Abstract
Background
Intracranial stenosis (ICS) is a major determinant of ischemic stroke in Asians. We determined the clinical significance of different risk factors and the role of ICS in Taiwanese patients with varied distributions of cervicocerebral stenosis.
Methods and Results
Presence of extracranial carotid stenosis (ECS, ≥70%) and ICS (>50%) was examined in 13 539 patients using ultrasonography and magnetic resonance angiography, respectively. Seven hundred thirty‐three patients with non‐ECS/ICS (n=372), isolated ICS (n=112), isolated ECS (n=121), or combined ECS/ICS (CEIS, n=128) were selected. Prevalence of ischemic stroke in each group was compared, and risk factors for stenosis were determined. The area under the receiver operating characteristic curve for each risk factor was calculated. Prevalence of ischemic stroke was highest in patients with CEIS (odds ratio 15.86; P<0.001), followed in decreasing order by those with isolated ICS (odds ratio 7.16; P<0.001), isolated ECS (odds ratio 1.77; P=0.011), and non‐ECS/ICS. Multivariate logistic regression analysis revealed that hypertension, coronary artery disease, and smoking were risk factors for isolated ECS; hypertension, diabetes mellitus, coronary artery disease, and smoking were risk factors for isolated ICS; and diabetes mellitus, coronary artery disease, and smoking were risk factors for CEIS. Smoking, diabetes mellitus, and coronary artery disease were the greatest contributors to CEIS, isolated ICS, and isolated ECS, respectively.
Conclusions
CEIS was associated with higher odds of ischemic stroke compared with isolated ICS and isolated ECS. Smoking and diabetes mellitus, major determinants of CEIS and isolated ICS, should be targeted in therapeutic strategies to reduce the risk of ischemic stroke.
Keywords: atherosclerosis, carotid arteries, risk factors, stenosis, stroke
Subject Categories: Ischemic Stroke, Stenosis, Risk Factors, Ultrasound, Magnetic Resonance Imaging (MRI)
Introduction
Intracranial stenosis (ICS) is a well‐known pathogenic factor for ischemic stroke. Previous studies have reported that the correlation of ICS and ischemic stroke is more prominent in Asians; in contrast, extracranial carotid stenosis (ECS) is more prevalent in whites.1, 2, 3 ICS is thought to be the cause of 33% to 84% of ischemic strokes in Asian populations4 and 8% to 10% of strokes in North America.5, 6 Additionally, the annual stroke recurrence rate in patients with symptomatic ICS may be as high as 10% to 24%, even for patients receiving medical therapy7; on the other hand, the stroke recurrence rate is 7% to 13% in patients with symptomatic ECS who are treated with the appropriate medication.8, 9
Variance in the distribution of cerebral atherosclerosis in different races is thought to be a result of differences in vascular risk factor profiles, lifestyles, and genetic susceptibility.6, 10, 11 Improving our understanding of the risk factors contributing to ICS in Asians with ischemic stroke is critical for the development and implementation of appropriate treatment strategies. While several vascular risk factors have been identified in ICS,6, 12, 13, 14 these risk factors have varied among studies, possibly due to differences in study populations and methodologies. Therefore, in this study, we aimed to investigate the risk factor stratification of ICS in Taiwanese patients with different distributions of cervicocerebral stenosis.
Methods
This retrospective cohort study was carried out within the certified Neurovascular Ultrasound Laboratory at Tri‐Service General Hospital, Taipei, Taiwan. Between January 1, 2006, and December 31, 2012, a total of 13 539 consecutive patients were referred to the Neurovascular Ultrasound Laboratory due to stroke‐related symptoms in 7191 (53.1%), dizziness or syncope in 5205 (38.4%), coronary artery disease (CAD) or high risk for stroke in 842 (6.2%), cancer in 29 (0.2%), and other conditions or symptoms in 272 (2.0%). All patients were screened for carotid stenosis by 3 certified sonographers. Each individual underwent color‐coded duplex ultrasonography of the cervical and retrobulbar vessels with the use of an ATL HDI 5000 ultrasound system (Philips) with an L12‐5 linear 38‐mm transducer. Degree of stenosis was determined according to the laboratory's ultrasound criteria,15 which have been validated to correspond to the degree of stenosis on cerebral angiography measured by using the European Carotid Surgery Trial method,16 along with carotid endarterectomy findings. The entire process for each patient was recorded by using a digital video system, and examination data were reviewed by 2 neurovascular specialists. Written consent was provided by every patient before the examinations. The study was approved by the Institutional Review Board for Human Studies of the Tri‐Service General Hospital (approval No. 2‐101‐05‐030).
