Abstract
Background
Air pollution is associated with greater cardiovascular event risk, but the types of events and specific persons at risk remain unknown. This analysis evaluates effects of short‐term exposure to fine particulate matter air pollution with risk of acute coronary syndrome events, including ST‐segment elevation myocardial infarction, non–ST‐segment elevation myocardial infarction, unstable angina, and non–ST‐segment elevation acute coronary syndrome.
Methods and Results
Acute coronary syndrome events treated at Intermountain Healthcare hospitals in urban areas of Utah's Wasatch Front were collected between September 1993 and May 2014 (N=16 314). A time‐stratified case‐crossover design was performed matching fine particulate matter air pollution exposure at the time of each event with referent periods when the event did not occur. Patients served as their own controls, and odds ratios were estimated using nonthreshold and threshold conditional logistic regression models. In patients with angiographic coronary artery disease, odds ratios for a 10‐μg/m3 increase in concurrent‐day fine particulate matter air pollution >25 μg/m³ were 1.06 (95% CI 1.02–1.11) for all acute coronary syndrome, 1.15 (95% CI 1.03–1.29) for ST‐segment elevation myocardial infarction, 1.02 (95% CI 0.97–1.08) for non–ST‐segment elevation myocardial infarction, 1.09 (95% CI 1.02–1.17) for unstable angina, and 1.05 (95% CI 1.00–1.10) for non–ST‐segment elevation acute coronary syndrome events. Excess risk from fine particulate matter air pollution exposure was not observed in patients without angiographic coronary artery disease.
Conclusions
Elevated fine particulate matter air pollution exposures contribute to triggering acute coronary events, especially ST‐segment elevation myocardial infarction, in those with existing seriously diseased coronary arteries but not in those with nondiseased coronary arteries.
Keywords: acute coronary syndrome, air pollution, cardiovascular disease, particulate matter, ST‐segment elevation myocardial infarction
Subject Categories: Epidemiology, Cardiovascular Disease, Acute Coronary Syndromes
Introduction
A substantial body of evidence indicates that exposure to ambient fine particulate matter air pollution (fine particulate matter ≤2.5 μm in aerodynamic diameter [PM2.5]) contributes to cardiovascular morbidity and mortality.1, 2 Various prospective cohort studies of long‐term exposure (years or decades) have found that elevated PM2.5 exposures are associated with increased risk of cardiovascular disease mortality3, 4, 5, 6, 7, 8, 9 and may contribute to the initiation and progression of related chronic diseases including atherosclerosis, hypertension, and diabetes.10, 11 The Global Burden of Disease 2010 analysis reported comparative burden of disease risk assessments from 67 risk factors. These assessments estimate that both ambient and household air pollution are among the top 10 contributors to global burden of disease, in large part because of the estimated effect of PM2.5 on ischemic heart disease.12
There is also evidence that short‐term exposure (hours to a few days) to PM2.5 may help trigger acute coronary syndrome (ACS) events including myocardial infarction (MI) and unstable angina (UA) events,13, 14 especially in persons with preexisting coronary artery disease (CAD).15, 16, 17 Furthermore, a recent study reported evidence that short‐term elevations in PM2.5 exposure trigger ST‐segment elevation MI (STEMI) but not non‐STEMI (NSTEMI).18
The present study used ≈20 years of ACS event data from a large, ongoing registry of well‐characterized patients who underwent coronary arteriography.19, 20 These data were linked with air pollution and weather data and analyzed using a case‐crossover design. This study had 3 specific objectives: (1) Evaluate the effects of elevations in short‐term exposure to PM2.5 on ACS events, including STEMI, NSTEMI, UA, and non–ST‐segment elevation ACS (NSTE‐ACS); (2) explore the potential triggering effects of PM2.5, specifically for persons with existing angiographic CAD, defined in this analysis as ≥1 coronary artery with ≥70% maximal stenosis as determined at angiography; and (3) explore potential effect modification of various other indicators of preexisting disease and patient characteristics. In addition, sensitivity analysis of the results of various modeling choices, including nonthreshold versus threshold models, was conducted.
