Abstract
Background
In the Apixaban for the Initial Management of Pulmonary Embolism and Deep‐Vein Thrombosis as First‐Line Therapy (AMPLIFY) trial, apixaban was noninferior to enoxaparin/warfarin in preventing recurrent symptomatic venous thromboembolism (VTE) or venous thromboembolism–related death, with significantly less bleeding. This analysis evaluated the effects of apixaban versus enoxaparin/warfarin on all‐cause hospitalizations during AMPLIFY.
Methods and Results
Of the 5365 patients included, 2676 received apixaban and 2689 received enoxaparin/warfarin. All‐cause hospitalizations during the treatment period after the index event were captured using dedicated case report forms. Outcomes included all‐cause hospitalizations and time from randomization to first hospitalization. Patients were censored at death, loss to follow‐up, or end of study, whichever came first. Treatment effects were assessed using Cox proportional hazards regression models. During the treatment period after the index event, 343 patients were hospitalized at least once: 153 (5.72%) in the apixaban group and 190 (7.07%) in the enoxaparin/warfarin group. Compared with enoxaparin/warfarin, apixaban significantly reduced all‐cause hospitalizations (hazard ratio 0.804, 95% CI=0.650–0.995, P=0.045). All‐cause hospitalization rates within the first 30 days after the index event were 2.28% and 3.35% in the apixaban and enoxaparin/warfarin groups, respectively (hazard ratio 0.676, 95% CI=0.488–0.935, P=0.018). For all patients, the average per‐patient estimated mean length of hospital stay was also shorter with apixaban than enoxaparin/warfarin (0.57 days versus 1.01 days, P<0.0001).
Conclusions
Apixaban significantly reduced all‐cause hospitalizations versus enoxaparin/warfarin, and shortened the length of hospital stay in patients with acute venous thromboembolism.
Clinical Trial Registration
URL: https://Clinicaltrials.Gov/. Unique identifier: NCT00643201.
Keywords: anticoagulants, hemorrhage, mortality, prevention, thrombosis
Subject Categories: Anticoagulants, Clinical Studies, Thrombosis
Introduction
Venous thromboembolism (VTE) is associated with significant morbidity and mortality, and is the third leading cause of cardiovascular death.1, 2, 3 Of those patients who experience a first VTE, ≈20% present with sudden death secondary to pulmonary embolism (PE), ≈30% have died within 30 days, and ≈30% develop recurrent VTE within 10 years.4 Additionally, ≈2% of patients with VTE treated with vitamin K antagonists experience major bleeding events within 3 months, of which up to 18% are fatal.5
The standard regimen of anticoagulation treatment for VTE is complex and inconvenient, particularly in outpatient settings, as it involves overlapping a vitamin K antagonist with parenteral anticoagulants that require daily subcutaneous injections for ≥5 days.6, 7, 8 Additionally, long‐term use of vitamin K antagonists for the treatment of VTE is limited by concerns of bleeding, the narrow therapeutic window, and patient inconvenience due to the need for frequent international normalized ratio monitoring and numerous drug–drug and food interactions.9
Apixaban is an oral factor Xa inhibitor administered in fixed doses without the need for laboratory monitoring.10, 11 The Apixaban for the Initial Management of Pulmonary Embolism and Deep‐Vein Thrombosis as First‐line Therapy (AMPLIFY; NCT00643201) trial showed that apixaban was noninferior to conventional therapy (enoxaparin/warfarin) for the primary efficacy outcome, recurrent symptomatic VTE or VTE‐related death (2.3% versus 2.7%, respectively), and was associated with fewer major bleeding events (0.6% versus 1.8%, respectively, P<0.001).12 The Apixaban after the Initial Management of Pulmonary Embolism and Deep‐Vein Thrombosis with First‐Line Therapy–Extended Treatment (AMPLIFY‐EXT; NCT00633893) trial showed that compared with placebo, both the 2.5‐mg and the 5‐mg doses of apixaban reduced the risk of recurrent fatal or nonfatal VTE over 12 months, and that the rates of major bleeding were both low and comparable to those in the placebo group.13 Our previous analysis of the AMPLIFY‐EXT trial showed that extended anticoagulation with apixaban reduced the risk of all‐cause hospitalizations compared with placebo in patients with VTE who had already received anticoagulant therapy for 6 to 12 months for their index event.14
The primary objective of this analysis was to evaluate the efficacy of apixaban compared with conventional therapy (enoxaparin/warfarin) for the reduction of all‐cause hospitalizations in patients with VTE who were enrolled in the AMPLIFY trial. Secondary end points included time to first hospitalization, length of hospital stay, and reasons for hospital admission.
