Abstract
Background
Impaired pulmonary function (IPF) and left ventricular systolic dysfunction (LVSD) are prevalent in the elderly and are associated with significant morbidity and mortality. The main objectives of this study were to examine the relative impact and joint association of IPF and LVSD with heart failure, cardiovascular mortality and all‐cause mortality, and their impact on risk classification using a continuous net reclassification index.
Methods and Results
We followed 2342 adults without prevalent cardiovascular disease (mean age, 76 years) from the Cardiovascular Health Study for a median of 12.6 years. LVSD was defined as LV ejection fraction <55%. IPF was defined as: forced expiratory volume in 1 second:forced vital capacity <70%, and predicted forced expiratory volume in 1 second <80%. Outcomes included heart failure hospitalization, cardiovascular mortality, all‐cause mortality, and composite outcome. LVSD was detected in 128 subjects (6%), IPF in 441 (19%) and both in 38 (2%). Compared to those without LVSD or IPF, there was a significantly increased cardiovascular risk for groups of LVSD only, IPF only, and LVSD plus IPF, adjusted hazard ratio (95% CI) 2.1 (1.5–3.0), 1.7 (1.4–2.1), and 3.2 (2.0–5.1) for HF; 1.8 (1.2–2.6), 1.4 (1.1–1.8), and 2.8 (1.7–4.7) for cardiovascular mortality; 1.3 (1.0–1.8), 1.7 (1.4–1.9), and 2.1 (1.5–3.0) for all‐cause mortality, and 1.6 (1.3–2.1), 1.7 (1.5–1.9), and 2.4 (1.7–3.3) for composite outcome, respectively. Risk classification improved significantly for all outcomes when IPF was added to the adjusted model with LVSD or LVSD to IPF.
Conclusions
While risk of cardiovascular outcomes was the highest among elderly with both LVSD and IPF, risk was comparable between subjects with IPF alone and those with LVSD alone. This observation, combined with improved risk classification by adding IPF to LVSD or LVSD to IPF, underscore the importance of comprehensive heart and lung evaluation in cardiovascular outcome assessment.
Keywords: elderly, epidemiology, heart failure, left ventricular systolic dysfunction, mortality, pulmonary function
Subject Categories: Epidemiology, Heart Failure, Imaging
Introduction
Left ventricular systolic dysfunction (LVSD) and impaired pulmonary function (IPF) are associated with significant morbidity and mortality,1, 2, 3, 4, 5, 6, 7 and prevalence of both conditions increases with age.1, 5, 8, 9, 10 While cardiovascular disease is a major cause of death in individuals with IPF,7 little is known about the relative impact and the joint association of LVSD and IPF in terms of risk for clinical outcomes. It is particularly important to examine this association in the elderly, as the burden of both diseases is high.
The main objective of this study was to prospectively examine the relative impact and joint association of LVSD and IPF with risk of heart failure, cardiovascular mortality, and all‐cause mortality in the elderly free of prevalent cardiovascular disease who were participants of the Cardiovascular Health Study (CHS), a large population‐based observational study. We also examined the impact of LVSD and IPF on risk classification using a continuous net reclassification index (NRI).
Methods
Study Population and Data Collection
CHS is a population‐based epidemiological study designed to investigate cardiovascular disease in the elderly. The CHS design and recruitment are described in detail elsewhere.11 Briefly, 5201 men and women aged 65 or older were initially recruited in 1989–1990 from Medicare eligibility lists in 4 US communities: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. An additional 687 black participants were recruited in a similar fashion between 1992 and 1993. All participants gave written informed consent and the institutional review board at each study site approved the protocol.
For the purpose of the present study, we selected years 1993–1995 of the CHS as the baseline visit since data on pulmonary function testing was available from years 1993–1994 and an echocardiographic examination12 was performed between 1994 and 1995 on both the original cohort and the African American cohort. Of 3625 participants with both echocardiography and pulmonary function testing data, we excluded participants with a technically inadequate echocardiographic examination for assessing LV function (n=213), those with prevalent coronary heart disease (myocardial infarction, angina, coronary artery bypass grafting, or angioplasty), prevalent heart failure or stroke13 (n=946), or those with missing data for any of the key variables (n=143). Our final sample included 2342 participants.