Methods
Study Area and Participants
The study area was Utah's Wasatch Front, which includes a narrow strip of land (≈80 miles long from north to south) bordered on the east by the Wasatch Mountain range and on the west by the Great Salt Lake, Utah Lake, and smaller mountain ranges. Approximately 80% of Utah's population lives in the Wasatch Front communities that are part of 3 nearly contiguous metropolitan areas: the city of Ogden and surrounding communities to the north, Salt Lake City and surrounding communities located in the center, and Provo/Orem and surrounding communities to the south. This relatively well‐defined area experiences substantial variability in air pollution resulting from densely populated mountain valley topography and frequent temperature inversions.
Study participants included patients who received coronary angiography within the Utah‐based Intermountain Healthcare system and who participated in the catheterization registry of the Intermountain Heart Collaborative Study.19, 20 Male and female patients of unrestricted age were included in the registry. All angiograms were performed during the ACS admission based on a referral due to clinical signs or symptoms and clinical laboratory evidence of acute MI or unstable chest pain. Consistent with the case‐crossover design, only patients with these ACS events were included in the study.
Patient information included residential address, age, sex, smoking history (active or previous, >10 pack‐years), and body mass index. Other information on preexisting disease and patient characteristics included angiographic CAD, defined as ≥1 coronary artery with ≥70% maximal stenosis as determined at angiography; congestive heart failure, as reported by a physician based on clinical symptoms; hypertension, as reported by a physician for systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive agents; hyperlipidemia, as reported by a physician for total cholesterol ≥200 mg/dL, low‐density lipoprotein level ≥130 mg/dL, or use of cholesterol‐lowering medication; diabetes, as determined based on physician‐reported fasting blood sugar level ≥126 mg/dL or use of an antidiabetic medication; and family history, based on self‐reported information that a first‐order relative suffered cardiovascular death, MI, or coronary revascularization at age <65 years.
Specific ACS event data used for this analysis included information on event onset date and the type of acute coronary event. The specific ACS events studied included STEMI,21 NSTEMI22 (including MI not specifically diagnosed as STEMI), and UA.22 The diagnoses of STEMI, NSTEMI, and UA were not based directly on billing codes but rather on a clinical diagnosis (overall clinical evidence including symptoms, electrocardiographic evidence, and cardiac troponin levels)22 made by the cardiologist at the time of angiography. In addition, as part of this analysis, NSTEMI and UA events were combined to approximately represent overall NSTE‐ACS events.22 Subsequent MI events identified by searching the Intermountain Healthcare electronic medical records database were also included. Between September 10, 1993, and May 15, 2014, a total of 27 486 ACS events were identified. Most patients lived in Utah and in neighboring western states. This analysis was restricted to the 16 314 eligible events experienced by patients who lived in the Wasatch Front study area (based on ZIP code of patient residency).
The study used deidentified data from the cardiac catheterization database registry and health care electronic medical records database under a data use agreement that required data security and confidentiality. The study was approved by the Intermountain Healthcare institutional review board and was granted a waiver of informed consent.
Pollution and Weather Data
Daily weather data were collected from the National Weather Service using the Salt Lake City International Airport Station from January 1, 1993, through September 29, 2014. Weather data collected included daily minimum, average, and maximum temperatures (Fahrenheit); dew point temperature (Fahrenheit); and barometric pressure (inches).
Particulate air pollution data for PM2.5 and inhalable particulate matter ≤10 μm in aerodynamic diameter (PM10) were compiled from the Utah Department of Environmental Quality, Division of Air Quality, and the U.S. Environmental Protection Agency Air Quality System for the time period January 1, 1993, through September 29, 2014. Monitoring was conducted in accordance with the Environmental Protection Agency federal reference method.23 Available data from monitoring sites in the Wasatch Front study area from January 1, 1993, to September 29, 2014, were collected. Observations of PM10 concentration >200 μg/m3 caused by extreme windstorms were deleted.