Methods
Study Population
The study design and results of the AMPLIFY trial have been reported previously.12 AMPLIFY was a randomized, active‐controlled, parallel‐group, double‐blind, noninferiority trial in which patients with VTE were enrolled from August 2008 through August 2012. The protocol was approved by the institutional review board at each center and written informed consent was obtained from all patients.12
Patients were included if they were aged ≥18 years; had objectively confirmed symptomatic proximal deep‐vein thrombosis (DVT) or PE (with or without DVT), and had provided written informed consent.12 Patients were excluded from the study if they had active bleeding or a high risk of bleeding, <6 months of anticoagulation planned for the most recent DVT or PE, or an indication other than VTE for which long‐term anticoagulation was planned. Other exclusion criteria included contraindications to treatment with either enoxaparin or warfarin, life expectancy <6 months, platelet count <100 000/mm3, hemoglobin <9 g/dL, serum creatinine >2.5 mg/dL, calculated creatinine clearance (CrCL) <25 mL/min, alanine aminotransferase or aspartate transaminase >2×the upper limit of normal or total bilirubin >1.5×upper limit of normal.12
Randomization
Patients were assigned to receive apixaban, 10 mg twice daily for the first 7 days, followed by 5 mg twice daily for 23 weeks, or enoxaparin 1 mg/kg of body weight every 12 hours for at least 5 days, followed by warfarin, begun concomitantly, for 6 months.12 Randomization was performed with the use of an interactive voice‐response system and stratified according to the qualifying diagnosis of either symptomatic proximal DVT or symptomatic PE (with or without DVT). Patients were assigned to receive apixaban tablets plus placebo enoxaparin injections and placebo warfarin tablets or conventional therapy plus placebo apixaban tablets.
Clinical Outcomes
Primary clinical outcomes
The primary efficacy outcome for AMPLIFY was the incidence of the adjudicated composite of recurrent symptomatic VTE or VTE‐related death, and the primary safety outcome was adjudicated major bleeding.12
Hospitalizations
All‐cause hospitalizations, emergency room visits, doctor's office visits, rehabilitation unit and nursing home admissions after the index event were captured by dedicated case report forms during the study conduct. The hospitalizations reported here are new hospitalizations that occurred either after the initial hospitalization or after commencing therapy for planned outpatient management. The outcomes of interest for this analysis were the rate of all‐cause hospitalizations, length of hospital stay, the time from randomization to first hospitalization, the reason for first hospitalization, and types of healthcare provider visit or admission. For hospitalization data, patients were censored at death, lost to follow‐up, or end of study, whichever came first.
Statistical Analysis
A time‐to‐event analysis was conducted for the first hospitalization according to treatment group. Time‐to‐event curves were calculated using the Kaplan–Meier method. Hospitalizations data were summarized using descriptive statistics. A Cox proportional hazards regression model or a stratified Cochran–Mantel–Haenszel test was used to examine the effects of treatment with apixaban versus enoxaparin/warfarin. A t test with Satterthwaite unequal variance adjustment was used to examine the statistical difference in mean length of stay for hospitalized patients after the index event between the 2 treatment groups. A zero‐inflated Poisson regression was used to estimate the mean length of stay after the index event for all patients in either treatment group because many patients were not readmitted. The intention‐to‐treat population comprised all randomized subjects with a nonmissing primary end point. All efficacy analyses included data for patients in the intention‐to‐treat population for whom the outcome status at 6 months was documented. All safety analyses included data from patients during study treatment, defined as the time from the administration of the first dose until 48 hours after the last dose was administered.
An analysis was conducted to test the interaction between treatment and the following subgroups using the same model: index event (DVT; PE), sex (male; female), age (<65 years; 65–75 years; >75 years), weight (<60 kg; 60–100 kg; >100 kg), and level of renal impairment (severe or moderate [CrCL ≤50 mL/min]; mild [CrCL >50 to ≤80 mL/min]; normal [CrCL >80 mL/min]). CrCL (mL/min) was calculated using the Cockcroft–Gault equation as ([140−age]×[weight at baseline (kg)])/(serum creatinine at baseline×72) for males; for females this calculation is adjusted by multiplying by a factor of 0.85. All statistical analyses were conducted using SAS® software version 9.