Assessment of Exposure and Covariates
The design of the echocardiographic examination in CHS has been published.14 All echocardiographic measurements were made at a core echo reading center using an off‐line image analysis system. To ensure reliable measures, technicians’ and readers’ training was standardized. Two‐dimensional echocardiography was used to assess LV ejection fraction qualitatively as normal (≥55%) or impaired (<55%), as previously described.
Centrally trained and certified technicians performed spirometry testing using a volume‐displaced water‐sealed spirometer.15, 16 In the current study, we included forced vital capacity and forced expiratory volume in 1 second (FEV1) as measures of pulmonary function. From a minimum of 5 forced expirations, at least 3 acceptable spirograms were obtained, in accordance with the American Thoracic Society guidelines.17 The best spirogram was identified by the computer and then confirmed by the technician. Predicted FEV1 was calculated from published reference equations derived from the Third National Health and Nutrition Examination Survey as a function of age and height for sex and race subgroups.18 In this study, IPF was defined as FEV1/forced vital capacity <70% and predicted FEV1 <80%.19
Diabetes was defined based on physician diagnosis of diabetes or use of oral antidiabetic medications or insulin. Hypertension was used as a dichotomous variable defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications. Smoking status was categorized as never, former, or current. Alcohol use was categorized as none, <0.5, 0.5 to 1, or >1 drink per day.
Assessment of Outcome
Methods utilized in the CHS to assess cardiovascular mortality and heart failure have been described previously.20 Participants were followed for adjudicated heart failure hospitalization, cardiovascular mortality, and all‐cause mortality from year 1994–1995 until the date of outcome of interest, loss to follow‐up, or the end of the follow‐up period on June 30, 2009. Data on all events of interest were gathered from annual examinations, as well as interim 6‐month phone contacts, and confirmed by independent review committee using information from hospital records, death certificates, autopsy reports, and interviews with physicians or next of kin.
Incident heart failure was confirmed by a centralized committee of physicians if all 3 of the following were met: (1) documented symptoms and signs or clinical findings from imaging (echocardiography, ventriculography, or chest radiograph); (2) physician's confirmed diagnosis; and (3) therapy for heart failure. Cardiovascular mortality included mortality due to coronary heart disease, cerebrovascular disease, atherosclerotic disease other than coronary or cerebrovascular disease, and other cardiovascular disease not codable as any of the above. The composite outcome included heart failure, cardiovascular mortality, and all‐cause mortality.
Statistical Analyses
We investigated the independent, as well as joint, association of LVSD and IPF with heart failure, cardiovascular mortality, all‐cause mortality, as well as the composite outcome (composite of heart failure and mortality). We divided the cohort into 4 exposure categories: (1) no LVSD or IPF (reference); (2) LVSD only, no IPF; (3) IPF only, no LVSD; and (4) both LVSD and IPF. Cox proportional hazards models were used to estimate hazards ratios for outcomes of interest, adjusting for conventional cardiovascular risk factors including age, race and sex, total cholesterol, diabetes, hypertension, smoking history, alcohol use, and body mass index. We also assessed interaction between LVSD and IPF using the likelihood ratio test to compare models with and without the interaction term (LVSD×IPF).
In our risk‐prediction analysis, we calculated the Harrell C statistic and continuous NRI to assess whether risk prediction improves with the addition of IPF to the fully adjusted model with LVSD and vice versa.21, 22 Continuous NRI was calculated as: ([Difference between number of individuals moving to the appropriate risk category and those moving to the inappropriate risk category among cases]/total number of cases+[Difference between number of individuals moving to the appropriate risk category and those moving to the inappropriate risk category among noncases]/total number of noncases). P values <0.05 were considered statistically significant. All statistical analyses were done using STATA 12.23
Results
Of the 2342 participants analyzed, 15% were African Americans and 63% were females. Mean age was 76 years (range 66–95 years). Diabetes and hypertension prevalence was 10% and 57%, respectively. There were 128 (5.5%) participants who had LVSD while 441 (18.8%) had IPF. There were 38 subjects (1.6%) who had both. As expected, those with IPF were more likely to be current or former smokers. Diabetes prevalence was higher among those with LVSD, while hypertension prevalence was the highest among those with both LVSD and IPF (Table 1).
