Abstract
Background
Multiple scores have been proposed to stratify bleeding risk, but their value to guide dual antiplatelet therapy duration has never been appraised. We compared the performance of the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines), ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) scores in 1946 patients recruited in the Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study (PRODIGY) and assessed hemorrhagic and ischemic events in the 24‐ and 6‐month dual antiplatelet therapy groups.
Methods and Results
Bleeding score performance was assessed with a Cox regression model and C statistics. Discriminative and reclassification power was assessed with net reclassification improvement and integrated discrimination improvement. The C statistic was similar between the CRUSADE score (area under the curve 0.71) and ACUITY (area under the curve 0.68), and higher than HAS−BLED (area under the curve 0.63). CRUSADE, but not ACUITY, improved reclassification (net reclassification index 0.39, P=0.005) and discrimination (integrated discrimination improvement index 0.0083, P=0.021) of major bleeding compared with HAS‐BLED. Major bleeding and transfusions were higher in the 24‐ versus 6‐month dual antiplatelet therapy groups in patients with a CRUSADE score >40 (hazard ratio for bleeding 2.69, P=0.035; hazard ratio for transfusions 4.65, P=0.009) but not in those with CRUSADE score ≤40 (hazard ratio for bleeding 1.50, P=0.25; hazard ratio for transfusions 1.37, P=0.44), with positive interaction (P int=0.05 and P int=0.01, respectively). The number of patients with high CRUSADE scores needed to treat for harm for major bleeding and transfusion were 17 and 15, respectively, with 24‐month rather than 6‐month dual antiplatelet therapy; corresponding figures in the overall population were 67 and 71, respectively.
Conclusions
Our analysis suggests that the CRUSADE score predicts major bleeding similarly to ACUITY and better than HAS BLED in an all‐comer population with percutaneous coronary intervention and potentially identifies patients at higher risk of hemorrhagic complications when treated with a long‐term dual antiplatelet therapy regimen.
Clinical Trial Registration
URL: http://clinicaltrials.gov. Unique identifier: NCT00611286.
Keywords: ACUITY, bleeding risk score, clopidogrel, CRUSADE, duration of dual antiplatelet therapy, HAS‐BLED
Subject Categories: Pharmacology, Secondary Prevention, Thrombosis
Introduction
Bleeding is a common adverse event after percutaneous coronary intervention and is associated with increased morbidity and mortality.1, 2, 3, 4 Bleeding predictors have been described extensively; they are related mostly to the patient's clinical characteristics, the invasiveness of the procedure, and the potency of the antithrombotic regimen. Antithrombotic therapies after coronary intervention reduce ischemic events but invariably increase bleeding risk, which in turn may adversely affect short‐ and long‐term outcomes.5, 6 International guidelines recommend careful evaluation of both ischemic and bleeding risk based on the patient's clinical characteristics7, 8; however, evidence supporting the individualization of antithrombotic therapy is still limited. In particular, the potency and duration of dual antiplatelet therapy (DAPT) after coronary stenting are currently based mainly on the patient's clinical presentation (ie, acute coronary syndrome or stable coronary artery disease) and the type of stent used (ie, drug‐eluting or bare metal stent), with evanescent indications based on the patient's bleeding risk.7, 8, 9 Many bleeding risk scores have been validated for the prediction of early and late bleeding events, and some have been tested on large cohorts with acute coronary syndrome, demonstrating reasonably good performance.10, 11, 12 Among them, the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA [American College of Cardiology/American Heart Association] Guidelines) score has been validated in 17 857 patients with non–ST‐segment elevation myocardial infarction (MI), and its predictive capability was consistent in terms of hemorrhagic risks in patients taking ≥2 antithrombotic medications.10 Our study sought to compare the predictive performance of the CRUSADE, ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HAS‐BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR [international normalized ratio], Elderly, Drugs/Alcohol Concomitantly) risk scores with respect to major bleeding events in an all‐comer population treated with coronary stent. We also intended to determine the incidence of major bleeding after 24‐month rather than 6‐month DAPT in the subgroups of patients with high and low to intermediate bleeding risk.
Methods
The design and main study findings for the Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study (PRODIGY) were reported previously.5 In brief, all patients receiving a balanced mixture of stents with varying anti–intimal hyperplasia potency and including both first‐ and second‐generation drug‐eluting stents at 3 Italian sites were randomly allocated at 30 days to either 6 or 24 months of DAPT. Selection criteria were broad, reflecting routine clinical practice. Randomization to 6‐ or 24‐month DAPT was performed at 30 days and stratified by center, ongoing ST‐segment elevation MI, presence of diabetes mellitus, and need for intervention on at least 1 in‐stent restenotic lesion. The study was conducted in accordance with the principles of the Declaration of Helsinki. The ethics committees of the 3 participating centers independently approved the protocol, and all participants gave written informed consent.
Treatment Protocol
All patients received aspirin (80–160 mg orally, indefinitely) and clopidogrel (75 mg/day) according to the following randomization scheme: either 6 months in the short DAPT group or 24 months in the prolonged DAPT arm, regardless of the previously implanted stent type or indication for the coronary procedure.
Follow‐up
All randomized patients returned for study visits at 30 days and then every 6 months for up to 2 years. During follow‐up visits, patients were examined and assessed for adverse events and asked about antiplatelet therapy compliance; in addition, 12‐lead ECG recordings were obtained.
Study End Points
The primary objective of this analysis was to compare the predictive performance of the CRUSADE, ACUITY, and HAS‐BLED bleeding risk scores with respect to major bleeding events, adjudicated according to Bleeding Academic Research Consortium (BARC) class 3 or 5, among patients recruited to the PRODIGY trial. Further sensitivity analysis evaluated the consistency of the results obtained with the BARC classification with other widely accepted bleeding definitions, including the Thrombolysis in Myocardial Infarction (TIMI) and Global Use of Strategies to Open Occluded Arteries (GUSTO) scales. The CRUSADE, ACUITY, and HAS‐BLED bleeding risk scores were calculated, as reported previously,10, 11, 12 taking into account the following exceptions: For the ACUITY score, given the exclusive use of unfractionated heparin as an anticoagulant in the PRODIGY trial, the “antithrombotic medication” variable was set to zero; for the HAS‐BLED score, given that patients with an indication for long‐term anticoagulation were not included in the PRODIGY trial, the “labile INR” variable was set to zero.
To assess the effect of high bleeding risk status in the 24‐ and 6‐month DAPT treatment arms, we selected the high‐risk cutoff value of 40 for the CRUSADE score, as reported previously.10 The incidence of major bleeding, red blood cell transfusion, and major adverse cardiac events—a composite of all‐cause death, MI, and cerebrovascular accident—was appraised in the subgroup of patients with high CRUSADE scores (HCSs; >40) versus those with low to intermediate scores (≤40) in the 2 DAPT duration arms. All study end point definitions were reported previously,13 confirmed on the basis of documentation collected at each hospital, and centrally adjudicated by the clinical events committee, the members of which were unaware of the patients’ treatment‐group assignments. The time frame of interest for the primary end point was from randomization (ie, 30 days after index procedure) to 24 months.