Selection of Study Patients With ECS or ICS
Among the 13 539 patients screened in this study, 9408 had <30% stenosis of the extracranial carotid artery (ECA), including the common carotid and internal carotid arteries (ICAs) on both sides, while 353 had ≥70% stenosis of the ECA, including unilateral or bilateral lesions. After exclusion of patients who did not undergo magnetic resonance imaging/magnetic resonance angiography (MRI/MRA), 2606 patients with stenosis of the ECA of <30% and 264 patients with stenosis of the ECA of ≥70% remained. Based on propensity scores that used age, sex, and examination dates as covariates, 528 patients were selected from the 2606 patients with stenosis of the ECA of <30%, with a ratio of 2:1, to match the 264 patients with stenosis of the ECA of ≥70%. All patients received detailed structural surveys for vascular risk factors in the neurovascular examination laboratory. After the exclusion of patients with incomplete data or risk factors that were not associated with atherosclerosis, such as cardiogenic embolism, coagulopathy, dissection, Takayasu disease, cancer, and extracranial‐to‐intracranial bypass, a total of 733 patients (484 with stenosis of the ECA of <30% and 249 with stenosis of the ECA of ≥70%) were recruited for investigation of stenosis of the ICA (Figure 1). Grade of ICS was determined by using brain MRA. All intracranial vessels were evaluated, and severity of ICS was classified as normal to mild stenosis (≤50%) or moderate stenosis to occlusion (>50%), as described in previous reports.15, 17, 18 Consequently, a total of 733 patients were classified into 4 subgroups: non‐ECS/ICS (NEIS; ECS <30% and ICS ≤50%; n=372), isolated ICS (IICS; ECS <30% and ICS >50%; n=112), isolated ECS (IECS; ECS ≥70% and ICS ≤50%; n=121), and combined ECS/ICS (CEIS; ECS ≥70% and ICS >50%; n=128).
Figure 1.
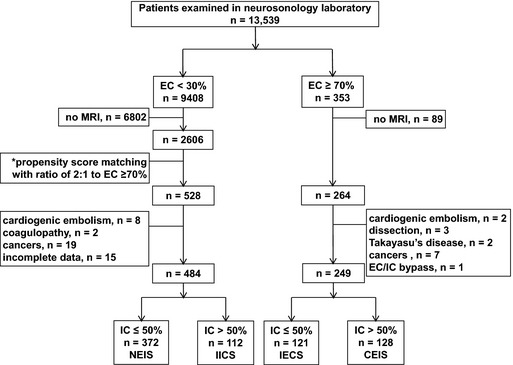
Study patient selection. *Age, sex, and examination dates were used as covariates to compute propensity score. CEIS indicates combined extracranial/intracranial stenosis; EC, extracranial carotid artery; IC, intracranial artery; IECS, isolated extracranial stenosis; IICS, isolated intracranial stenosis; MRI, magnetic resonance imaging; NEIS, nonextracranial/intracranial stenosis.
Demographic Features and Clinical Characteristics
Ischemic stroke was evaluated by using brain MRI. All ischemic stroke types were included except for cardioembolism and nonatherosclerotic etiologies. Acute, recurrent, and old ischemic stroke were defined by using different MRI modalities, including T1/T2‐weighted imaging, diffusion‐weighted imaging, apparent diffusion coefficient, and fluid‐attenuated inversion recovery. Patients were diagnosed as having acute first‐ever stroke, recurrent stroke, or old stroke according to the presence of typical stroke symptoms and positive brain MRI findings. Demographic features and risk factors were recorded using a structured checklist that included hypertension (HTN), diabetes mellitus (DM), CAD, hypercholesterolemia, and hyperuricemia, defined as described previously.17 Smoking habit was defined as being a current smoker or having quit smoking <6 months earlier, while alcohol drinking was classified as either nonheavy (<1 drink per day for women or <2 drinks per day for men) or heavy (>1 drink per day for women or >2 drinks per day for men).