Results
The clinical efficacy and safety results of the AMPLIFY trial have been published previously.12 Briefly, a total of 5614 patients were enrolled at 358 centers in 28 countries; 5 patients (apixaban, n=2; enoxaparin, n=3) were excluded due to an absence of source documentation. The intention‐to‐treat population included 2691 patients assigned to apixaban and 2704 assigned to enoxaparin/warfarin. The safety population included 2676 patients assigned to apixaban and 2689 assigned to enoxaparin/warfarin. For the primary efficacy outcome, recurrent VTE, apixaban was noninferior to enoxaparin/warfarin (2.3% versus 2.7%; relative risk 0.84, 95% CI=0.60–1.18, P<0.001).12 The rate of major bleeding, the primary safety outcome, was significantly reduced with apixaban compared with enoxaparin/warfarin (0.6% versus 1.8%; relative risk 0.31, 95% CI=0.17–0.55, P<0.001).12
Hospitalizations Outcomes
Over the 6‐month period, there were 182 and 218 all‐cause hospitalizations after and not including the index event in the apixaban and enoxaparin/warfarin groups, respectively. Of these, a significantly lower proportion of apixaban‐treated patients had ≥1 hospitalization after and not including the index event compared with those treated with enoxaparin/warfarin (5.72% versus 7.07%, hazard ratio 0.804, 95% CI=0.650–0.995, P=0.045; Table 1). Similar results were seen for hospitalizations that occurred in the first 30 days (2.28% and 3.35% in the apixaban and enoxaparin/warfarin groups, respectively; hazard ratio 0.676, 95% CI=0.488–0.935, P=0.018; Table 1). The total length of hospital stay for hospitalizations after and not including the index event was 1535 days for patients treated with apixaban versus 2197 days for those treated with enoxaparin/warfarin (Table 2). The mean (SD) length of hospital stay per hospitalized patient was shorter with apixaban than with enoxaparin/warfarin (10.2 [13.7] days versus 11.7 [28.2] days) (Table 2). For all patients, the average estimated number of days spent in the hospital per patient after and not including the index event was also shorter with apixaban than with enoxaparin/warfarin (0.57 days versus 1.01 days, P<0.0001). The median time to first hospitalization was longer for patients receiving apixaban than for those receiving enoxaparin/warfarin (63.0 days versus 34.5 days; Figure 1).
Table 1.
Hospitalizations by Treatment Group
| Apixaban (n=2676) | Enoxaparin/Warfarin (n=2689) | Hazard Ratio (95% CI) | P Value | NNT | |
|---|---|---|---|---|---|
| Number of all‐cause hospitalizations after and not including the index event | 182 | 218 | |||
| Number of patients with ≥1 hospitalization after and not including the index event (%) | 153 (5.72) | 190 (7.07) | 0.804 (0.650–0.995) | 0.045 | 74 |
| Number of patients with ≥1 hospitalization in the first 30 days after and not including the index event (%) | 61 (2.28) | 90 (3.35) | 0.676 (0.488–0.935) | 0.018 | 93 |
A Cox proportional hazards regression model was used to examine the effects of treatment with apixaban vs enoxaparin/warfarin. NNT indicates number needed to treat.
Table 2.
Length of Hospital Stay by Treatment Group
| Apixaban (n=2676) | Enoxaparin/Warfarin (n=2689) | P Value | |
|---|---|---|---|
| Length of stay in hospitalization | |||
| Total, days | 1535 | 2197 | |
| Mean (SD) length of hospital stay per hospitalized patient, days | 10.2 (13.7) | 11.7 (28.2) | 0.5002 |
| Average estimated length of hospital stay per patient, days | 0.57 | 1.01 | <0.0001 |
A zero‐inflated Poisson regression was used to estimate the mean length of stay because many patients were not readmitted.
Figure 1.