Statistical Analysis
Categorical variables were expressed as frequency (percentage), whereas continuous variables were expressed as median (interquartile range). Continuous variables were compared between randomized groups using the Wilcoxon rank sum test, whereas for binary variables, the χ2 test was used.
In its original derivation, the CRUSADE score assigned patients to 5 risk strata (very low risk ≤20, low risk 21–30, moderate risk 31–40, high risk 41–50, very high risk >50). The ACUITY score defined 4 risk strata (low risk <10, moderate risk 10–14, high risk 15–19, very high risk ≥20), whereas HAS‐BLED stratified patients into 3 risk strata (low risk <2, intermediate risk 2, high risk >2). For the purpose of this analysis, patients were categorized into 3 bleeding risk strata across all scores by jointly considering the very high risk and high risk (high risk) and low risk and very low risk categories (low risk) as 1 each. A detailed report of the components of each score is presented in Table S1.
The predictive value of CRUSADE, ACUITY, and HAS‐BLED scores was assessed in Cox regression models and with receiver operating characteristics area under the curve (AUC) and category‐free net reclassification improvement and integrated discrimination improvement.14 The calibration of the models was evaluated using the Hosmer–Lemeshow goodness‐of‐fit statistical analysis. Net reclassification improvement and integrated discrimination improvement were calculated by analyzing the differences in patients’ individual estimated probability of experiencing major bleeding events after the addition of the CRUSADE score result to the models containing the aforementioned bleeding risk scores. Net reclassification improvement represents the average weighted improvement in discrimination. Integrated discrimination improvement considers the change in the estimated prediction probabilities as a continuous variable and represents the average improvement in predicted probability.
Estimation of the cumulative incidence of events was performed using the Kaplan–Meier method, and events were compared with the log‐rank test. Absolute risk difference with 95% CI was calculated for long‐term versus short‐term clopidogrel with the Newcombe–Wilson method without continuity correction. The Mantel–Haenszel χ2 test was used to assess the evidence of statistical interaction on an additive scale between randomized DAPT duration and bleeding risk status, according to CRUSADE. The number needed to treat for harm (NNTH) was calculated as 1 divided by the absolute risk difference (ARD). All analyses were performed on the basis of the intention‐to‐treat principle with Review Manager version 5.3 (RevMan; Cochrane Collaboration) and SPSS version 21.0 (IBM Corp).
Results
In the PRODIGY trial, a total of 1970 patients were randomly allocated at 30 days postprocedure to receive clopidogrel therapy for 6 or 24 months. Complete data regarding the 3 bleeding risk scores were available in 1946 patients (98.8%).
The 2‐year cumulative risk of major bleeding and need for red blood cell transfusion was 2.7% and 2.0%, respectively (2.2% and 2.6% for TIMI minor or major and GUSTO moderate to severe bleeding, respectively). Patients randomized to 24‐month DAPT duration, compared with those allocated to 6‐month DAPT, experienced a significant increase in major bleeding (3.5% versus 2.0%, P=0.042; NNTH 66.7) and received blood transfusions more frequently (2.7% versus 1.3%, P=0.041; NNTH 71.4). No bleeding event occurred in the 24 patients for whom bleeding scores were missing.
The median CRUSADE score was 25 (interquartile range 18–35; mean±SD: 26.5±12.8), whereas the median ACUITY and HAS‐BLED scores were 15 (interquartile range 10–21; mean±SD: 15.8±7.9) and 1 (interquartile range 1–2; mean±SD: 1.3±0.7), respectively (Table 1 and Figure 1). By applying previously validated cutoffs, 307 patients (15.8%) based on CRUSADE, 991 patients (50.9%) based on ACUITY, and 55 patients (2.8%) based on HAS‐BLED met the threshold for the high or very high bleeding risk category. Most patients with HCS also satisfied high bleeding risk criteria according to both HAS‐BLED and ACUITY, whereas the vast majority of patients at high bleeding risk according to ACUITY did not reach the same risk category for the other 2 scores (Figure 1).
Table 1.
Baseline Characteristics
| Characteristic | Major Bleeding (n=53) | No Major Bleeding (n=1893) | P Value |
|---|---|---|---|
| Age, y | 76.3 (71.3–81.3) | 68.9 (59.8–76.1) | <0.0001 |
| Female sex | 28.3% (15/53) | 23.1% (438/1893) | 0.38 |
| Body mass index, kg/m2 | 25.4 (24.0–28.7) | 26.6 (24.3–29.4) | 0.30 |
| Diabetes | 34.0% (18/53) | 24.0% (455/1893) | 0.09 |
| Insulin dependent | 7.5% (4/53) | 5.8% (110/1893) | |
| Hypertension | 77.4% (41/53) | 71.7% (1358/1893) | 0.37 |
| Hyperlipidemia | 52.8% (28/53) | 54.9% (1039/1893) | 0.76 |
| Current cigarette use | 13.2% (7/53) | 24.3% (459/1893) | 0.06 |
| Creatinine clearance, mL/min | 49.5 (36.3–65.5) | 75.7 (57.0–96.5) | <0.0001 |
| Prior myocardial infarction | 41.5% (22/53) | 25.9% (491/1893) | 0.01 |
| Prior PCI | 24.2% (13/53) | 18.3% (343/1893) | 0.23 |
| Prior CABG | 5.7% (3/53) | 11.0% (208/1893) | 0.21 |
| LVEF | 45.0 (35.75–55.0) | 52.0 (45–60.0) | 0.001 |
| Clinical presentation | |||
| Stable angina pectoris | 24.5% (13/53) | 25.6% (485/1893) | 0.85 |
| Acute coronary syndrome | 80.6% (40/53) | 74.4% (1408/1893) | |
| Multivessel disease | 79.2% (42/53) | 69.7% (1319/1893) | 0.17 |
| No. of treated lesions | 1 (1–2) | 1 (1–2) | 0.30 |
| ≥2 treated lesions | 35.8% (19/53) | 37.6% (712/1893) | 0.76 |
| ≥3 treated lesions | 7.5% (4/53) | 11.5% (218/1893) | |
| Multivessel intervention | 24.5% (13/53) | 26.9% (509/1893) | 0.70 |
| At least 1 complex lesion (type B2 or C)a | 66.0% (35/53) | 66.0% (1250/1893) | 0.99 |
| Total ACC/AHA scoreb | 3 (2–5) | 3 (2–5) | 0.48 |
| Bleeding risk score | |||
| CRUSADE score | 38 (24–43) | 25 (18–35) | <0.0001 |
| ACUITY score | 20 (14–28) | 15 (9–20) | <0.0001 |
| HAS‐BLED score | 2 (1–2) | 1 (1–2) | <0.0001 |
ACC indicates American College of Cardiology; ACUITY, Acute Catheterization and Urgent Intervention Triage Strategy; AHA, American Heart Association; CABG, coronary artery bypass grafting; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention.