Statistical Analysis
Continuous variables were presented as mean±SD values, while categorical variables were expressed as numbers and percentages. Univariate logistic regression was performed to determine the risk of ischemic stroke in patients with IECS, IICS, and CEIS compared with that in patients with NEIS and to estimate unadjusted odds ratios (ORs) and corresponding 95% CIs. Multivariate logistic regression was performed to identify significant independent risk factors for IECS, IICS, and CEIS compared with NEIS and to estimate ORs and corresponding 95% CIs. Discrimination of significant risk factors for IECS, IICS, and CEIS was assessed by using area under the receiver operating characteristic (ROC) curves (AUC). An ROC curve area of 0.5 indicated no discrimination, whereas an ROC curve area of 1.0 indicated perfect discrimination. Statistical analyses were performed with SPSS version 19 (SPSS). Values were considered statistically significant at P<0.05.
Results
A total of 13 539 patients were screened for ECS in the neurosonology laboratory (Figure 1). Of these patients, 9408 (69.5%) were found to have no stenosis (ECA <30%), whereas 353 (2.6%) had ECS. Patients with ECS had higher rates of concomitant ICS compared with patients without ECS (51.4% [128/249 patients] versus 23.1% [112/484 patients]). According to brain MRA findings in patients with ICS (CEIS, n=128; IICS, n=112), 123 patients (96.1%) with CEIS had ICS in the anterior circulation; 81 (63.3%) had an isolated lesion in the anterior circulation, and 42 (32.8%) had lesions in both the anterior and posterior circulation. Eighty‐three patients (74.1%) with IICS had ICS in the anterior circulation; 46 (41.1%) had an isolated lesion in the anterior circulation, and 37 (33.0%) had lesions in both the anterior and posterior circulation. Patients with CEIS had a significantly higher incidence of ICS in the anterior circulation compared with those with IICS (P<0.001).
Table 1 and Figure 2 show that the prevalence of total ischemic stroke was highest in the CEIS group (95.3%, OR 15.86, 95% CI, 6.81 to 36.91, P<0.001), followed in decreasing order by the IICS (90.2%, OR 7.16, 95% CI, 3.72 to 13.79, P<0.001), IECS (69.4%, OR 1.77, 95% CI, 1.14 to 2.74, P=0.011), and NEIS (56.2%, reference) groups.
Table 1.
Odds Ratios of Ischemic Stroke in Patients With Different Distributions of Cervicocerebral Stenosis
| NEIS (n=372) | IECS (n=121) | OR (95% CI) | P Value | IICS (n=112) | OR (95% CI) | P Value | CEIS (n=128) | OR (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ischemic stroke | 209 (56.2) | 84 (69.4) | 1.77 (1.14–2.74) | 0.011 | 101 (90.2) | 7.16 (3.72–13.79) | <0.001 | 122 (95.3) | 15.86 (6.81–36.91) | <0.001 |
| Acute, first‐ever | 113 (30.4) | 29 (24.0) | 0.72 (0.45–1.16) | 0.177 | 59 (52.7) | 2.55 (1.66–3.93) | <0.001 | 64 (50.0) | 2.29 (1.52–3.46) | <0.001 |
| Recurrent | 53 (14.2) | 12 (9.9) | 0.66 (0.34–1.29) | 0.224 | 26 (23.2) | 1.82 (1.08–3.08) | 0.026 | 31 (24.2) | 1.92 (1.17–3.17) | 0.010 |
| Old | 43 (11.6) | 43 (35.5) | 4.22 (2.59–6.88) | <0.001 | 16 (14.3) | 1.28 (0.69–2.36) | 0.440 | 27 (21.1) | 2.05 (1.20–3.48) | 0.008 |
Values are expressed as n (%). Statistically significant differences were determined using univariate logistic regression. CEIS indicates combined extracranial/intracranial stenosis; IECS, isolated extracranial stenosis; IICS, isolated intracranial stenosis; NEIS, nonextracranial/intracranial stenosis, OR odds ratio.
Figure 2.
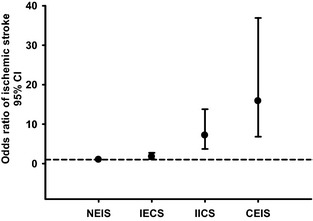
Odds ratio of ischemic stroke in IECS, IICS, and CEIS groups. The odds ratio of ischemic stroke was highest in the CEIS group (OR 15.86, 95% CI 6.81 to 36.91), followed in decreasing order by the IICS (OR 7.16, 95% CI 3.72 to 13.79), IECS (OR 1.77, 95% CI 1.14 to 2.74), and NEIS (reference) groups. CEIS indicates combined extracranial/intracranial stenosis; IECS, isolated extracranial stenosis; IICS, isolated intracranial stenosis; NEIS, nonextracranial/intracranial stenosis, OR odds ratio.