Table 1.
Baseline Characteristics of the Study Population
| All | No LVSD or IPF | LVSD Only, No IPF | IPF Only, No LVSD | Both LVSD and IPF | |
|---|---|---|---|---|---|
| N=2342 | n=1811 (77.3%) | n=90 (3.8%) | n=403 (17.2%) | n=38 (1.6%) | |
| Age, y | 76 (5) | 76 (5) | 76 (5) | 76 (5) | 76 (5) |
| Black, % | 15 | 15 | 9 | 15 | 13 |
| Female, % | 63 | 66 | 49 | 58 | 40 |
| Body mass index, kg/m2 | 26 (4) | 27 (4) | 27 (5) | 26 (4) | 27 (5) |
| Smoking, % | |||||
| Never | 48 | 54 | 54 | 24 | 21 |
| Former | 42 | 39 | 38 | 57 | 63 |
| Current | 10 | 7 | 8 | 19 | 16 |
| Alcohol use, % | |||||
| None | 77 | 79 | 69 | 74 | 66 |
| ≥1 drink/day | 10 | 9 | 11 | 12 | 16 |
| Hypertension, % | 57 | 57 | 50 | 59 | 66 |
| Diabetes, % | 10 | 10 | 16 | 10 | 16 |
| Total cholesterol, mg/dL | 203 (39) | 203 (38) | 198 (39) | 202 (39) | 197 (39) |
Values for continuous variables expressed as mean (SD); values for categorical variables expressed as proportion. IPF indicates impaired pulmonary function; LVSD, left ventricular systolic dysfunction.
Median follow‐up time was 12.6 years (interquartile range: 7.6–14.6 years). Incidence rates for heart failure, cardiovascular mortality, all‐cause mortality, as well as the composite outcome were higher in those with either LVSD or IPF compared to those without LVSD and IPF. When both conditions were present, incidence rates were the highest (Figure 1). Findings remained similar after adjusting for demographic characteristics and traditional cardiovascular risk factors (Table 2, Table S1). There was a lack of multiplicative interaction between LVSD and IPF with interaction P values: 0.649, 0.748, 0.809, and 0.556 for heart failure, cardiovascular mortality, all‐cause mortality, and the composite outcome, respectively.
Figure 1.
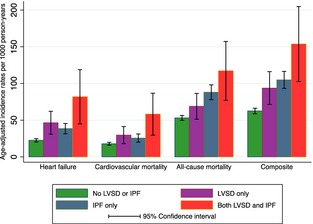
Crude incidence rates per 1000 person‐years with 95% CI for the 4 groups. IPF indicates impaired pulmonary function; LVSD, left ventricular systolic dysfunction.
Table 2.
Relative Risk and Joint Association of LVSD and IPF With Outcomes (Adjusted Hazard Ratios With 95% CI)
| No LVSD or IPF (n=1811) | LVSD Only, No IPF (n=90) | IPF Only, No LVSD (n=120) | Both LVSD and IPF (n=38) | |
|---|---|---|---|---|
| Heart failure (events) | 401 | 34 | 120 | 19 |
| Reference | 2.1 (1.5, 3.1) | 1.7 (1.4, 2.1) | 3.2 (2.0, 5.1) | |
| Cardiovascular mortality (events) | 332 | 25 | 85 | 16 |
| Reference | 1.8 (1.2, 2.6) | 1.4 (1.1, 1.8) | 2.8 (1.7, 4.7) | |
| All‐cause mortality (events) | 999 | 59 | 299 | 33 |
| Reference | 1.3 (1.0, 1.7) | 1.7 (1.4, 1.9) | 2.1 (1.5, 3.0) | |
| Composite (events) | 1103 | 68 | 322 | 35 |
| Reference | 1.6 (1.3, 2.1) | 1.7 (1.5, 1.9) | 2.3 (1.7, 3.3) |
All hazard ratios adjusted for age, race, sex, total cholesterol, history of diabetes, hypertension, smoking, body mass index, and time difference between the 2 exposure measurements. Events indicates number of outcome events; IPF, impaired pulmonary function; LVSD, left ventricular systolic dysfunction.