According to the ACC/AHA coronary lesion classification.
Type A stenoses were coded as 1 point, type B1 stenoses were coded as 2 points, type B2 stenoses were coded as 3 points, and type C stenoses were coded as 4 points.
Figure 1.
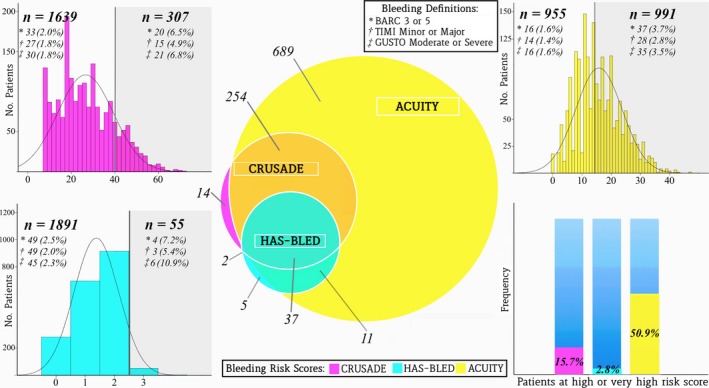
Distribution of bleeding risk scores and major bleeding events in the PRODIGY population. The Venn diagram (center) shows the patients included in the high bleeding risk category by each score. The ACUITY score had broader inclusion in the high‐risk category, whereas CRUSADE and HAS‐BLED were more restrictive (bottom right corner). Bleeding risk score distribution is presented for CRUSADE (top left corner), ACUITY (top right corner), and HAS‐BLED (bottom left corner), with the number of patients with major bleeding in the high‐risk category (gray section) and in the low‐ to intermediate‐risk category according to 3 bleeding definitions. ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; BARC, Bleeding Academic Research Consortium; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; GUSTO, Global Use of Strategies to Open Occluded Arteries; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; PRODIGY, Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study; TIMI, Thrombolysis in Myocardial Infarction.
The patients who bled were older, had reduced renal function and left ventricular ejection fraction, had more frequent history of MI and diabetes mellitus, and underwent left main coronary artery intervention more frequently. All 3 bleeding risk scores were significantly higher for patients with hemorrhagic events at follow‐up compared with those without, consistently across bleeding definitions (Table 1).
Predictive Performance of the Bleeding Risk Scores
The transition from a lower to a higher risk category carried a significant increase in bleeding rates across bleeding risk scores (Table 2). This result was consistent among all explored bleeding definitions (Tables 3 and 4) and in the 6‐ and 24‐month DAPT groups when assessed separately. The ACUITY score best classified patients with major bleeding in the high‐risk group (higher sensitivity), but it was also the least specific, classifying only 25% of patients without events to the low‐risk group. In contrast, the HAS‐BLED score showed the lowest sensitivity, classifying 7.5% of those who eventually bled as high risk. The CRUSADE score provided reasonable sensitivity and specificity, correctly classifying 67% of patients without events in the low‐risk category (Table 5).
Table 2.
Incidence of Major Bleeding Among Bleeding Risk Categories
| Major Bleeding | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|
| All patients | 2.7% (53/1946) | — | — |
| CRUSADE score | |||
| Low (≤30) | 1.4% (18/1282) | Reference | — |
| Intermediate (31–40) | 4.2% (15/357) | 3.10 (1.56–6.15) | 0.001 |
| High (>40) | 6.5% (20/307) | 5.62 (2.99–10.55) | <0.0001 |
| ACUITY score | |||
| Low (<10) | 0.8% (4/480) | Reference | — |
| Intermediate (10–14) | 2.5% (12/475) | 3.08 (0.99–9.54) | 0.052 |
| High (>14) | 3.7% (37/991) | 4.93 (1.76–13.82) | 0.002 |
| HAS‐BLED score | |||
| Low (<2) | 1.5% (15/977) | Reference | — |
| Intermediate (2) | 3.7% (34/914) | 2.56 (1.40–4.69) | 0.002 |
| High (>2) | 7.3% (4/55) | 5.45 (1.81–16.43) | 0.003 |
Each hazard ratio is considered as compared with the reference low bleeding risk category. ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly.
Table 3.
Incidence of TIMI Minor and Major Bleeding Among Bleeding Risk Categories
| Events (n/N) | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|
| All patients | 2.1% (42/1946) | — | — |
| CRUSADE score | |||
| Low (≤30) | 1.2% (16/1282) | Reference | — |
| Intermediate (31–40) | 3.0% (11/357) | 2.50 (1.16–5.39) | 0.019 |
| High (>40) | 4.9% (15/307) | 4.20 (2.08–8.51) | <0.0001 |
| ACUITY score | |||
| Low (<10) | 0.8% (4/480) | Reference | — |
| Intermediate (10–14) | 2.1% (10/475) | 2.56 (0.80–8.17) | 0.112 |
| High (>14) | 2.8% (28/991) | 3.47 (1.22–9.89) | 0.02 |
| HAS‐BLED score | |||
| Low (<2) | 1.3% (13/977) | Reference | — |
| Intermediate (2) | 2.8% (26/914) | 2.17 (1.11–4.22) | 0.023 |
| High (>2) | 5.4% (3/55) | 4.79 (1.36–16.83) | 0.015 |
Each hazard ratio is considered as compared to the reference low bleeding risk category. ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; TIMI, Thrombolysis in Myocardial Infarction.
Table 4.
Incidence of GUSTO Moderate and Severe Bleeding Among Bleeding Risk Categories
| Events (n/N) | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|
| All patients | 2.6% (52/1946) | — | — |
| CRUSADE score | |||
| Low (≤30) | 1.3% (18/1282) | Reference | — |
| Intermediate (31–40) | 3.6% (13/357) | 2.80 (1.36–5.76) | 0.005 |
| High (>40) | 6.8% (21/307) | 5.58 (2.94–10.58) | <0.0001 |
| ACUITY score | |||
| Low (<10) | 0.8% (4/480) | Reference | — |
| Intermediate (10–14) | 2.5% (12/475) | 3.08 (0.99–9.56) | 0.052 |
| High (>14) | 3.5% (35/991) | 4.35 (1.55–12.24) | 0.005 |
| HAS‐BLED score | |||
| Low (<2) | 1.4% (14/977) | Reference | — |
| Intermediate (2) | 3.4% (31/914) | 2.40 (1.28–4.52) | 0.006 |
| High (>2) | 10.9% (6/55) | 9.05 (3.47–23.60) | <0.0001 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; GUSTO, Global Use of Strategies to Open Occluded Arteries; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly.
Table 5.