PM2.5 exposure estimates were based primarily on data from 3 monitoring sites located in the 3 Wasatch Front metro areas: the Ogden site in Ogden, the Hawthorne site in Salt Lake City, and the Lindon site in the Provo/Orem area. PM2.5 and PM10 data were collected from each of these 3 primary sites and from nearby secondary sites (North Provo, Spanish Fork, Bountiful, Salt Lake City–Rose Park, and Salt Lake City–AMC monitoring sites). Daily PM2.5 concentrations for each of the 3 primary monitoring sites were regressed on PM2.5 data collected from surrounding sites, yielding highly correlated results (R 2 values 0.74–0.94). Missing daily PM2.5 concentrations at each central site were imputed using the regression results and the PM2.5 concentrations from the most highly correlated monitor with nonmissing PM2.5 data. PM2.5 concentrations that were still missing after imputations using nearby PM2.5 monitors were estimated using winter (December to February) and nonwinter ratios of PM2.5 to PM10 from the nearest monitoring site with nonmissing data. During winter months, low‐level temperature inversion episodes become more common, trapping local emissions in a stagnant air mass near the valley floor and leading to elevated particulate matter concentrations. These stagnant air conditions result in a relatively high PM2.5/PM10 ratio, whereas summer months have lower PM2.5/PM10 ratios. Assignments of exposure for the event period and the referent periods are based on the Wasatch Front metropolitan area of the patient's residence (based on ZIP code) and the PM2.5 exposure estimates for that metro area. Overall, 79% of PM2.5 concentrations for all eligible event and referent days were from central‐site monitoring, 20% were imputed (as indicated above), and 1% were missing.
Statistical Analysis
The primary statistical analysis of ACS events and ambient PM2.5 is based on a time‐stratified case‐crossover design.24, 25, 26 This approach matches exposures at the time of event with ≥1 period when the event did not occur (control or referent periods) and estimates the potential excess risk using conditional logistic regression. Control or referent periods are matched for day of the week in the same month and year as the event, resulting in up to 3 or 4 control periods per event date. By choosing these matching referent periods close in time and on the same day of the week, time‐dependent risk factors (including day of week, seasonality, and long‐term time trends) were controlled for by design. Furthermore, because patients served as their own controls, there was near‐perfect matching for participant‐specific characteristics that do not vary over time (eg, age, smoking status, health history).
The initial model used to analyze the data was a conditional logistic regression model that included concurrent‐day PM2.5 (central monitor plus imputed values) as a simple linear nonthreshold model and controlled for weather variables (minimum temperature, dew point temperature, barometric pressure, and squared terms for each). Based on the Akaike information criterion, alternative threshold models were also estimated that allowed for no effect up to a certain threshold and a linear effect thereafter. In addition, we estimated a threshold model with the threshold specifically set at the current U.S. National Ambient Air Quality Standard for PM2.5 of 35 μg/m3. Odds ratios using both the standard linear and threshold models were estimated for all ACS, STEMI, NSTEMI, UA, and NSTE‐ACS events for all patients and for patients with and without CAD. For comparison purposes, and consistent with reporting from various studies and reviews of the literature,1, 2, 3, 4, 5, 6, 7, 8, 11, 14, 15, 16 odds ratios associated with 10‐μg/m3 incremental increases in PM2.5 concentration are presented.
To explore the sensitivity of the results, odds ratios were estimated and compared from models that did not control for weather variables, that excluded imputed PM2.5 data, that excluded subsequent ACS events that occurred in the same month as a previous event, that included control or referent periods matched on the same day of week for the 3 weeks prior to the event with no referent periods following the event, and that had alternative lagged moving average exposures. In addition, analyses stratified by various indicators of preexisting disease and patient characteristics were conducted.