Risk factors for different distributions of cervicocerebral stenosis are shown in Table 2. Multivariate logistic regression analysis revealed that CAD (OR 3.32, 95% CI, 2.01 to 5.51, P<0.001), HTN (OR 2.40, 95% CI, 1.34 to 4.29, P=0.003), and smoking (OR 1.75, 95% CI, 1.05 to 2.92, P=0.033) were significant risk factors for IECS. DM (OR 2.50, 95% CI, 1.59 to 3.96, P<0.001), smoking (OR 2.26, 95% CI, 1.34 to 3.82, P=0.002), HTN (OR 2.21, 95% CI, 1.24 to 3.94, P=0.007), and CAD (OR 2.03, 95% CI, 1.14 to 3.60, P=0.016) were significant risk factors for IICS. Smoking (OR 4.41, 95% CI, 2.70 to 7.22, P<0.001), CAD (OR 3.30, 95% CI, 1.92 to 5.69, P<0.001), and DM (OR 1.77, 95% CI, 1.10 to 2.83, P=0.018) were significant risk factors for CEIS.
Table 2.
Multivariate Logistic Regression Analysis for Vascular Risk Factors in Patients With Different Distributions of Cervicocerebral Stenosis
| NEIS (n=372) | IECS (n=121) | aOR (95% CI) | P Value | IICS (n=112) | aOR (95% CI) | P Value | CEIS (n=128) | aOR (95% CI) | P Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 301 (80.9) | 97 (80.2) | 88 (78.6) | 99 (77.3) | ||||||
| Age, mean±SD, y | 71.1±13.4 | 73.1±11.3 | 72.4±11.6 | 70.9±11.6 | ||||||
| HTN | 253 (68.0) | 103 (85.1) | 2.40 (1.34–4.29) | 0.003 | 94 (83.9) | 2.21 (1.24–3.94) | 0.007 | 99 (77.3) | 1.44 (0.85–2.43) | 0.176 |
| DM | 98 (26.3) | 36 (29.8) | 1.03 (0.63–1.68) | 0.912 | 56 (50.0) | 2.50 (1.59–3.96) | <0.001 | 49 (38.3) | 1.77 (1.10–2.83) | 0.018 |
| CAD | 47 (12.6) | 44 (36.4) | 3.32 (2.01–5.51) | <0.001 | 25 (22.3) | 2.03 (1.14–3.60) | 0.016 | 39 (30.5) | 3.30 (1.92–5.69) | <0.001 |
| H/C | 106 (28.5) | 51 (42.1) | 1.51 (0.95–2.39) | 0.080 | 32 (28.6) | 0.80 (0.48–1.33) | 0.392 | 46 (35.9) | 1.09 (0.68–1.76) | 0.714 |
| Hyperuricemia | 41 (11.0) | 27 (22.3) | 1.65 (0.92–2.96) | 0.093 | 8 (7.1) | 0.48 (0.21–1.12) | 0.089 | 29 (22.7) | 1.74 (0.96–3.16) | 0.070 |
| Smoking | 84 (22.6) | 40 (33.1) | 1.75 (1.05–2.92) | 0.033 | 40 (35.7) | 2.26 (1.34–3.82) | 0.002 | 69 (53.9) | 4.41 (2.70–7.22) | <0.001 |
| Alcohol drinking | ||||||||||
| Nonheavy | 35 (9.4) | 22 (18.2) | 1.73 (0.90–3.34) | 0.102 | 15 (13.4) | 1.06 (0.51–2.22) | 0.872 | 29 (22.7) | 1.45 (0.77–2.73) | 0.245 |
| Heavy | 20 (5.4) | 3 (2.5) | 0.50 (0.14–1.81) | 0.291 | 5 (4.5) | 0.66 (0.23–1.95) | 0.455 | 8 (6.3) | 0.90 (0.36–2.25) | 0.820 |
Values are expressed as n (%) unless otherwise noted. Statistically significant differences were determined using multivariate logistic regression after adjusting for other factors. aOR indicates adjusted odds ratio; CAD, coronary artery disease; CEIS, combined extracranial/intracranial stenosis; DM, diabetes mellitus; H/C: hypercholesterolemia; HTN, hypertension; IECS, isolated extracranial stenosis; IICS, isolated intracranial stenosis; NEIS, nonextracranial/intracranial stenosis.