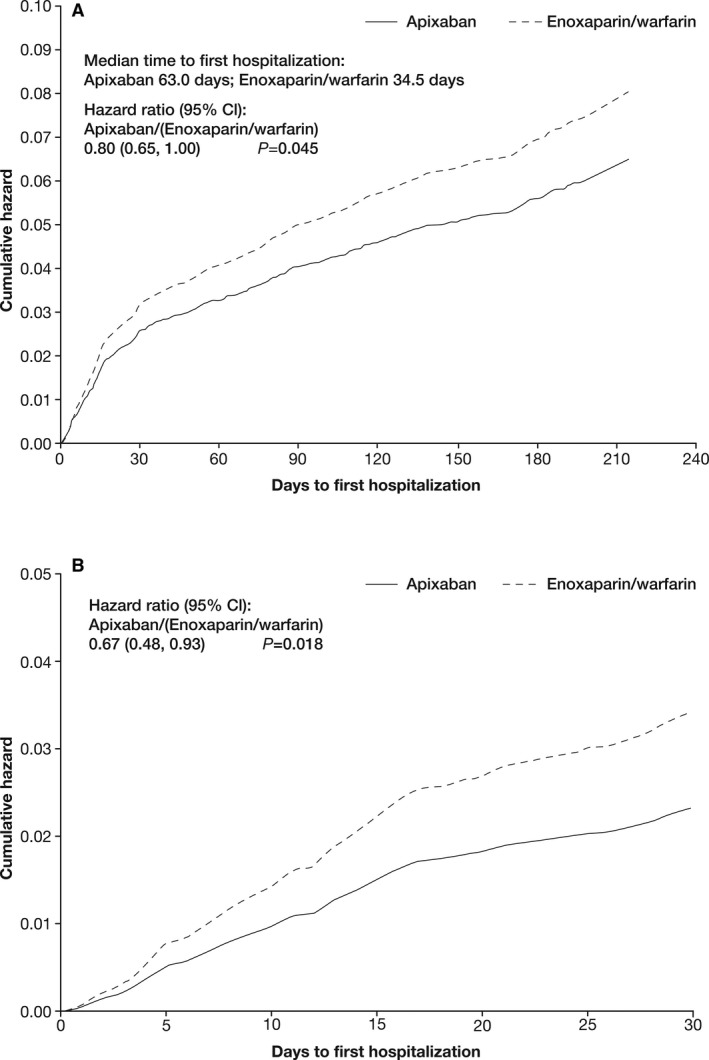
Time to first hospitalization by treatment group (A) during the trial after an index event and (B) during the first 30 days after an index event.
Irrespective of the subgroups analyzed, the treatment effect of apixaban versus enoxaparin/warfarin for the outcome of time to first hospitalization was consistent with the overall results (Figure 2).
Figure 2.
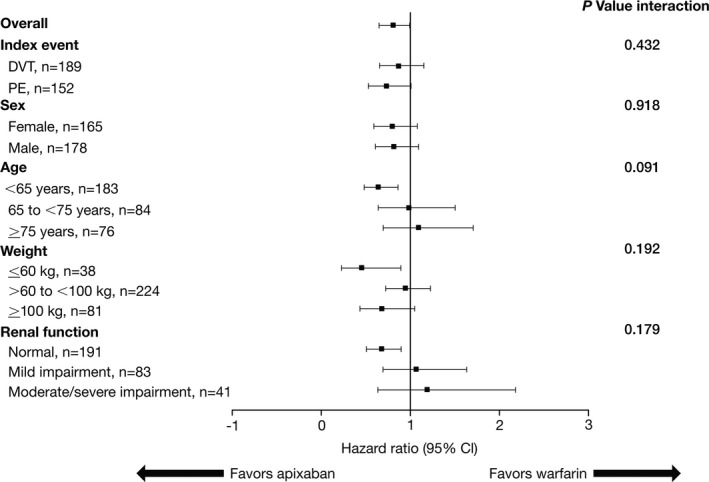
Comparison of the treatment effect of apixaban vs warfarin for time to first hospitalization in key subgroups. DVT indicates deep‐vein thrombosis; PE, pulmonary embolism.
Reasons for Hospitalizations
The numbers of patients hospitalized for VTE recurrence was lower in patients receiving apixaban than in those receiving enoxaparin/warfarin (37 versus 48; Table 3). Similarly, the number of patients hospitalized for bleeding events was also lower in patients receiving apixaban than in those receiving enoxaparin/warfarin (31 versus 59; Table 3). The number of patients hospitalized for cardiovascular reasons was 23 in the apixaban group and 29 in the enoxaparin/warfarin group (Table 3). Of the other reasons for hospitalizations in apixaban‐treated versus enoxaparin/warfarin‐treated patients (80 versus 73), the most common causes were sepsis (15 versus 10), cancer (10 versus 7), respiratory (6 versus 5), and surgery (6 versus 2).
Table 3.