Results
Summary information for daily PM2.5 concentrations is provided in Table 1. Figure 1 presents the daily PM2.5 concentrations at the most central monitor (Salt Lake City–Hawthorne) for the full study period (Figure 1A) and, for more detailed illustration, a sample shorter period from September 2003 through April 2004 (Figure 1B). On average, the air pollution in the study area during the study period was not high (≈10 μg/m3), with most days <20 or <25 μg/m3; however, there were days, especially during wintertime temperature inversions, when PM2.5 concentrations were substantially elevated. This large variability in PM2.5 concentrations provided an excellent research opportunity (natural experiment) to evaluate whether short‐term elevations in PM2.5 were associated with elevated risk of acute coronary events.
Table 1.
Summary of Available Daily Particulate Matter Concentrations at the 3 Primary Monitoring Sites (January 1993 to September 2014)
| Monitoring Sites | n | Mean | SD | Maximum | Median | IQR |
|---|---|---|---|---|---|---|
| Ogden | ||||||
| PM2.5 monitored | 3443 | 9.9 | 9.2 | 108 | 7.2 | 5.0–10.7 |
| +imputed | 7698 | 10.6 | 9.1 | 108 | 7.9 | 5.8–11.5 |
| Salt Lake City, Hawthorne | ||||||
| PM2.5 monitored | 5668 | 10.6 | 11.0 | 94 | 7.0 | 5.0–10.9 |
| +imputed | 7855 | 10.5 | 10.4 | 94 | 7.2 | 5.0–11.0 |
| Provo/Orem, Lindon | ||||||
| PM2.5 monitored | 5415 | 10.0 | 10.2 | 123 | 6.9 | 4.8–10.8 |
| +imputed | 7697 | 10.7 | 10.8 | 123 | 7.4 | 5.1–11.7 |
IQR indicates interquartile range; PM2.5, fine particulate matter ≤2.5 μm in aerodynamic diameter.
Figure 1.
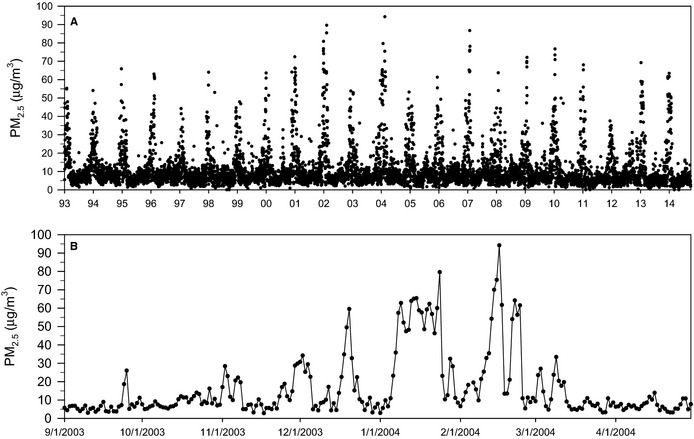
Daily particulate matter concentrations (μg/m3) at Salt Lake City (Hawthorne monitor, plus imputed) from January 1993 through September 2014 (A) and, for a more detailed illustration, a shorter sample period from September 2003 through April 2004 (B). PM 2.5 indicates fine particulate matter ≤2.5 μm in aerodynamic diameter.
Table 2 presents baseline participant characteristics for ACS events, separated by STEMI, NSTEMI, and UA events. Note that there is substantial preexisting cardiovascular disease, and 76% of all events and 95% of STEMI events occurred in patients with clinically significant CAD.
Table 2.