Risk Classification of Major Bleeding According to the 3 Bleeding Risk Scores
| CRUSADE | ACUITY | HAS‐BLED | CRUSADE vs ACUITY | ACUITY vs HAS‐BLED | CRUSADE vs HAS‐BLED | ||||
|---|---|---|---|---|---|---|---|---|---|
| Difference | P Value | Difference | P Value | Difference | P Value | ||||
| True‐positive ratea | 37.7% (20/53) | 69.8% (37/53) | 7.5% (4/53) | −32.1 | <0.0001 | 62.3 | <0.0001 | 30.2 | <0.0001 |
| False‐positive rateb | 15.2% (287/1893) | 50.3% (954/1893) | 2.7% (51/1893) | −35.1 | <0.0001 | 47.6 | <0.0001 | 12.5 | <0.0001 |
| False‐negative ratec | 34.0% (18/53) | 7.5% (4/53) | 28.3% (15/53) | 26.5 | <0.0001 | −20.8 | <0.0001 | 5.7 | <0.0001 |
| True‐negative rated | 66.7% (1264/1893) | 25.1% (476/1893) | 50.8% (962/1893) | 41.6 | <0.0001 | −25.7 | <0.0001 | 15.9 | <0.0001 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly.
Proportion of events categorized as CRUSADE >40, ACUITY >14, and HAS‐BLED>2.
Proportion of events categorized as CRUSADE >40, ACUITY >14, and HAS‐BLED>2.
Proportion of events categorized as CRUSADE ≤30, ACUITY <10, and HAS‐BLED<2.
Proportion of events categorized as CRUSADE ≤30, ACUITY <10, and HAS‐BLED<2.
At the C statistic analysis, the point estimate of the AUC for the prediction of major bleeding was similar between the CRUSADE risk score (AUC 0.71, 95% CI 0.64–0.77) and ACUITY (AUC 0.68, 95% CI 0.61–0.75) and numerically higher than HAS‐BLED (AUC 0.63, 95% CI 0.56–0.70), as both continuous and 3‐risk categories, although 95% CIs remained partially overlapping among the 3 bleeding scores (Table 6). All 3 risk models were well calibrated according to the Hosmer–Lemershow test for goodness of fit (CRUSADE P=0.27; ACUITY P=0.33; HAS‐BLED P=0.69) (Figure 2). These observations remained consistent when TIMI and GUSTO bleeding definitions were applied (Table 7). CRUSADE, but not ACUITY, successfully reclassified the risk of major bleeding compared with HAS‐BLED, with better discriminatory power. When compared with ACUITY, CRUSADE was not significantly superior on net reclassification improvement and integrated discrimination improvement (Table 8 and Figure 3). These results were largely consistent across different bleeding scales (Table 9). In addition, the bleeding risk scores, especially CRUSADE, showed reasonably good discriminatory capability for ischemic events, including the composite of death, MI, or cerebrovascular accident; for MI alone; and for stent thrombosis alone (Table 10).
Table 6.
ROC: Predictive Performance of Major Bleeding With the 3 Risk Scores Used as Continuous Variables and as 3 Risk Score Categories (Low, Intermediate, and High Risk)
| Major Bleeding | ||
|---|---|---|
| AUC (95% CI) | P Value | |
| CRUSADE score | ||
| Continuous parameter | 0.71 (0.64–0.77) | <0.0001 |
| 3 Categories | 0.68 (0.60–0.75) | <0.0001 |
| ACUITY score | ||
| Continuous parameter | 0.68 (0.61–0.75) | <0.0001 |
| 3 Categories | 0.61 (0.55–0.68) | 0.004 |
| HAS‐BLED score | ||
| Continuous parameter | 0.63 (0.56–0.70) | 0.001 |
| 3 Categories | 0.62 (0.55–0.70) | 0.002 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; AUC, area under the curve; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; ROC, receiver operating characteristics.
Figure 2.
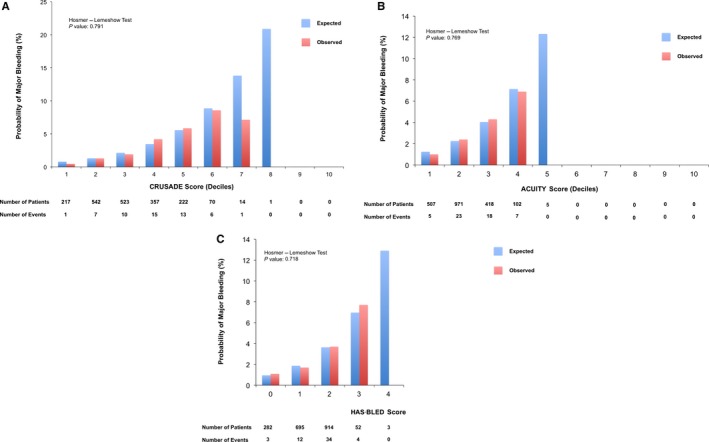
Calibration plots comparing the expected and observed probabilities of major bleeding. A, CRUSADE score. B, ACUITY score. C, HAS‐BLED score. ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly.
Table 7.
ROC: Predictive Performance of TIMI Minor or Major and GUSTO Moderate or Severe Bleeding for the 3 Risk Scores Used as Continuous Variables and as 3 Risk Score Categories (Low, Intermediate, and High Risk)
| TIMI Minor or Major | GUSTO Moderate or Severe | |||
|---|---|---|---|---|
| AUC (95% CI) | P Value | AUC (95% CI) | P Value | |
| CRUSADE score | ||||
| Continuous parameter | 0.68 (0.60–0.76) | <0.0001 | 0.71 (0.63–0.82) | <0.0001 |
| 3 Categories | 0.65 (0.56–0.74) | 0.001 | 0.68 (0.58–0.79) | <0.0001 |
| ACUITY score | ||||
| Continuous parameter | 0.65 (0.57–0.73) | 0.001 | 0.67 (0.58–0.77) | <0.0001 |
| 3 Categories | 0.59 (0.52–0.67) | 0.036 | 0.61 (0.51–0.69) | 0.009 |
| HAS‐BLED score | ||||
| Continuous parameter | 0.62 (0.53–0.69) | 0.010 | 0.65 (0.55–0.73) | <0.0001 |
| 3 Categories | 0.61 (0.52–0.69) | 0.019 | 0.64 (0.53–0.73) | 0.001 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; AUC, area under the curve; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; GUSTO, Global Use of Strategies to Open Occluded Arteries; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; ROC, receiver operating characteristics; TIMI, Thrombolysis in Myocardial Infarction.
Table 8.