Kaplan–Meier curves demonstrated that increased risk of heart failure was present early and persisted throughout the follow‐up duration in all 3 subgroups when compared to the reference group (P<0.001) (Figure 2A). The highest cardiovascular mortality risk was seen in the group with both LVSD and IPF, although it was also increased in the other 2 groups compared to the reference group (P<0.001) (Figure 2B). Those with IPF showed a steady increase in risk of all‐cause mortality, which clearly exceeded the risk among those with LVSD in the second half of the follow‐up, while the risk remained the highest among those with both LVSD and IPF (P<0.001) (Figure 2C). The risk of the composite outcome was significantly increased and nearly identical in the LVSD and IPF group when compared to the reference group. Event‐free survival was substantially compromised when both LVSD and IPF were present (P<0.001) (Figure 2D).
Figure 2.
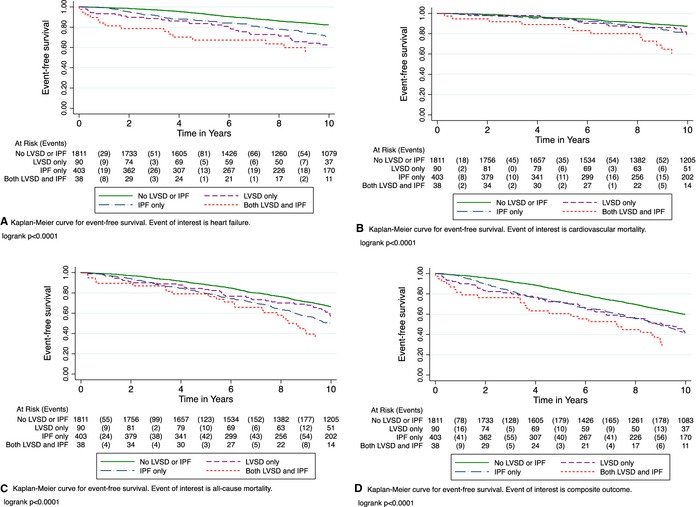
Kaplan–Meier plots for the association of each of the 4 exposure categories with (A) heart failure, (B) cardiovascular mortality, (C) all‐cause mortality, and (D) composite outcome. IPF indicates impaired pulmonary function; LVSD, left ventricular systolic dysfunction.
In the risk‐prediction analyses, addition of IPF to the fully adjusted model including LVSD improved risk prediction for all outcomes (NRI P<0.0001 for heart failure, all‐cause mortality and composite outcome; P=0.05 for cardiovascular mortality). Similarly, when LVSD was added to the fully adjusted model including IPF, there was significant improvement in risk prediction for all outcomes (NRI P<0.01 for all outcomes evaluated) (Table 3). While NRI was similar for the prediction models for cardiovascular mortality and composite outcome, there was a greater risk prediction improvement for heart failure and all‐cause mortality when IPF was added to LVSD compared to the model when LVSD was added to IPF. Similar findings were observed for the C‐statistic.
Table 3.
Risk Prediction Comparing Harrell C‐Statistic and Continuous Net Reclassification Index
| Adding IPF to LVSD | Adding LVSD to IPF | |||||||
|---|---|---|---|---|---|---|---|---|
| C‐Statistic | P Valuea | NRI (95% CI) | C‐Statistic | P Valuea | NRI (95% CI) | |||
| Heart failure | LVSD | 0.693 | IPF | 0.694 | ||||
| +IPF | 0.701 | 0.03 | 14.5 (7.1, 21.9) | +LVSD | 0.701 | 0.02 | 7.9 (1.7, 4.1) | |
| Cardiovascular mortality | LVSD | 0.708 | IPF | 0.706 | ||||
| +IPF | 0.712 | 0.17 | 7.9 (−0.1, 15.9) | +LVSD | 0.712 | 0.03 | 8.1 (2.3, 13.9) | |
| All‐cause mortality | LVSD | 0.670 | IPF | 0.678 | ||||
| +IPF | 0.679 | <0.001 | 24.7 (18.2, 31.1) | +LVSD | 0.679 | 0.09 | 5.4 (1.02, 9.7) | |
| Composite | LVSD | 0.665 | IPF | 0.671 | ||||
| +IPF | 0.674 | <0.001 | 25.8 (19.2, 32.5) | +LVSD | 0.674 | 0.01 | 25.3 (17.3, 33.4) | |
Baseline model includes LVSD, age, race, sex, total cholesterol, and history of diabetes, hypertension, smoking, and body mass index. +IPF=Baseline model+IPF; +LVSD=Baseline model+LVSD. C‐statistic indicates Harrell C‐statistic; IPF, impaired pulmonary function; LVSD, left ventricular systolic dysfunction; NRI, continuous net reclassification index.