Figure 3 shows ROC curves demonstrating the sensitivity and specificity of different risk factors with respect to prediction of IECS, IICS, and CEIS. For prediction of IECS, the AUCs of CAD, HTN, and smoking were 0.619, 0.586, and 0.552, respectively. DM was the best factor for predicting IICS, with an AUC of 0.618, followed by HTN, smoking, and CAD (AUCs of 0.580, 0.566, and 0.548, respectively). Smoking was the best factor for predicting CEIS, with an AUC of 0.657, followed by CAD and DM (AUCs of 0.589 and 0.560, respectively).
Figure 3.

Receiver operating characteristic curve, demonstrating the associations of risk factors and prediction of IECS, IICS, and CEIS. A, CAD was the most predictive factor (highest AUC value) for IECS, followed in decreasing order by HTN and smoking. B, DM was the most predictive factor for IICS, followed in decreasing order by HTN, smoking, and CAD. C, Smoking was the most predictive factor for CEIS, followed in decreasing order by CAD and DM. AUC indicates area under the receiver operating characteristic curve; CAD, coronary artery disease; CEIS, combined extracranial/intracranial stenosis; DM, diabetes mellitus; HTN, hypertension; IECS, isolated extracranial stenosis; IICS, isolated intracranial stenosis.
Discussion
Our study demonstrated that CEIS was more strongly associated with odds of ischemic stroke than were IICS and IECS in Taiwanese patients. Patients with IECS had lower odds of ischemic stroke compared with patients with IICS or CEIS. Moreover, while CAD was significantly associated with IECS, DM was the major determinant of IICS. Smoking, being a common risk factor for IECS, IICS, and CEIS, was the most important contributor to CEIS. These data provide important insights into the risk factors associated with ICS in Taiwanese patients.
Angiographic and pathologic evidence has shown that racial and ethnic differences exist in the distribution of cervicocerebral stenosis in patients with ischemic stroke.1 ICS is the most common vascular lesion in Asians with ischemic stroke.2, 3, 4 The estimated prevalence of middle cerebral artery stenosis among asymptomatic Asian patients ranges from 7.2% to 29.6%, while Asian patients with ischemic stroke have a higher ICS incidence of 33% to 84%.4, 19 Further, concurrent atherosclerosis of intracranial and extracranial vessels is not uncommon in Asian populations13, 20, 21, 22; in fact, ≈20% to 50% of patients with ECS are reported to also have ICS.22, 23, 24 In this study, we found that 51.4% of patients with ECS had concurrent ICS. Compared with the IICS and IECS groups, the CEIS group had the highest prevalence of ischemic stroke. Moreover, IICS was more significantly associated with ischemic stroke compared with IECS. All of these findings are in agreement with those of previous studies showing that CEIS is associated with the highest rate of stroke and poor prognoses20 and that ICS has worse prognoses than ECS in Asian populations.2, 10, 11, 12, 14 Recently, one study found a much higher prevalence of ICS in Europeans,25 suggesting that ICS‐related stroke is a major problem worldwide and is likely the most common stroke subtype.
Several risk factors, such as age, HTN, hyperlipidemia, metabolic syndrome, smoking, and DM, are regarded as major contributors to ICS.1, 6, 12, 13, 14 In particular, DM has been identified as a common vascular risk factor for ICS, even in different races.6, 14, 21, 25, 26 After multivariate logistic regression analysis, our study showed that DM was the most important determinant of IICS and an independent risk factor for both IICS and CEIS but not for IECS (Table 2, Figure 3). The mechanisms through which DM contributes preferentially to the pathogenesis of ICS rather than ECS remain unclear. However, the distinct structures between extracranial and intracranial arteries may be involved. Intracranial arteries are muscular‐type arteries with few elastic fibers and greater antioxidant enzyme activity.27 Risk factors for ICS, such as DM, may be associated with reduced antioxidant capacity and increased oxidative stress, resulting in endothelial dysfunction and increased inflammation to accelerate atherogenesis.27 However, further research is needed to investigate the pathologic mechanisms responsible for the differential effects of DM on ICS and ECS. Given the poor prognoses in patients with ICS,28 particularly in Asian populations, aggressive control of DM is very important for the prevention of IICS‐ and CEIS‐related ischemic stroke.
