeheffer RJ, Burnett JC Jr. The prognostic value of N‐terminal pro‐B‐type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfucntion in the community. J Am Med Assoc. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 14. Mayes D, Furuyama S, Kem DC, Nugent CA. A radioimmunoassay for plasma aldosterone. J Clin Endocrinol Metab. 1970;30:682–685. [DOI] [PubMed] [Google Scholar]
- 15. Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, Burnett JC Jr. Circulating N‐terminal atrial natriuretic peptide as a marker for symptomless left‐ventricular dysfunction. Lancet. 1993;341:1105–1109. [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 17. Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PWF, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 18. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 19. Schiffrin EL. Effects of aldosterone on the vasculature. Hypertension. 2006;47:312–318. [DOI] [PubMed] [Google Scholar]
- 20. Calhoun DA, Sharma K. The role of aldosteronism in causing obesity‐related cardiovascular risk. Cardiol Clin. 2010;28:517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Briet M, Schiffrin EL. The role of aldosterone in the metabolic syndrome. Curr Hypertens Rep. 2011;13:163–172. [DOI] [PubMed] [Google Scholar]
- 22. Boscaro M, Giacchetti G, Ronconi V. Visceral adipose tissue: emerging role of gluco‐ and mineralocorticoid hormones in the setting of cardiometabolic alterations. Ann N Y Acad Sci. 2012;1264:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarzani R, Salvi F, Dessi‐Fulgheri P, Rappelli A. Renin‐angiotensin system, natriuretic peptides, obesity, metabolic syndrome, and hypertension: an integrated view in humans. J Hypertens. 2008;26:831–843. [DOI] [PubMed] [Google Scholar]
- 24. Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, Fabbri A, Zennaro MC, Feve B. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152:113–125. [DOI] [PubMed] [Google Scholar]
- 25. Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, Carpinelli G, Canese R, Pagotto U, Quarta C, Malorni W, Matarrese P, Marconi M, Fabbri A, Rosano G, Cinti S, Young MJ, Caprio M. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high‐fat‐diet‐fed mice. FASEB J. 2014;28:3745–3757. [DOI] [PubMed] [Google Scholar]
- 26. Reincke M, Fischer E, Gerum S, Merkle K, Schulz S, Pallauf A, Quinkler M, Hanslik G, Lang K, Hahner S, Allolio B, Meisinger C, Holle R, Beuschlein F, Bidlingmaier M, Endres S. Observational study mortality in treated primary aldosteronism: the German Conn's registry. Hypertension. 2012;60:618–624. [DOI] [PubMed] [Google Scholar]
- 27. Luther JM. Effects of aldosterone on insulin sensitivity and secretion. Steroids. 2014;91:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whaley‐Connell A, Johnson MS, Sowers JR. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Prog Cardiovasc Dis. 2010;52:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mehdi UF, Adams‐Huet B, Raskin P, Vega GL, Toto RD. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin‐converting enzyme inhibition in diabetic nephropathy. J Am Soc Nephrol. 2009;20:2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(suppl 1):S3–S8. [DOI] [PubMed] [Google Scholar]
- 31. Herder C, Kowall B, Tabak AG, Rathmann W. The potential of novel biomarkers to improve risk prediction of type 2 diabetes. Diabetologia. 2014;57:16–29. [DOI] [PubMed] [Google Scholar]
- 32. Catena C, Verheyen N, Pilz S, Kraigher‐Krainer E, Tomaschitz A, Sechi LA, Pieske B. Plasma aldosterone and left ventricular diastolic function in treatment‐naive patients with hypertension: tissue‐Doppler imaging study. Hypertension. 2015;65:1231–1237. [DOI] [PubMed] [Google Scholar]
- 33. Meyer KA, Harnack LJ, Luepker RV, Zhou X, Jacobs DR, Steffen LM. Twenty‐two‐year population trends in sodium and potassium consumption: the Minnesota Heart Survey. J Am Heart Assoc. 2013;2:e000478 doi: 10.1161/JAHA.113.000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ III, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Musani SK, Vasan RS, Bidulescu A, Liu J, Xanthakis V, Sims M, Gawalapu RK, Samdarshi TE, Steffes M, Taylor HA, Fox ER. Aldosterone, C‐reactive protein, and plasma B‐type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes Care. 2013;36:3084–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitt B. Mineralocorticoid receptor antagonists for the treatment of hypertension and the metabolic syndrome. Hypertension. 2015;65:41–42. [DOI] [PubMed] [Google Scholar]
- 37. Nakagawa H, Oberwinkler H, Nikolaev VO, Gaßner B, Umbenhauer S, Wagner H, Saito Y, Baba HA, Frantz S, Kuhn M. Atrial natriuretic peptide locally counteracts the deleterious effects of cardiomyocyte mineralocorticoid receptor activation. Circ Heart Fail. 2014;7:814–821. [DOI] [PubMed] [Google Scholar]
- 38. McKie PM, Cataliotti A, Huntley BK, Martin FL, Olson TM, Burnett JC Jr. A human atrial natriuretic peptide gene mutation reveals a novel peptide with enhanced blood pressure‐lowering, renal‐enhancing, and aldosterone‐suppressing actions. J Am Coll Cardiol. 2009;54:1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. ROC curves for aldosterone at Visit 1 predictor of disease at Visit 2.
Figure S2. ROC curves for the associations between aldosterone at Visit 2 and disease at Visit 2.