Reasons for First Hospitalization
| Apixaban (N=153) | Enoxaparin/Warfarin (N=190) | |
|---|---|---|
| VTE (DVT, PE) | 37 (24.2) | 48 (25.3) |
| Bleeding | 31 (20.2) | 59 (31.1) |
| Cardiovascular | 23 (15.0) | 29 (15.3) |
| Other | 80 (52.3) | 73 (38.4) |
| Potential bleed | 1 (0.7) | 1 (0.5) |
| Potential VTE | 5 (3.3) | 5 (2.6) |
| Cancer | 10 (6.5) | 7 (3.7) |
| Chest pain | 8 (5.2) | 6 (3.2) |
| Generala | 12 (7.8) | 15 (7.9) |
| Hepatic | 2 (1.3) | 2 (1.1) |
| INR | 0 | 2 (1.1) |
| Lab abnormality | 4 (2.6) | 0 |
| Leg edema | 1 (0.7) | 3 (1.6) |
| Leg pain | 0 | 3 (1.6) |
| Neurologic | 7 (4.6) | 1 (0.5) |
| Psychiatric | 2 (1.3) | 3 (1.6) |
| Renal | 0 | 4 (2.1) |
| Respiratory | 6 (3.9) | 5 (2.6) |
| Rheumatologic | 0 | 3 (1.6) |
| Sepsis | 15 (9.8) | 10 (5.3) |
| Surgery | 6 (3.9) | 2 (1.1) |
| Trauma | 1 (0.7) | 1 (0.5) |
Data are n (%). Hospitalizations data were summarized using descriptive statistics. DVT indicates deep‐vein thrombosis; INR, international normalized ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
General was a category for events that did not fit into other categories.
Types of, and Reasons for, Healthcare Provider Visits or Rehabilitation Unit Admissions Other Than Hospitalizations
The numbers of emergency room visits and rehabilitation unit admissions were similar in both treatment groups (P=0.631 and 0.692, respectively); however, the number of doctor's office visits was significantly lower in apixaban‐treated than in enoxaparin/warfarin‐treated patients (P=0.026; Table 4). Compared with enoxaparin/warfarin, apixaban was associated with fewer healthcare provider visits or rehabilitation unit admissions for VTE and bleeding events (35 versus 61 and 71 versus 130, respectively; Table 5). Healthcare provider visits or rehabilitation unit admissions for cardiovascular reasons were similar in all treatment groups. Of other reasons for healthcare provider visits or rehabilitation unit admissions (172 versus 176) in apixaban‐treated versus enoxaparin/warfarin‐treated patients, the most common causes were potential VTE (29 versus 25), respiratory (15 versus 22), sepsis (12 versus 18), chest pain (11 versus 16), and hepatic (4 versus 13) (Table 5).
Table 4.
Types of Healthcare Provider Visit or Rehabilitation Unit Admission Other Than Hospitalization
| Apixaban (n=2676) | Enoxaparin/Warfarin (n=2689) | Relative Risk (95% CI) | P Value | |
|---|---|---|---|---|
| Total number of visits/admissions | 307 | 389 | ||
| ER visits | ||||
| Number of visits | 102 | 117 | ||
| Number of patients (%) | 91 (3.4) | 97 (3.6) | 0.93 (0.70–1.25) | 0.631 |
| Doctor's office visits | ||||
| Number of visits | 198 | 269 | ||
| Number of patients (%) | 156 (5.8) | 196 (7.3) | 0.78 (0.63–0.97) | 0.026 |
| Rehabilitation unit admission | ||||
| Number of visits | 5 | 3 | ||
| Number of patients (%) | 4 (0.1) | 3 (0.1) | 1.35 (0.30–6.07) | 0.692 |
| Days in rehabilitation unit (SD) | 13.8 (8.85) | 16.0 (11.53) | ||
| Nursing home admissions | ||||
| Number of visits | 2 | 0 | N/A | N/A |
| Number of patients (%) | 2 (<0.1) | 0 (0) | ||
A Cox proportional hazards regression model was used to examine the effects of treatment with apixaban vs enoxaparin/warfarin. ER indicates emergency room; N/A, not applicable.
Table 5.