We demonstrated that smoking was a common risk factor for IECS, IICS, and CEIS (Table 2). Moreover, smoking was the most important contributor to CEIS (Figure 3). A previous study demonstrated that both past and current smoking (passive and active) are associated with carotid atherosclerosis.29 Additionally, there is a significant dose–response relationship between pack‐years of smoking and severity of stenosis.30 Given the high smoking rates in Asian countries,31 immediate action is needed to increase public and political awareness of this issue for better tobacco control.
Notably, CAD was found to be significantly associated with IECS, IICS, and CEIS and was the best predictor for IECS. CAD is thought to be associated with the same pathologic mechanism of atherosclerosis as that seen in cervicocerebral vessel stenosis. Previous studies have shown that the degree of carotid stenosis is significantly related to the extent of CAD.32, 33 Although our study showed that the association between IECS and ischemic stroke was far less significant than that between IICS or CEIS and ischemic stroke, the significance of IECS in clinical practice is still relevant. Based on our results, which showed a close correlation between CAD and IECS, patients with ECS should undergo detailed examinations for presence of CAD to facilitate early diagnosis and treatment, potentially reducing CAD‐related morbidity and mortality.
This study provided strong evidence in a large patient pool (n=13 539), and all included patients were strictly evaluated by using color‐coded duplex ultrasonography and brain MRI/MRA. Additionally, we enrolled 4 distinct patient groups: CEIS, IICS, IECS, and NEIS. This allowed for clear identification of the risk factors for each distribution of atherosclerosis. However, when interpreting data from an institutional series, the limitations of a retrospective analysis, such as patient selection biases as well as lack of a temporal relationship and adequate outcome assessment, must be considered. We used propensity scores with age and sex as covariates to match the patients. Therefore, the impact of age and sex on distribution of atherosclerosis could not be evaluated. Most of the patients referred for color‐coded duplex ultrasonography were symptomatic suspect of relating to a vascular lesion, which may reflect the high prevalence of ischemic stroke in our population. Moreover, patients who did not undergo an MRI/MRA study were excluded, which could influence the results regarding prevalence of ICS and ischemic stroke. Meanwhile, it is possible that some patients experienced a stroke after ECS or ICS, and then a new lesion developed after the stroke. However, duration from onset of stroke to date of examination was not recorded in this study.
Some diseases have been proved to be independent predictors for cardiovascular disorders34, 35 but were not discussed in this study. For example, obstructive sleep apnea and chronic obstructive pulmonary disease are related to a systemic inflammatory condition and are significantly associated with cardiovascular events.34 Dolichocarotids, an anatomic variation of cervical ICA, are also a relative risk factor for stroke.35 Some medications have been linked to risk control against atherosclerotic progression. Therefore, history of medication use, including drug types and treatment durations, may be an influential factor for the varied distribution of cervicocranial vessel lesions. Future clinical trials are warranted to test the benefit of medication against vascular progression and for better clinical outcomes. This study population was hospital based rather than community based; thus, generalization of the results may be limited. In addition, MRA may overestimate stenosis of the major arteries. Conventional digital subtraction angiography is still the gold standard for confirming cervicocerebral stenosis. Finally, embolic disease could not be completely excluded in this study. Embolic occlusion with partial recanalization can angiographically resemble stenosis.
Conclusion
In this study, we found that patients with CEIS had the highest odds of ischemic stroke. Moreover, patients with CEIS or IICS had higher odds of ischemic stroke than those with IECS. The identification of risk factors contributing to the varied distribution of cervicocerebral stenosis in this study may provide important insights into appropriate clinical practices and methods. Patients with newly diagnosed DM may benefit from undergoing advanced neuroimaging or neurosonology tests to screen for ICS. Smoking, which was a common risk factor for CEIS, IICS, and IECS, should be strictly controlled. Patients with ECS should undergo detailed examinations for the presence of CAD. Early identification of these risk factors and aggressive management are essential for reducing the risk of ischemic stroke and improving outcomes.
Sources of Funding
Tri‐Service General Hospital (TSGH‐C100‐11‐S01‐3, TSGH‐C102‐076, and TSGH‐C103‐085), Ministry of Science and Technology (MOST 103‐2314‐B‐016‐012), and Ministry of National Defense (MAB 101‐57).
Disclosures
None.