Risk Reclassification and Integrated Discriminatory Improvement for Major Bleeding
| Bleeding Correctly Reclassified, P (n1) | No Bleeding Correctly Reclassified, P (n2) | Net Reclassification Improvementb | P Value | Integrated Discriminatory Improvementc | P Value | |
|---|---|---|---|---|---|---|
| CRUSADE vs ACUITYa | 0.57 (30) | 0.49 (921) | 0.12 | 0.49 | 0.0015 | 0.488 |
| ACUITY vs HAS‐BLEDa | 0.49 (26) | 0.57 (1076) | 0.12 | 0.40 | 0.0067 | 0.069 |
| CRUSADE vs HAS‐BLEDa | 0.62 (33) | 0.57 (1087) | 0.39 | 0.005 | 0.0083 | 0.021 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly.
The model considered each bleeding risk score as a reference value for the others.
The net reclassification improvement was defined as (A+B)−([1−A]+[1−B]), in which A is the probability of bleeding correctly reclassified and B is the probability of no bleeding correctly reclassified.
The integrated discrimination improvement was defined as
Figure 3.
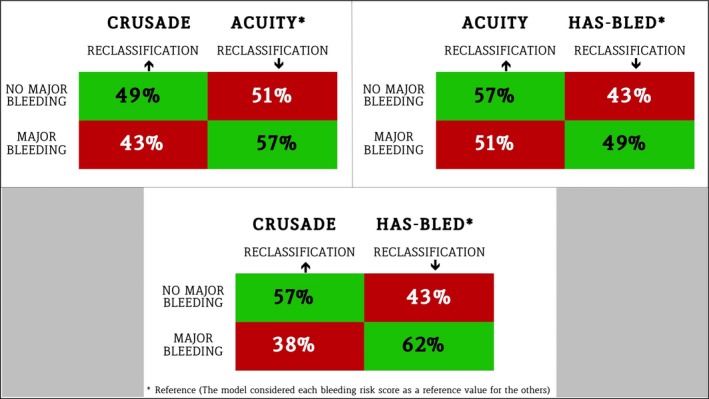
Reclassification tables. The 3 bleeding risk scores are compared using each score as reference for the others: The first score mentioned is the score to be tested, the second is considered the reference. The percentage of patients correctly reclassified by each score is displayed in green, whereas the percentage of patients not correctly reclassified is in red. ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly.
Table 9.
Risk Reclassification and Integrated Discriminatory Improvement for TIMI Minor or Major and GUSTO Moderate or Severe Bleeding
| Bleeding Correctly Reclassified P (n1) | No Bleeding Correctly Reclassified, P (n2) | Net Reclassification Improvementb | P Value | Integrated Discriminatory Improvement c | P Value | |
|---|---|---|---|---|---|---|
| TIMI Minor or Major | ||||||
| CRUSADE vs ACUITYa | 0.55 (23) | 0.51 (974) | 0.12 | 0.53 | 0.0022 | 0.198 |
| ACUITY vs HAS‐BLEDa | 0.45 (19) | 0.55 (1055) | 0.012 | 1.00 | 0.0031 | 0.256 |
| CRUSADE vs HAS‐BLEDa | 0.62 (26) | 0.56 (1065) | 0.37 | 0.03 | 0.0053 | 0.069 |
| GUSTO Moderate or Severe | ||||||
| CRUSADE vs ACUITYa | 0.53 (27) | 0.55 (1044) | 0.16 | 0.26 | 0.004 | 0.11 |
| ACUITY vs HAS‐BLEDa | 0.47 (24) | 0.53 (998) | −0.004 | 1.00 | 0.002 | 0.62 |
| CRUSADE vs HAS‐BLEDa | 0.59 (30) | 0.54 (1029) | 0.26 | 0.066 | 0.006 | 0.11 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; GUSTO, Global Use of Strategies to Open Occluded Arteries; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; TIMI, Thrombolysis in Myocardial Infarction.
The model considered each bleeding risk score as a reference value for the others.
The net reclassification improvement was defined as (A+B)−([1−A]+[1−B]), in which A is the probability of bleeding correctly reclassified and B is the probability of no bleeding correctly reclassified.
The integrated discrimination improvement was defined as
Table 10.
Incidence of Ischemic Events Among Bleeding Risk Categories
| MACEa | Myocardial Infarction | Definite/probable STb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Events (n/N) | HR (95% CI) | P Value | Events (n/N) | HR (95% CI) | P Value | Events (n/N) | HR (95% CI) | P Value | |
| All patients | 9.9% (193/1946) | — | — | 3.9% (77/1946) | — | — | 1.4% (27/1946) | — | — |
| CRUSADE score | |||||||||
| Low (≤30) | 5.7% (73/1282) | Reference | — | 2.3% (29/1282) | Reference | — | 0.9% (12/1282) | Reference | — |
| Intermediate (31–40) | 10.4% (37/357) | 1.87 (1.26–2.77) | 0.002 | 4.2% (15/357) | 1.86 (1.00–3.48) | 0.05 | 0.5% (2/357) | 0.60 (0.13–2.71) | 0.509 |
| High (>40) | 27.0% (83/307) | 5.45 (3.98–7.46) | <0.0001 | 10.7% (33/307) | 4.92 (2.99–8.11) | <0.0001 | 4.2% (13/307) | 4.82 (2.20–10.6) | <0.0001 |
| ACUITY score | |||||||||
| Low (<10) | 4.2% (20/480) | Reference | — | 1.7% (8/480) | Reference | — | 0.8% (4/480) | Reference | — |
| Intermediate (10–14) | 5.3% (25/475) | 1.28 (0.71–2.30) | 0.413 | 1.7% (8/475) | 1.02 (0.38–2.71) | 0.97 | 0.4% (2/475) | 0.51 (0.09–2.79) | 0.438 |
| High (>14) | 14.9% (148/991) | 3.83 (2.40–6.11) | <0.0001 | 6.1% (61/991) | 3.71 (1.78–7.76) | <0.0001 | 2.1% (21/991) | 2.58 (0.89–7.52) | 0.082 |
| HAS‐BLED score | |||||||||
| Low (<2) | 5.5% (54/977) | Reference | — | 2.2% (22/977) | Reference | — | 1.0% (10/977) | Reference | — |
| Intermediate (2) | 13.0% (119/914) | 2.44 (1.77–3.36) | <0.0001 | 5.4 (50/914) | 2.47 (1.5–4.09) | <0.0001 | 1.5% (14/914) | 1.51 (0.67–3.40) | 0.318 |
| High (>2) | 36.4% (20/55) | 7.82 (4.68–13.07) | <0.0001 | 9.0 (5/55) | 4.50 (1.70–11.8) | 0.002 | 5.4% (3/55) | 6.13 (1.69–22.3) | 0.006 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; MACE, major adverse cardiovascular events; ST, stent thrombosis.
MACE is intended as a composite of death from all causes, myocardial infarction, and cerebrovascular accident.
Definite or probable ST was defined according to the academic research consortium.