Reasons for Healthcare Provider Visit or Rehabilitation Unit Admission Other Than Hospitalization
| Apixaban (N=307) | Enoxaparin/Warfarin (N=389) | |
|---|---|---|
| Reasons for visit or admission other than hospitalization | ||
| VTE (DVT, PE) | 35 (11.4) | 61 (15.7) |
| Bleeding | 71 (23.1) | 130 (33.4) |
| Cardiovascular | 25 (8.1) | 19 (4.9) |
| Other | 176 (57.3) | 179 (46.0) |
| Potential bleed | 6 (2.0) | 3 (0.8) |
| Potential VTE | 29 (9.4) | 25 (6.4) |
| Stroke | 2 (0.7) | 2 (0.5) |
| Cancer | 3 (1.0) | 2 (0.5) |
| Chest pain | 11 (3.6) | 16 (4.1) |
| Generala | 34 (11.1) | 27 (6.9) |
| Hepatic | 4 (1.3) | 13 (3.3) |
| INR | 0 | 1 (0.3) |
| Lab abnormality | 3 (1.0) | 4 (1.0) |
| Leg edema | 7 (2.3) | 4 (1.0) |
| Leg pain | 8 (2.6) | 7 (1.8) |
| Neurologic | 11 (3.6) | 3 (0.8) |
| Psychiatric | 7 (2.3) | 1 (0.3) |
| Renal | 3 (1.0) | 1 (0.3) |
| Respiratory | 15 (4.9) | 22 (5.7) |
| Rheumatologic | 5 (1.6) | 7 (1.8) |
| Sepsis | 12 (3.9) | 18 (4.6) |
| Surgery | 4 (1.3) | 2 (0.5) |
| Trauma | 1 (0.3) | 13 (3.3) |
| Anticoagulant consult | 1 (0.3) | 0 |
| Management of anticoagulation therapy | 1 (0.3) | 0 |
| Cardiac | 1 (0.3) | 0 |
| Diabetes | 1 (0.3) | 4 (1.0) |
| Lab draw | 1 (0.3) | 0 |
| Genitourinary | 4 (1.3) | 2 (0.5) |
| Superficial VT | 0 | 1 (0.3) |
| Fracture | 1 (0.3) | 0 |
| Reason not provided | 1 (0.3) | 1 (0.3) |
Data are n (%). Hospitalizations data were summarized using descriptive statistics. DVT indicates deep‐vein thrombosis; INR, international normalized ratio; PE, pulmonary embolism; VT, venous thrombus; VTE, venous thromboembolism.
General was a category for events that did not fit into other categories.
Discussion
This analysis of the double‐blind AMPLIFY trial demonstrated the benefits of apixaban versus enoxaparin/warfarin with respect to hospitalizations in patients being treated for VTE. The analysis demonstrated that apixaban significantly reduced both the risk of all‐cause hospitalizations and other healthcare provider visits compared with enoxaparin/warfarin over 6 months. All‐cause hospitalization was also significantly reduced with apixaban versus enoxaparin/warfarin within the first 30 days after the index event. Additionally, apixaban reduced the length of stay in hospital per patient compared with enoxaparin/warfarin. These results were consistent across key subgroups. The main reasons for hospitalizations were VTE recurrence and bleeding events, both of which were lower in apixaban‐treated than enoxaparin/warfarin‐treated patients.
The reduction in all‐cause hospitalizations by apixaban versus enoxaparin/warfarin may be explained by the overall trial results that showed apixaban significantly reduced major bleeding compared with enoxaparin/warfarin (relative risk 0.31, 95% CI=0.17–0.55) and had a numerically lower risk of VTE recurrence or VTE‐related death (relative risk 0.84, 95% CI=0.60–1.18).12 Patients randomized to apixaban did not receive bridging therapy with low‐molecular‐weight heparin. However, this did not compromise the rates of VTE recurrence or VTE‐related death, or the rate of all‐cause hospitalizations. This suggests that bridging therapy with low‐molecular‐weight heparin is not necessary with apixaban. Therefore, the increased risk of bleeding associated with switching treatments between parenteral anticoagulants and oral anticoagulants such as warfarin is likely to be reduced.
These results are consistent with an analysis from the AMPLIFY‐EXT trial, which showed that extended anticoagulation with apixaban reduced the risk of all‐cause hospitalizations in patients with VTE who had already received anticoagulant therapy for 6 to 12 months for their index event.14 This result was also consistent across key subgroups including index event, sex, age, level of renal impairment, and weight, and also with the overall trial results that showed both doses of apixaban reduced the risk of recurrent VTE or all‐cause death compared with placebo.12 In addition, apixaban was shown to extend the mean time to first hospitalization compared with placebo by 43 days in the 2.5‐mg twice‐daily group and 49 days in the 5‐mg twice‐daily group, and to reduce the median length of stay of first hospitalization (apixaban 5 mg, 4.5 days; apixaban 2.5 mg, 5.0 days; placebo, 7.0 days).14 In the AMPLIFY‐EXT trial, the rates of major bleeding were low and similar in the apixaban and placebo groups (apixaban 2.5 mg, 0.2%; apixaban 5 mg, 0.1%; placebo 0.5%).13 VTE recurrence accounted for 51.6% of hospitalizations in the placebo group, which was reduced with both apixaban 2.5 mg and 5 mg (26.2% and 17.7%, respectively). Although bleeding rates were low and similar in AMPLIFY‐EXT, and not a main reason for hospitalization, apixaban still reduced the risk of hospitalization compared with placebo, possibly due to reducing the risk of VTE recurrence.