Acknowledgments
We thank Li‐Hui Chu, Chin‐Mei Hsiung, and Ya‐Yu Hsu for performing vascular sonography during the study period.
(J Am Heart Assoc.2015;4:e002692 doi: 10.1161/JAHA.115.002692)
References
- 1. Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet. 2014;383:984–998. [DOI] [PubMed] [Google Scholar]
- 2. De Silva DA, Woon FP, Lee MP, Chen CP, Chang HM, Wong MC. South Asian patients with ischemic stroke: intracranial large arteries are the predominant site of disease. Stroke. 2007;38:2592–2594. [DOI] [PubMed] [Google Scholar]
- 3. Moussouttas M, Aguilar L, Fuentes K, Anyanwu B, Manassarians H, Papamitsakis N, Shi Q, Visintainer P. Cerebrovascular disease among patients from the Indian subcontinent. Neurology. 2006;67:894–896. [DOI] [PubMed] [Google Scholar]
- 4. Sung YF, Tsai CL, Lee JT, Chu CM, Hsu CH, Lin CC, Peng GS. Reversal of ophthalmic artery flow and stroke outcomes in Asian patients with acute ischemic stroke and unilateral severe cervical carotid stenosis. PLoS One. 2013;8:e80675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. [DOI] [PubMed] [Google Scholar]
- 6. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race‐ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke. 1995;26:14–20. [DOI] [PubMed] [Google Scholar]
- 7. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35:1388–1392. [DOI] [PubMed] [Google Scholar]
- 8. Collaborators NASCET . Beneficial effect of carotid endarterectomy in symptomatic patients with high‐grade carotid stenosis. N Engl J Med. 1991;325:445–453. [DOI] [PubMed] [Google Scholar]
- 9. Group ECSTC . Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 10. Nishimaru K, McHenry LC Jr, Toole JF. Cerebral angiographic and clinical differences in carotid system transient ischemic attacks between American Caucasian and Japanese patients. Stroke. 1984;15:56–59. [DOI] [PubMed] [Google Scholar]
- 11. Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, Caplan LR. Chinese‐White differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40:1541–1545. [DOI] [PubMed] [Google Scholar]
- 12. Park KY, Chung CS, Lee KH, Kim GM, Kim YB, Oh K. Prevalence and risk factors of intracranial atherosclerosis in an asymptomatic Korean population. J Clin Neurol. 2006;2:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suwanwela NC, Chutinetr A. Risk factors for atherosclerosis of cervicocerebral arteries: intracranial versus extracranial. Neuroepidemiology. 2003;22:37–40. [DOI] [PubMed] [Google Scholar]
- 14. Leung SY, Ng TH, Yuen ST, Lauder IJ, Ho FC. Pattern of cerebral atherosclerosis in Hong Kong Chinese. Severity in intracranial and extracranial vessels. Stroke. 1993;24:779–786. [DOI] [PubMed] [Google Scholar]
- 15. Peng GS, Lee CC, Hsu CH, Lee JT, Lee FY, Tsao WL. Diagnostic assessment of carotid stenosis: comparison of color duplex ultrasonography with magnetic resonance angiography, cerebral angiography and carotid endarterectomy. J Med Ultrasound. 1996;4:174–179. [Google Scholar]
- 16. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70‐99%) or with mild (0‐29%) carotid stenosis. European Carotid Surgery Trialists’ Collaborative Group. Lancet. 1991;337:1235–1243. [PubMed] [Google Scholar]
- 17. Peng GS, Yin SJ, Cheng CA, Chiu SW, Lee JT, Lin WW, Lin JC, Hsu YD. Increased risk of cerebral hemorrhage in Chinese male heavy drinkers with mild liver disorder. Cerebrovasc Dis. 2007;23:309–314. [DOI] [PubMed] [Google Scholar]
- 18. Yao CT, Cheng CA, Wang HK, Chiu SW, Chen YC, Wang MF, Yin SJ, Peng GS. The role of ALDH2 and ADH1B polymorphism in alcohol consumption and stroke in Han Chinese. Hum Genomics. 2011;5:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wong KS, Ng PW, Tang A, Liu R, Yeung V, Tomlinson B. Prevalence of asymptomatic intracranial atherosclerosis in high‐risk patients. Neurology. 2007;68:2035–2038. [DOI] [PubMed] [Google Scholar]