CRUSADE Score and DAPT Duration
Bleeding events
Patients meeting the threshold for HCS showed an almost 3‐fold greater rate of major bleeding when treated with 24‐ versus 6‐month DAPT (9.7% versus 3.7%; ARD 6%; 95% CI 0.4% to 12.3%; P=0.04); patients with low to intermediate CRUSADE scores did not experience a significant increase in major bleeding when treated with long versus short DAPT duration (2.4% versus 1.6%; ARD 0.8%; 95% CI −0.6% to 2.2%; P=0.25) (Figure 4A and Table 11). A quantitative interaction was noted between bleeding risk and duration of antiplatelet therapy with respect to major bleeding (P int=0.05) (Figure 5). The NNTH to experience major bleeding with prolonged DAPT in the HCS group was 17 (Figure 6). These findings remained consistent across bleeding scales (Table 11). Patients with HCS experienced an almost 5‐fold increase in red blood cell transfusion in the 24‐ versus 6‐month DAPT duration arms (8.3% versus 1.8%; ARD 6.5%; 95% CI 1.6% to 12.3%; P=0.02; NNTH: 15.4), whereas this did not differ in patients with low to intermediate CRUSADE score (1.7% versus 1.2%; ARD 0.5%; 95% CI −0.6% to 1.7%; P=0.45) (Figure 4B and Table 11), with positive interaction testing (P int=0.01) (Figure 5).
Figure 4.
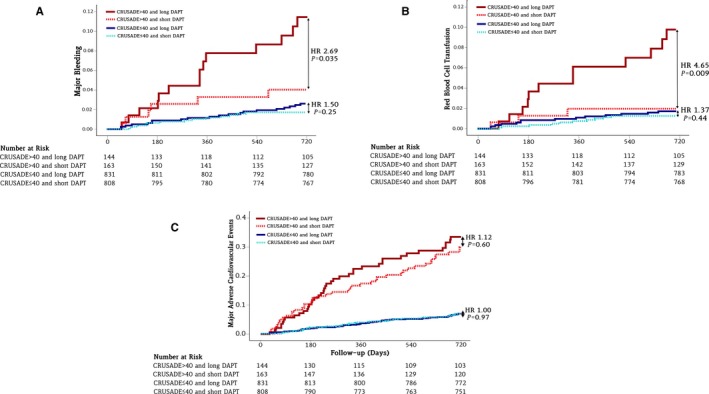
Kaplan–Meier curves during follow‐up for hemorrhagic and ischemic events in the high and low to intermediate CRUSADE score categories after 24‐ or 6‐month DAPT. A, Major bleeding. B, Red blood cell transfusion. C, Major adverse cardiovascular events including death for all causes, myocardial infarction, and cerebrovascular accident. CRUSADE indicates Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; DAPT, dual antiplatelet therapy; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; HR, hazard ratio.
Table 11.
Hemorrhagic and Ischemic Outcomes in the High and Low to Intermediate CRUSADE Score Groups After 24‐ or 6‐Month Dual Antiplatelet Therapy
| HCS (>40) | LICS (≤40) | P int | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 24‐Month DAPT (n=144) | 6‐Month DAPT (n=163) | ARD (95% CI) | P Value | 24‐Month DAPT (n=831) | 6‐Month DAPT (n=808) | ARD (95% CI) | P Value | ||
| Major Bleedinga | 9.7% (14) | 3.7% (6) | 6% (0.4%, 12.3%) | 0.04 | 2.4% (20) | 1.6% (13) | 0.8% (−0.6%, 2.2%) | 0.25 | 0.05 |
| Red blood cell transfusion | 8.3% (12) | 1.8% (3) | 6.5% (1.6%, 12.3%) | 0.02 | 1.7% (14) | 1.2% (10) | 0.5% (−0.6%, 1.7%) | 0.45 | 0.01 |
| MACE | 28.5% (41) | 25.8% (42) | 2.7% (−7.2%, 12.6%) | 0.59 | 6.7% (56) | 6.7% (54) | 0.0% (−2.5, 2.4%) | 0.96 | 0.58 |
ACUITY indicates Acute Catheterization and Urgent Intervention Triage Strategy; ARD, absolute risk difference; CRUSADE, Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; DAPT, dual antiplatelet therapy; GUSTO, Global Use of Strategies to Open Occluded Arteries; HAS‐BLED, Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly; HCS, high CRUSADE score; int, interaction; LICS, low to intermediate CRUSADE score; MACE, major adverse cardiovascular events consistent with death from all causes, myocardial infarction, and cerebrovascular accident; TIMI, Thrombolysis in Myocardial Infarction.
Results consistent among other bleeding definitions: TIMI minor or major and HCS (7.6% vs 2.4%; ARD 5.2%; 95% CI 0.2% to 10.9%; P: 0.05) and LICS (1.9% vs 1.4%; ARD 0.5%; 95% CI −0.6% to 1.9%; P=0.37) (P int=0.02). GUSTO moderate or severe and HCS (9.7% vs 4.3%; ARD 5.4%; 95% CI 0.3% to 11.8%; P=0.08) and LICS (2.2% vs 1.5%; ARD 0.7%; 95% CI −0.6% to 2.1%; P=0.30) (P int=0.08).
Figure 5.
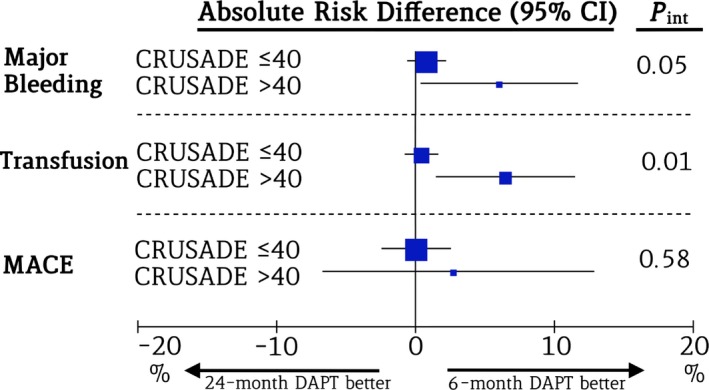
Hemorrhagic and ischemic outcomes in patients with high and low to intermediate CRUSADE scores. The forest plot shows the absolute risk difference and the P value of the interaction effect for major bleeding, red blood cell transfusion, and MACE after 24‐ versus 6‐month DAPT in the groups of patients with high and low to intermediate CRUSADE scores. CRUSADE indicates Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; DAPT, dual antiplatelet therapy; int, interaction; MACE, major adverse cardiovascular events.
Figure 6.