Baseline Participant Characteristics for Acute Coronary Syndrome Events Studied
| Characteristic | All Events (n=16 314) | STEMI (n=1274) | NSTEMI (n=9515) | UA (n=5525) | NSTE‐ACS (n=15 040) |
|---|---|---|---|---|---|
| Age, y | 64±13 | 62±13 | 64±13 | 63±12 | 64±13 |
| Male, % | 67 | 73 | 66 | 68 | 67 |
| Smoking, % | 26 | 33 | 25 | 26 | 25 |
| BMI, kg/m2 | 29±6 | 30±6 | 29±6 | 30±6 | 29±6 |
| CHF, % | 17 | 13 | 21 | 10 | 17 |
| Hypertension, % | 60 | 49 | 57 | 67 | 61 |
| Hyperlipidemia, % | 59 | 44 | 54 | 70 | 60 |
| Diabetes, % | 24 | 19 | 24 | 26 | 25 |
| Family history, % | 40 | 38 | 35 | 50 | 41 |
| CAD, % | 76 | 95 | 75 | 74 | 75 |
BMI indicates body mass index; CAD, coronary artery disease; CHF, congestive heart failure; NSTE‐ACS, non–ST‐segment elevation acute coronary syndrome; NSTEMI, non–ST‐segment elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction; UA, unstable angina.
Table 3 presents the odds ratios (and 95% CIs) associated with a 10‐μg/m3 increase in concurrent‐day PM2.5 for nonthreshold and threshold models. Based on the Akaike information criterion, the alternative threshold model (which allowed for no effect up to 25 μg/m3 PM2.5, with a linear effect thereafter) fit the data better than the linear model. Table 3 presents results for all events and for the subgroups of STEMI, NSTEMI, UA, and NSTE‐ACS events stratified by those with and without CAD for both the nonthreshold and threshold models. In general, elevated concurrent‐day PM2.5 exposures were associated with greater risk of ACS events, but the results differed substantially depending on the type of event and whether or not the patient had existing CAD. Significant PM2.5 effects were observed only for patients with preexisting CAD (potentially in part due to the lower sample size). PM2.5 effects were observed for STEMI events but not for NSTEMI events. PM2.5 effects were also observed for UA events, particularly for those with CAD. Based on results presented in Table 3, it is also apparent that the effect estimates were larger for increases in PM2.5 >25 μg/m3 based on the threshold model. The nonthreshold model suggests that for those with existing CAD, a 10‐μg/m3 increase in PM2.5 is associated with the risk for all ACS, STEMI, and UA events by ≈3%, 9%, and 6%, respectively. The threshold model suggests that there is minimal or no greater risk up to 25 μg/m3, but every 10‐μg/m3 increase in PM2.5 over that threshold is associated with greater risk for all ACS, STEMI, UA, and NSTE‐ACS events by ≈6%, 15%, 9%, and 5%, respectively.
Table 3.
Number of ACS Events and Adjusted ORs (and 95% CIs) Associated With a 10‐μg/m3 Increase in Concurrent‐Day PM2.5 Concentration for Nonthreshold and Threshold Models
| Event | No. of Eventsa | OR (95% CI) per 10 μg/m3, >0 μg/m3 | OR (95% CI) per 10 μg/m3, >25 μg/m3 |
|---|---|---|---|
| All events | 16 148 | 1.02 (1.00–1.05)c | 1.05 (1.01–1.09)e |
| All, with CADb | 12 290 | 1.03 (1.00–1.06)d | 1.06 (1.02–1.11)e |
| All, without CAD | 3858 | 1.00 (0.96–1.05) | 1.00 (0.93–1.08) |
| STEMI, All | 1262 | 1.08 (1.01–1.16)d | 1.13 (1.01–1.26)d |
| STEMI, with CAD | 1196 | 1.09 (1.01–1.17)d | 1.15 (1.03–1.29)d |
| STEMI, without CAD | 66 | 0.95 (0.66–1.35) | 0.68 (0.32–1.41) |
| NSTEMI, All | 9418 | 1.00 (0.97–1.03) | 1.01 (0.96–1.06) |
| NSTEMI, with CAD | 7032 | 1.00 (0.96–1.03) | 1.02 (0.97–1.08) |
| NSTEMI, without CAD | 2386 | 1.01 (0.95–1.07) | 0.98 (0.89–1.08) |
| UA, All | 5468 | 1.04 (1.00–1.09)d | 1.09 (1.02–1.16)e |
| UA, with CAD | 4062 | 1.06 (1.01–1.11)d | 1.09 (1.02–1.17)d |
| UA, without CAD | 1406 | 1.00 (0.92–1.09) | 1.08 (0.94–1.23) |
| NSTE‐ACS, All | 14 886 | 1.02 (0.99–1.04) | 1.04 (1.00–1.08)c |
| NSTE‐ACS, with CAD | 11 094 | 1.02 (0.99–1.05) | 1.05 (1.00–1.10)d |
| NSTE‐ACS, without CAD | 3792 | 1.01 (0.96–1.06) | 1.01 (0.93–1.09) |
ACS indicates acute coronary syndrome; CAD, coronary artery disease; NSTE‐ACS, non–ST‐segment elevation ACS; NSTEMI, non–ST‐segment elevation myocardial infarction; OR, odds ratio; PM2.5, fine particulate matter ≤2.5 μm in aerodynamic diameter; STEMI, ST‐segment elevation myocardial infarction; UA, unstable angina.