- 20. Man BL, Fu YP, Chan YY, Lam W, Hui CF, Leung WH, Wong KS. Use of magnetic resonance angiography to predict long‐term outcomes of ischemic stroke patients with concurrent stenoses in Hong Kong. Cerebrovasc Dis. 2009;28:112–118. [DOI] [PubMed] [Google Scholar]
- 21. Lee SJ, Cho SJ, Moon HS, Shon YM, Lee KH, Kim DI, Lee BB, Byun HS, Han SH, Chung CS. Combined extracranial and intracranial atherosclerosis in Korean patients. Arch Neurol. 2003;60:1561–1564. [DOI] [PubMed] [Google Scholar]
- 22. Liu HM, Tu YK, Yip PK, Su CT. Evaluation of intracranial and extracranial carotid steno‐occlusive diseases in Taiwan Chinese patients with MR angiography: preliminary experience. Stroke. 1996;27:650–653. [DOI] [PubMed] [Google Scholar]
- 23. Tsai CL, Lee JT, Cheng CA, Liu MT, Chen CY, Hu HH, Peng GS. Reversal of ophthalmic artery flow as a predictor of intracranial hemodynamic compromise: implication for prognosis of severe carotid stenosis. Eur J Neurol. 2013;20:564–570. [DOI] [PubMed] [Google Scholar]
- 24. Kappelle LJ, Eliasziw M, Fox AJ, Sharpe BL, Barnett HJ. Importance of intracranial atherosclerotic disease in patients with symptomatic stenosis of the internal carotid artery. The North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1999;30:282–286. [DOI] [PubMed] [Google Scholar]
- 25. Bos D, van der Rijk MJ, Geeraedts TE, Hofman A, Krestin GP, Witteman JC, van der Lugt A, Ikram MA, Vernooij MW. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke. 2012;43:1878–1884. [DOI] [PubMed] [Google Scholar]
- 26. Miyazawa N, Akiyama I, Yamagata Z. Analysis of incidence and risk factors for progression in patients with intracranial steno‐occlusive lesions by serial magnetic resonance angiography. Clin Neurol Neurosurg. 2007;109:680–685. [DOI] [PubMed] [Google Scholar]
- 27. Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130:1407–1414. [DOI] [PubMed] [Google Scholar]
- 28. Marzewski DJ, Furlan AJ, St Louis P, Little JR, Modic MT, Williams G. Intracranial internal carotid artery stenosis: longterm prognosis. Stroke. 1982;13:821–824. [DOI] [PubMed] [Google Scholar]
- 29. Diez‐Roux AV, Nieto FJ, Comstock GW, Howard G, Szklo M. The relationship of active and passive smoking to carotid atherosclerosis 12‐14 years later. Prev Med. 1995;24:48–55. [DOI] [PubMed] [Google Scholar]
- 30. Tell GS, Polak JF, Ward BJ, Kittner SJ, Savage PJ, Robbins J. Relation of smoking with carotid artery wall thickness and stenosis in older adults. The Cardiovascular Health Study. The Cardiovascular Health Study (CHS) Collaborative Research Group. Circulation. 1994;90:2905–2908. [DOI] [PubMed] [Google Scholar]
- 31. Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer‐Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD, Murray CJ, Gakidou E. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. [DOI] [PubMed] [Google Scholar]
- 32. Steinvil A, Sadeh B, Arbel Y, Justo D, Belei A, Borenstein N, Banai S, Halkin A. Prevalence and predictors of concomitant carotid and coronary artery atherosclerotic disease. J Am Coll Cardiol. 2011;57:779–783. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka H, Nishino M, Ishida M, Fukunaga R, Sueyoshi K. Progression of carotid atherosclerosis in Japanese patients with coronary artery disease. Stroke. 1992;23:946–951. [DOI] [PubMed] [Google Scholar]
- 34. Durgan DJ, Bryan RM Jr. Cerebrovascular consequences of obstructive sleep apnea. J Am Heart Assoc. 2012;1:e000091 doi: 10.1161/JAHA.111.000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ciccone MM, Sharma RK, Scicchitano P, Cortese F, Salerno C, Berchialla P, Frasso G, Sassara M, Carbone M, Palmiero P, Maiello M, Voelker DJ, Reddy HK, Federico F. Dolichocarotids: echo‐color Doppler evaluation and clinical role. J Atheroscler Thromb. 2014;21:56–63. [DOI] [PubMed] [Google Scholar]