In addition to reducing all‐cause hospitalizations, apixaban also reduced the number of doctor's office visits and was associated with fewer healthcare provider visits or rehabilitation unit admissions for VTE and bleeding events compared with enoxaparin/warfarin. In the AMPLIFY trial, apixaban was noninferior to enoxaparin/warfarin for the treatment of acute VTE with significantly less major bleeding,12 which may be one of the reasons for the reduction in healthcare provider visits or rehabilitation unit admissions. Warfarin also carries a substantial risk of hemorrhage, a narrow therapeutic margin necessitating frequent monitoring of the international normalized ratio level, and interactions with numerous drugs and foods that necessitate dose adjustments that are not required with apixaban.
The estimated annual cost of VTE treatment is $2 billion (US$, 2013) in the United States, which rises to $15.6 to $34.8 billion upon inclusion of complications, productivity loss, and other societal costs.15 Of these figures, hospitalizations and inpatient management of VTE account for more than 50% of total costs.16, 17 Therefore, taking both the AMPLIFY and AMPLIFY‐EXT studies into account, the reduction in all‐cause hospitalizations with apixaban versus enoxaparin/warfarin could lead to significant savings in the annual costs of VTE treatment. In addition, from a UK National Health Service perspective, 6‐month treatment with apixaban following a VTE event was found to be cost‐effective compared with dabigatran, rivaroxaban, and low‐molecular‐weight heparin/warfarin.18
This study was performed in the setting of a randomized, double‐blind, active‐controlled clinical trial, minimizing the possibility of treatment selection bias and treatment‐related management decisions. Although the results are likely to be generalizable, as a wide spectrum of patients were recruited, most of whom had unprovoked VTE, further studies are needed to examine whether the results could be generalized to the real‐world clinical practice setting. Randomized controlled trials include patients and participating centers that may not be entirely representative of the real‐world setting. For example, hospital admission and length of stay may be influenced by healthcare system factors, such as diagnostic or treatment protocols, patient access to outpatient thrombosis services, and cost incentives for early discharge.19 Physician and patient preference may be another important factor influencing hospitalizations and length of stay. Furthermore, additional information is needed regarding patients with cancer, low body weight, or CrCL <50 mL/min. There are penalties in some clinical venues for 30‐day readmissions after discharge from index hospitalization. Although 30‐day readmissions after the index event are reported here, data for time to rehospitalizations from discharge date were not collected, which is also a limitation of this study.
Conclusions
This analysis demonstrated that compared with enoxaparin/warfarin, apixaban reduced all‐cause hospitalizations, resulted in a lower number of hospitalized patients, and reduced the mean length of hospital stay in patients treated for acute VTE. This reduction in all‐cause hospitalizations was observed within the first 30 days after the index event, and was mainly due to fewer recurrent VTE and bleeding events in apixaban‐treated patients compared with enoxaparin/warfarin‐treated patients. Additionally, the mean length of hospital stay was shorter for patients treated with apixaban than for patients treated with enoxaparin/warfarin. These findings indicate that apixaban therapy for VTE could lead to significant cost savings.
Sources of Funding
The trial was sponsored by Pfizer Inc and Bristol‐Myers Squibb Company. The sponsors collected and maintained the data, and the academic author had access to the data at all times through the sponsors. All authors contributed to the interpretation of the results, wrote and approved all versions of the manuscript and made the decision to submit for publication, and vouch for the accuracy and completeness of the data.
Disclosures
Liu and Mardekian are employees of Pfizer Inc, New York, NY. Thompson and Johnson are employees of Pfizer Inc, Groton, CT. When this study was conducted, Phatak was an employee of Bristol‐Myers Squibb, Princeton, NJ, and currently owns significant BMS stocks. AC was a paid consultant to Pfizer Inc and Bristol‐Myers Squibb in connection with the development of this manuscript and has received research support from Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sanko, GlaxoSmithKline, Merck Serono, Johnson and Johnson, Mitsubishi Pharma, Pfizer Inc, Sanofi, and Schering Plough. Additionally, Cohen has received consultant fees and/or honoraria from Astellas, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Johnson and Johnson, Merck Serono, Mitsubishi Pharma, Pfizer Inc, Portola Pharmaceuticals, Sanofi, Schering Plough, Takeda, and XO1.