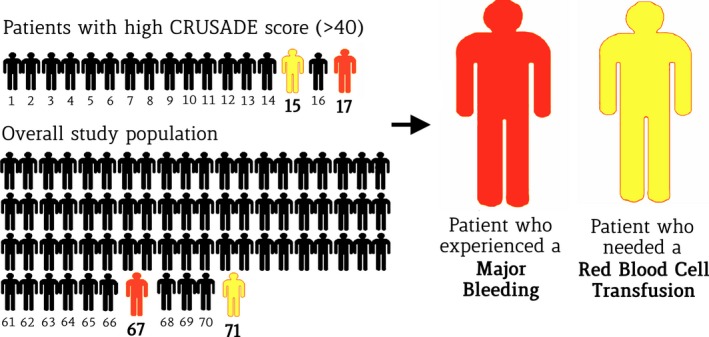
Effects of long‐ and short‐term DAPT on patients with a high CRUSADE score and in the overall population. The number of patients needed to treat to experience major bleeding or red blood cell transfusion after 24‐month DAPT compared with 6‐month treatment is significantly lower in the group of patients with a high CRUSADE score (>40) than in the overall study population. CRUSADE indicates Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; DAPT, dual antiplatelet therapy.
Ischemic events
The risk of death, MI, or cerebrovascular accident did not differ in the 24‐ versus 6‐month DAPT groups, both in patients with HCS (28.5% versus 25.8%; ARD 2.7%; 95% CI −7.2% to 12.6%; P=0.59) and with low to intermediate CRUSADE score (6.7% versus 6.7%; ARD 0%; 95% CI −2.5% to 2.4%; P=0.96) (P int=0.58). (Figure 4C and Table 11) Similarly, when separately assessed, the risk of all‐cause death, MI, or definite or probable stent thrombosis remained homogeneously distributed between DAPT groups in patients with and without HCS (Table 12).
Table 12.
Other Ischemic Outcomes in the High and Low to Intermediate CRUSADE Score Groups After 24‐ or 6‐Month Dual Antiplatelet Therapy
| HCS (>40) | LICS (≤40) | P int | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 24‐Month Clopidogrel (n=144) | 6‐Month Clopidogrel (n=163) | ARD (95% CI) | P Value | 24‐Month Clopidogrel (n=831) | 6‐Month Clopidogrel (n=808) | ARD (95% CI) | P Value | ||
| Death from all causes | 20.1% (29) | 20.9% (34) | −0.8% (−9.6% to 8.4%) | 0.87 | 4.1% (34) | 3.7% (30) | 0.4% (−1.5% to 2.3%) | 0.69 | 0.56 |
| MI | 11.1% (16) | 10.4%(17) | 0.7% (−6.3% to 7.9%) | 0.86 | 2.5% (21) | 2.8% (23) | −0.3% (−1.9% to 1.2%) | 0.69 | 0.71 |
| Definite/probable STa | 1.4% (2) | 1.2% (2) | 0.2% (−3.1 to 3.8%) | 0.90 | 1.3% (11) | 1.6% (13) | −0.3% (−1.5 to 0.9%) | 0.63 | 0.83 |
CRUSADE indicates Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the ACC/AHA Guidelines; MI, myocardial infarction; ST, stent thrombosis.
Definite or probable ST defined according to the academic research consortium.
Discussion
The main findings of this study can be summarized as follows. First, the CRUSADE, HAS‐BLED, and ACUITY risk scores demonstrated reasonably good predictive value with respect to major bleeding in the PRODIGY all‐comer population, regardless of the bleeding definition used. Second, bleeding risk scores also displayed a significant capability to predict ischemic events in terms of major adverse cardiac events, MI, or stent thrombosis. Third, the CRUSADE risk score, predicted bleeding significantly better than HAS‐BLED, with improved reclassification and discrimination performance. Fourth, patients with HCS treated with 24‐month DAPT experienced a 3‐fold higher risk of major bleeding and a 5‐fold risk of red blood cell transfusion compared with 6‐month DAPT, without clear evidence of benefit. The NNTH with an HCS was as low as 17 for major bleeding and 15 for red blood cell transfusion; these values were lower than corresponding values in the unselected patient cohort, suggesting that long‐term DAPT has a narrow therapeutic window and high potential for harm in this selected patient population with high bleeding risk. Fifth, conversely, patients not meeting the threshold for the HCS category—corresponding to as many as 84.2% of the patients originally included in our study—did not have higher bleeding risk, consistently across bleeding risk scales, if treated with 24‐ versus 6‐month DAPT duration.
There is consensus currently about the need to choose intensity and/or duration of potent antithrombotic therapy after percutaneous coronary intervention through the assessment of actual individual bleeding risk. Nevertheless, it remains undefined how bleeding risk should be properly assessed and whether it should truly influence therapeutic decisions in clinical practice. The ultimate goal of this analysis was to select 1 bleeding risk score that could guide duration of DAPT in clinical practice to maximize benefits over risks. Among the risk scores explored, we found CRUSADE to have a better predictive profile for major bleeding compared with HAS‐BLED and a similar profile compared to ACUITY. This observation is consistent with some previous studies and with the European Society of Cardiology guidelines that recommended the CRUSADE score for bleeding risk stratification in non–ST‐segment elevation MI.9, 15, 16 It might be speculated that the set of covariates used to predict bleeding risk for the CRUSADE score better reflects the bleeding risk in patients undergoing stent implantation and subsequent DAPT. Accordingly, we stratified the PRODIGY patient population into high versus nonhigh bleeding risk status based on CRUSADE and assessed whether a priori bleeding risk could be a treatment modifier with respect to DAPT duration. We failed to identify a specific patient population (eg, those at low or intermediate bleeding risk) for which long‐term DAPT was associated with lower rates of ischemic end points compared with a shortened DAPT regimen. This may reflect the null finding of the PRODIGY trial with respect to the benefit of long‐term DAPT on death, MI, or stroke. In contrast, our study, which recruited an all‐comer patient population, observed a distinct increase in bleeding end points in patients treated with 24‐month DAPT. The current stratified analysis largely expands on previous findings by showing that in patients with low to intermediate risk, prolonging DAPT was not associated with a significant bleeding risk consistently across bleeding scales. Conversely, we observed bleeding and blood transfusion hazards associated with long‐term DAPT in the selected cohort of patients with high bleeding risk. Given the magnitude of this association on both relative and absolute scales, it may be reasonable to stop DAPT after 6 months in this selected patient population, given that the risks seem to largely outweigh the potential benefits. At the same time, in patients not meeting high bleeding risk criteria according to the CRUSADE score, bleeding risk appears acceptable and not different from those undergoing 6‐month therapy duration. This may be the ideal patient population in which to prolong DAPT for long‐term secondary prevention.
The recent DAPT trial6 demonstrated that 30‐month DAPT with clopidogrel or prasugrel resulted in a significant reduction of both stent thrombosis and major adverse cardiac event rates compared with patients treated with 12‐month DAPT. Importantly, patients were eligible for randomization only if they were free of both ischemic and bleeding events at 12 months. The implementation of the DAPT study results into practice would imply that clinicians should adopt a 2‐step strategy for deciding whether DAPT should or should not be prolonged beyond 12 months and that only patients free from bleeding events may be selected to continue DAPT. This approach may expose patients already identifiable as potential bleeders to treatment‐related side effects that could be prevented by stopping DAPT earlier. Current European Society of Cardiology revascularization guidelines call for a shorter DAPT duration in patients at high bleeding risk.8 ACC/AHA guidelines state that if the bleeding risk is greater than the anticipated benefit, a shortened duration of DAPT should be considered.7 The results of our study may help standardize risk assessment for bleeding in clinical practice and may have implications for tailored DAPT duration.