P value is for C‐statistic (comparing the 2 C‐statistics).
Discussion
In this large observational study representative of community‐dwelling elderly in the United States, we found that the association of IPF with the risk of heart failure, mortality, and composite outcome was as strong as the association of LVSD with these clinical outcomes. The risk was the highest when both LVSD and IPF were present. Adding IPF to LVSD or LVSD to IPF significantly improved risk classification for all outcomes, underscoring the interdependence of heart and lung function in clinical outcomes.
Our results are consistent with prior studies, which have reported that LVSD is associated with an increased risk of adverse outcomes.1, 3, 4, 5, 24 Similarly, IPF confers a significantly increased risk of poor clinical outcomes.7, 25 Prior observation also suggests that low FEV1 is associated with heart failure risk.26 However, there are limited reports comprehensively comparing the relative impact of LVSD versus IPF on cardiovascular morbidity and mortality. Furthermore, the joint association of LVSD and IPF with outcomes has not been fully examined. IPF is highly prevalent in the elderly population and even more so among those with cardiovascular disease.27, 28, 29, 30 Of note, the prevalence of IPF far exceeded the prevalence of LVSD in this elderly cohort without prevalent cardiovascular disease. Considering the relative risk from IPF, which is comparable to LVSD, the absolute impact of IPF on clinical outcomes is therefore much greater than LVSD in the elderly. Not only the all‐cause mortality, but also heart failure and cardiovascular mortality risk are increased significantly in elderly with IPF. Hence, pulmonary function evaluation proves to be important in risk stratification for cardiovascular outcomes in the elderly. Nonetheless, we view our findings as hypothesis generating in light of the observational nature rather than hypothesis testing. While the changes of C‐statistic and NRI are relatively modest, it is important to recognize that IPF adds additional risk of heart failure and mortality to the already strong predictive power of LVSD for those outcomes, a finding of important clinical relevance because identification of added risk is essential to risk reduction. While there was lack of multiplicative interaction between IPF and LVSD, our findings of improved risk classification by adding IPF to LVSD or LVSD to IPF suggests that LVSD and IPF risks are additive.
The causes of heart failure in the elderly are complex. LVSD is just one of them. Prior publication from the Cardiovascular Heart Study suggests that subclinical contractile dysfunction and diastolic filling abnormalities are predictors of future heart failure, supporting the importance of heart failure with preserved ejection in this cohort.31 It would have been valuable if we had information on diastolic function. However, given that the echocardiography was performed 20 years ago, many of the diastolic parameters such as those from tissue Doppler imaging are not available for this cohort. Therefore, we focused on LVSD, a condition with significant morbidity and mortality risk supported by a large number of clinical as well as epidemiological studies, which provides a valid point of reference for risk estimation, making the relative risk of IPF more important and clinically relevant. Given the comparable relative risk of IPF and LVSD for heart failure and mortality, the joint risk of LVSD and IPF would in fact be greater if we had data on both systolic and diastolic dysfunction at baseline. Hence, we have likely underestimated the true risk.