Acknowledgments
Professional medical writing and editorial assistance were provided by Claire Hall, PhD, and Dana Fox, PhD, CMPP, at Caudex, and funded by Pfizer Inc and Bristol‐Myers Squibb Company.
(J Am Heart Assoc. 2015;4:e002340 doi: 10.1161/JAHA.115.002340)
References
- 1. Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, Greer IA, Heit JA, Hutchinson JL, Kakkar AK, Mottier D, Oger E, Samama MM, Spannagl M; VTE Impact Assessment Group in Europe (VITAE) . Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–764. [DOI] [PubMed] [Google Scholar]
- 2. Goldhaber SZ. Preventing pulmonary embolism and deep vein thrombosis: a ‘call to action’ for vascular medicine specialists. J Thromb Haemost. 2007;5:1607–1609. [DOI] [PubMed] [Google Scholar]
- 3. Heit J, Cohen A, Anderson FJ. Estimated annual number of incident and recurrent, non‐fatal and fatal venous thromboembolism (VTE) events in the US. Blood (ASH Annual Meeting Abstracts). 2005;106:Abstract 910. [Google Scholar]
- 4. Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, de Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 5. Nieto JA, Camara T, Gonzalez‐Higueras E, Ruiz‐Gimenez N, Guijarro R, Marchena PJ, Monreal M; RIETE Investigators . Clinical outcome of patients with major bleeding after venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:789–796. [PubMed] [Google Scholar]
- 6. Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e44S–e88S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR; American College of Chest Physicians . Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e419S–e494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van EJ, Eerenberg ES, Kamphuisen PW, Buller HR. How to prevent, treat, and overcome current clinical challenges of VTE. J Thromb Haemost. 2011;9(suppl 1):265–274. [DOI] [PubMed] [Google Scholar]
- 9. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:160S–198S. [DOI] [PubMed] [Google Scholar]
- 10. Frost C, Wang J, Nepal S, Schuster A, Barrett YC, Mosqueda‐Garcia R, Reeves RA, LaCreta F. Apixaban, an oral, direct factor Xa inhibitor: single‐dose safety, pharmacokinetics, pharmacodynamics and food effect in healthy subjects. Br J Clin Pharmacol. 2013;75:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd RA, Yu Z, Shenker A, Barrett YC, Mosqueda‐Garcia R, Lacreta F. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor Xa inhibitor, in healthy subjects. Br J Clin Pharmacol. 2013;76:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Masiukiewicz U, Pak R, Thompson J, Raskob GE, Weitz JI; AMPLIFY Investigators . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 13. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, Porcari A, Raskob GE, Weitz JI; AMPLIFY‐EXT Investigators . Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 14. Liu X, Thompson J, Phatak H, Mardekian J, Porcari AR, Johnson M. Apixaban reduces hospitalization in patients with venous thromboembolism: an analysis of the AMPLIFY‐EXT trial. Thromb Haemost. 2015 Oct 8;115(1). [Epub ahead of print]. [DOI] [PubMed]
- 15. Clafin AB, Mitchell CR, Liu X, Phatak H, Mitchell SA. Economic burden of venous thromboembolism across patient populations: a literature review. Presentation at ISPOR Annual European Congress 2013; Dublin, Ireland. [Google Scholar]
- 16. Khorana AA, Dalal MR, Lin J, Connolly GC. Health care costs associated with venous thromboembolism in selected high‐risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon Outcomes Res. 2013;5:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Belt AG, Bossuyt PM, Prins MH, Gallus AS, Buller HR. Replacing inpatient care by outpatient care in the treatment of deep venous thrombosis–an economic evaluation. TASMAN Study Group. Thromb Haemost. 1998;79:259–263. [PubMed] [Google Scholar]
- 18. Lanitis T, Leipold R, Hamilton M, Rublee D, Quon P, Browne C, Cohen A. Cost‐effectiveness of apixaban compared to other anticoagulants for the acute (6‐month) treatment of venous thromboembolism. Eur Heart J. 2014;35(abstract suppl):1064. [DOI] [PubMed] [Google Scholar]
- 19. Aujesky D, Stone RA, Kim S, Crick EJ, Fine MJ. Length of hospital stay and post discharge mortality in patients with pulmonary embolism: a statewide perspective. Arch Intern Med. 2008;168:706–712. [DOI] [PubMed] [Google Scholar]