Our study has several limitations. First, as a retrospective analysis, the results provided are hypothesis generating, and a specifically designed randomized trial is needed to confirm or refute our findings. Second, the scores evaluated in this study were not validated in an all‐comer population and were designed mostly to predict events in the first 30 days after the index procedure. When assessed individually, all variables included in each score were independent bleeding predictors. Third, the outcome of interest for the current analysis was clinically significant major bleeding defined according to the BARC class 3 or 5 definition. These events are relatively rare in modern clinical trials and occurred in only 2.7% of the PRODIGY population. As such, bigger sample sizes are needed to further corroborate our findings. Fourth, CRUSADE, ACUITY, and HAS‐BLED scores were validated using a bleeding definition that was different from BARC class 3 or 5, as used in the PRODIGY trial; however, at sensitivity analysis, the result observed for BARC were confirmed using TIMI minor or major and GUSTO moderate and severe definitions. Fifth, we did not evaluate the performance of other bleeding risk scores apart from those presented in this analysis; consequently, their incremental value in an all‐comer population should be investigated. Sixth, the bleeding risk scores were collected only on admission. Considering the sudden variability of clinical status in this population, the result of the scores at the moment of randomization or during follow‐up may change over time. Continuous and progressive evaluation of bleeding risk would be ideal but, unfortunately, hardly feasible.
Conclusions
The CRUSADE, ACUITY, and HAS‐BLED bleeding risk scores displayed reasonable predictive performance in an all‐comer population treated with coronary stenting; among them, CRUSADE showed the best predictive profile in our dataset. DAPT for 24 months was associated with a higher risk of major bleeding in patients at high risk based on the CRUSADE score but not in those with low or intermediate risk profiles. The CRUSADE score has potential to guide DAPT duration based on standardization of bleeding risk assessed for each individual patient.
Disclosures
None.
Supporting information
Table S1. Bleeding Risk Score Calculation in the Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study (PRODIGY) Trial
(J Am Heart Assoc. 2015;4:e002524 doi: 10.1161/JAHA.115.002524)
An accompanying Table S1 is available at http://jaha.ahajournals.org/content/4/12/e002524/suppl/DC1
References
- 1. Vranckx P, Leonardi S, Tebaldi M, Biscaglia S, Parrinello G, Rao SV, Mehran R, Valgimigli M. Prospective validation of the Bleeding Academic Research Consortium classification in the all‐comer PRODIGY trial. Eur Heart J. 2014;35:2524–2529. [DOI] [PubMed] [Google Scholar]
- 2. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 3. Hamon M, Lemesle G, Tricot O, Meurice T, Deneve M, Dujardin X, Brufau JM, Bera J, Lamblin N, Bauters C. Incidence, source, determinants, and prognostic impact of major bleeding in outpatients with stable coronary artery disease. J Am Coll Cardiol. 2014;64:1430–1436. [DOI] [PubMed] [Google Scholar]
- 4. Rao SV, O'Grady K, Pieper KS, Granger CB, Newby LK, Van de Werf F, Mahaffey KW, Califf RM, Harrington RA. Impact of bleeding severity on clinical outcomes among patients with acute coronary syndromes. Am J Cardiol. 2005;96:1200–1206. [DOI] [PubMed] [Google Scholar]
- 5. Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fuca G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R; Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study I . Short‐ versus long‐term duration of dual‐antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–2026. [DOI] [PubMed] [Google Scholar]
- 6. Mauri L, Kereiakes DJ, Yeh RW, Driscoll‐Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM; Investigators DS . Twelve or 30 months of dual antiplatelet therapy after drug‐eluting stents. N Engl J Med. 2014;371:2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:2574–2609. [DOI] [PubMed] [Google Scholar]
- 8. Authors/Task Force m , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2014;35:2541–2619. [DOI] [PubMed] [Google Scholar]
- 9. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D; Guidelines ESCCfP . ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: the Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 10. Subherwal S, Bach RG, Chen AY, Gage BF, Rao SV, Newby LK, Wang TY, Gibler WB, Ohman EM, Roe MT, Pollack CV Jr, Peterson ED, Alexander KP. Baseline risk of major bleeding in non‐ST‐segment‐elevation myocardial infarction: the CRUSADE (Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines) Bleeding Score. Circulation. 2009;119:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol. 2010;55:2556–2566. [DOI] [PubMed] [Google Scholar]
- 12. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 13. Valgimigli M, Campo G, Percoco G, Monti M, Ferrari F, Tumscitz C, Zuffi A, Colombo F, Kubbajeh M, Cavazza C, Cangiano E, Tebaldi M, Minarelli M, Arcozzi C, Scalone A, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R. Randomized comparison of 6‐ versus 24‐month clopidogrel therapy after balancing anti‐intimal hyperplasia stent potency in all‐comer patients undergoing percutaneous coronary intervention Design and rationale for the PROlonging Dual‐antiplatelet treatment after Grading stent‐induced intimal hyperplasia study (PRODIGY). Am Heart J. 2010;160:804–811. [DOI] [PubMed] [Google Scholar]
- 14. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐112. [DOI] [PubMed] [Google Scholar]
- 15. Abu‐Assi E, Raposeiras‐Roubin S, Lear P, Cabanas‐Grandio P, Girondo M, Rodriguez‐Cordero M, Pereira‐Lopez E, Romani SG, Gonzalez‐Cambeiro C, Alvarez‐Alvarez B, Garcia‐Acuna JM, Gonzalez‐Juanatey JR. Comparing the predictive validity of three contemporary bleeding risk scores in acute coronary syndrome. Eur Heart J Acute Cardiovasc Care. 2012;1:222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flores‐Rios X, Couto‐Mallon D, Rodriguez‐Garrido J, Garcia‐Guimaraes M, Gargallo‐Fernandez P, Pinon‐Esteban P, Aldama‐Lopez G, Salgado‐Fernandez J, Calvino‐Santos R, Vazquez‐Gonzalez N, Castro‐Beiras A. Comparison of the performance of the CRUSADE, ACUITY‐HORIZONS, and ACTION bleeding risk scores in STEMI undergoing primary PCI: insights from a cohort of 1391 patients. Eur Heart J Acute Cardiovasc Care. 2013;2:19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Bleeding Risk Score Calculation in the Prolonging Dual Antiplatelet Treatment After Grading Stent‐Induced Intimal Hyperplasia Study (PRODIGY) Trial