Among the elderly with both LVSD and IPF, the risk of heart failure and cardiovascular mortality was increased by about 3‐fold compared to those without either condition. Nonetheless, the mechanism of added risk of LVSD and IPF is yet to be elucidated. The conventional cardiovascular risk factors are not likely the explanation for the added risk, as their prevalence was comparable among the subgroups. However, pulmonary hypertension is possibly an important comorbidity, which can result from LVSD32, 33, 34 or IPF.35, 36 Whether due to LVSD37, 38 or IPF,39, 40, 41 pulmonary hypertension is associated with significantly increased morbidity and mortality. In addition to the postulated role of pulmonary hypertension, there is evidence demonstrating extensive redistribution of pulmonary blood flow in individuals with hemodynamically significant LVSD, underscoring a complex heart–lung interaction.42 Fundamentally, it is the intricate interplay of the heart and lung function that determines clinical outcomes among patients with LVSD. Cardiopulmonary exercise testing, which comprehensively measures the expiratory ventilation, pulmonary gas exchange, and cardiac output is one of the best predictors of adverse clinical outcomes for patients with chronic heart failure supporting the critical role of heart and lung interaction in heart failure pathophysiology.43, 44, 45, 46, 47, 48 Our cohort consisted of community‐dwelling older adults without history of heart failure. Nonetheless, impaired pulmonary and cardiac function collectively contributed to the risk of heart failure and mortality, an observation that extends the implication of heart and lung interaction beyond patients with chronic heart failure.
Our study has several strengths. We studied a large, well‐established elderly cohort with a relatively long‐term follow‐up period. All outcomes were carefully adjudicated. However, the study limitations must also be acknowledged. First, since this was an elderly cohort with a mean age of 76 years, the generalizability of our findings to younger age groups may be limited. Second, the pulmonary function evaluation was performed 1 year ahead of LV function assessment. Given that IPF typically develops over time, it is unlikely that pulmonary function changed substantially within a year. Our analysis was based on a 1‐time assessment of cardiac and pulmonary function. The development of LVSD and IPF during the follow‐up period was not ascertained. Therefore, it is possible that the effect of LVSD and IPF on outcomes was underestimated. Third, we lacked data on pulmonary pressure estimation by echocardiography in most of the cohort, which could have provided further insight into the potential mechanisms by which these 2 conditions were associated with adverse outcomes, possibly through a common intermediary pathway such as pulmonary hypertension. Fourth, the etiologies that contribute to IPF were not well characterized in this epidemiological cohort. In light of the prevalent smoking history in the majority of those having IPF, chronic obstructive pulmonary disease likely played an important role. Fifth, we had a relatively smaller sample size in the joint category (both LVSD and IPF), although still reasonably powered (80%) because of the high outcome rate (92%). Limited power also prevented risk assessment analyses stratified by the severity of LVSD. Last, we acknowledge that a relatively small number of participants had significant LVSD, which may contribute to some underestimation of the association of LVSD with outcomes. It is also important to acknowledge the limitations of the echocardiography data reported in this article. First, LVSD was based on qualitative assessment of left ventricular ejection fraction and not quantitative assessment. Second, left ventricular ejection fraction is load dependent and not a perfect measure for LVSD. Last, newer measures such as tissue Doppler were not available at the time of this study, thus limiting assessment of diastolic function.
In conclusion, there was increased and comparable risk of heart failure, mortality, and the composite outcome in a community‐dwelling elderly with LVSD or IPF. The risk was the highest when both conditions were present. Adding IPF to LVSD or LVSD to IPF significantly improved risk classification for all outcomes, underscoring the interdependence of heart and lung function in clinical outcomes. Our findings demonstrate the importance of comprehensive heart and lung evaluation in cardiovascular outcome assessment in the elderly.
Sources of Funding
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Disclosures
None.
Supporting information
Table S1. Relative Risk and Joint Association of LVSD and IPF With Outcomes.
(J Am Heart Assoc. 2015;4:e002308 doi: 10.1161/JAHA.115.002308)
An accompanying Table S1 is available at http://jaha.ahajournals.org/content/4/12/e002308/suppl/DC1
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population‐based cohort. J Am Coll Cardiol. 1999;33:1948–1955. [DOI] [PubMed] [Google Scholar]
- 2. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. [DOI] [PubMed] [Google Scholar]
- 3. Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. [DOI] [PubMed] [Google Scholar]
- 4. Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. [DOI] [PubMed] [Google Scholar]
- 5. Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. [DOI] [PubMed] [Google Scholar]
- 6. Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. Prognosis of all‐cause heart failure and borderline left ventricular systolic dysfunction: 5 year mortality follow‐up of the Echocardiographic Heart of England Screening Study (ECHOES). Eur Heart J. 2007;28:1128–1134. [DOI] [PubMed] [Google Scholar]
- 7. Mannino DM, Doherty DE, Sonia Buist A. Global Initiative on Obstructive Lung Disease (GOLD) classification of lung disease and mortality: findings from the Atherosclerosis Risk in Communities (ARIC) study. Respir Med. 2006;100:115–122. [DOI] [PubMed] [Google Scholar]
- 8. Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population. The Rotterdam Study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 9. Mosterd A. Heart failure in the population at large; news from the real world. Eur Heart J. 1999;20:398–399. [PubMed] [Google Scholar]
- 10. Nielsen OW, Hilden J, Larsen CT, Hansen JF. Cross sectional study estimating prevalence of heart failure and left ventricular systolic dysfunction in community patients at risk. Heart. 2001;86:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O'Leary DH, Psaty B, Rautaharju P, Tracy RP, Weiler PG, for the Cardiovascular Health Study Research Group. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 12. Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, Manolio TA, Dries DL, Siscovick DS. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol. 2004;43:2207–2215. [DOI] [PubMed] [Google Scholar]
- 13. Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. [DOI] [PubMed] [Google Scholar]
- 14. Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton‐Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free‐living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. [DOI] [PubMed] [Google Scholar]
- 15. Enright PL, Kronmal RA, Higgins M, Schenker M, Haponik EF. Spirometry reference values for women and men 65 to 85 years of age. Cardiovascular Health Study. Am Rev Respir Dis. 1993;147:125–133. [DOI] [PubMed] [Google Scholar]
- 16. Enright PL, Arnold A, Manolio TA, Kuller LH. Spirometry reference values for healthy elderly blacks. The Cardiovascular Health Study Research Group. Chest. 1996;110:1416–1424. [DOI] [PubMed] [Google Scholar]
- 17. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 18. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. [DOI] [PubMed] [Google Scholar]
- 19. Global Strategy for Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Available at: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Sept2.pdf. Accessed December 1, 2015.
- 20. Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. [DOI] [PubMed] [Google Scholar]
- 21. Newson RB. Comparing the predictive powers of survival models using Harrell's C or Somers’ D. Stata J. 2010;10:339–358. [Google Scholar]
- 22. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207‐12. [DOI] [PubMed] [Google Scholar]
- 23. StataCorp LP . Statistics/data analysis. 4905 Lakeway Drive CS, TX. [Google Scholar]
- 24. Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin. 2007;3:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gershon AS, Warner L, Cascagnette P, Victor JC, To T. Lifetime risk of developing chronic obstructive pulmonary disease: a longitudinal population study. Lancet. 2011;378:991–996. [DOI] [PubMed] [Google Scholar]
- 26. Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. [DOI] [PubMed] [Google Scholar]
- 27. Mullerova H, Agusti A, Erqou S, Mapel DW. Cardiovascular comorbidity in chronic obstructive pulmonary disease: systematic literature review. Chest. 2013;144:1163–1178. [DOI] [PubMed] [Google Scholar]
- 28. Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65:956–962. [DOI] [PubMed] [Google Scholar]
- 29. Iversen KK, Kjaergaard J, Akkan D, Kober L, Torp‐Pedersen C, Hassager C, Vestbo J, Kjoller E; Group EC‐LFS . Chronic obstructive pulmonary disease in patients admitted with heart failure. J Intern Med. 2008;264:361–369. [DOI] [PubMed] [Google Scholar]
- 30. Mascarenhas J, Lourenco P, Lopes R, Azevedo A, Bettencourt P. Chronic obstructive pulmonary disease in heart failure. Prevalence, therapeutic and prognostic implications. Am Heart J. 2008;155:521–525. [DOI] [PubMed] [Google Scholar]
- 31. Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2001;37:1042–1048. [DOI] [PubMed] [Google Scholar]
- 32. Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34:1802–1806. [DOI] [PubMed] [Google Scholar]
- 33. Guazzi M, Arena R. Pulmonary hypertension with left‐sided heart disease. Nat Rev Cardiol. 2010;7:648–659. [DOI] [PubMed] [Google Scholar]
- 34. Georgiopoulou VV, Kalogeropoulos AP, Borlaug BA, Gheorghiade M, Butler J. Left ventricular dysfunction with pulmonary hypertension: part 1: epidemiology, pathophysiology, and definitions. Circ Heart Fail. 2013;6:344–354. [DOI] [PubMed] [Google Scholar]
- 35. Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducolone A, Ehrhart M, Kessler R, Weitzenblum E. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–194. [DOI] [PubMed] [Google Scholar]
- 36. Minai OA, Chaouat A, Adnot S. Pulmonary hypertension in COPD: epidemiology, significance, and management: pulmonary vascular disease: the global perspective. Chest. 2010;137:39S–51S. [DOI] [PubMed] [Google Scholar]
- 37. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. [DOI] [PubMed] [Google Scholar]
- 38. Szwejkowski BR, Elder DH, Shearer F, Jack D, Choy AM, Pringle SD, Struthers AD, George J, Lang CC. Pulmonary hypertension predicts all‐cause mortality in patients with heart failure: a retrospective cohort study. Eur J Heart Fail. 2012;14:162–167. [DOI] [PubMed] [Google Scholar]
- 39. Oswald‐Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, Kessler R. Prognostic factors in COPD patients receiving long‐term oxygen therapy. Importance of pulmonary artery pressure. Chest. 1995;107:1193–1198. [DOI] [PubMed] [Google Scholar]
- 40. Doi M, Nakano K, Hiramoto T, Kohno N. Significance of pulmonary artery pressure in emphysema patients with mild‐to‐moderate hypoxemia. Respir Med. 2003;97:915–920. [DOI] [PubMed] [Google Scholar]
- 41. Sims MW, Margolis DJ, Localio AR, Panettieri RA, Kawut SM, Christie JD. Impact of pulmonary artery pressure on exercise function in severe COPD. Chest. 2009;136:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cao JJ, Wang Y, McLaughlin J, Rhee P, Passick M, Ngai N, Cheng J, Gulotta RJ, Berke AD, Petrossian GA, Reichek N. Effects of hemodynamics on global and regional lung perfusion: a quantitative lung perfusion study by magnetic resonance imaging. Circ Cardiovasc Imaging. 2012;5:693–699. [DOI] [PubMed] [Google Scholar]
- 43. Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. [DOI] [PubMed] [Google Scholar]
- 44. Cohn JN, Johnson GR, Shabetai R, Loeb H, Tristani F, Rector T, Smith R, Fletcher R. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V‐HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI5–VI16. [PubMed] [Google Scholar]
- 45. Balady GJ, Arena R, Sietsema K, Myers J, Coke L, Fletcher GF, Forman D, Franklin B, Guazzi M, Gulati M, Keteyian SJ, Lavie CJ, Macko R, Mancini D, Milani RV; American Heart Association Exercise CR, Prevention Committee of the Council on Clinical C, Council on E, Prevention, Council on Peripheral Vascular D, Interdisciplinary Council on Quality of C and Outcomes R . Clinician's Guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. [DOI] [PubMed] [Google Scholar]
- 46. Szlachcic J, Massie BM, Kramer BL, Topic N, Tubau J. Correlates and prognostic implication of exercise capacity in chronic congestive heart failure. Am J Cardiol. 1985;55:1037–1042. [DOI] [PubMed] [Google Scholar]
- 47. Likoff MJ, Chandler SL, Kay HR. Clinical determinants of mortality in chronic congestive heart failure secondary to idiopathic dilated or to ischemic cardiomyopathy. Am J Cardiol. 1987;59:634–638. [DOI] [PubMed] [Google Scholar]
- 48. Arena R, Sietsema KE. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011;123:668–680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relative Risk and Joint Association of LVSD and IPF With Outcomes.