The number of events used in each of the conditional logistic regressions may be slightly smaller than the total available, as presented in Table 2, because of missing air pollution or weather data.
CAD indicates seriously diseased coronary vessels, defined as ≥1 coronary artery with ≥70% maximal stenosis as determined at angiography.
P<0.10.
P<0.05.
P<0.01.
Figure 2 presents comparisons of estimated odds ratios for those with existing CAD compared across various models and alternative lagged exposures. The size and pattern of PM2.5 effects are not highly sensitive to the inclusion or exclusion of weather variables in the models or to whether or not the imputed PM2.5 data are included in the analysis. There were ≈560 subsequent ACS events that occurred in the same month as a previous event. When these events were excluded, results were nearly identical. Similarly, the results were not sensitive to the selection of only referent periods 3 weeks prior to the event. PM2.5 effect estimates were similar for concurrent‐day, previous‐day, and 2‐day lagged moving average, but fit was somewhat better for concurrent‐day exposures. PM2.5 effects declined gradually for longer lagged moving average exposures. Based on the Akaike information criterion, threshold models that allowed for no effect up to 25 μg/m3 of PM2.5 and then for a linear effect thereafter generally fit the data best. A threshold model that allowed for no effect up to the current U.S. National Ambient Air Quality Standard for PM2.5 of 35 μg/m3 did not fit the data quite as well as the model with a threshold at 25 μg/m3, but it indicated significant increase in risk of ACS at levels above the national standard.
Figure 2.
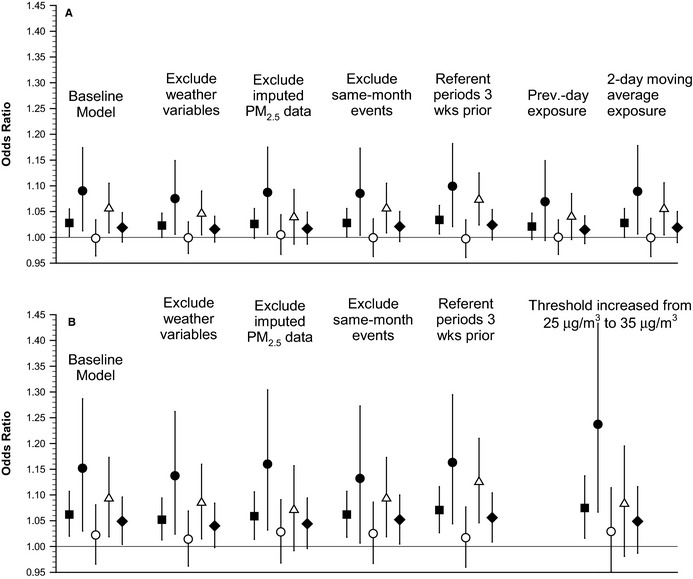
Odds ratios (and 95% CIs) associated with a 10‐μg/m3 increase in PM 2.5 for ACS events for persons with coronary artery disease compared across various models. Filled squares represent all ACS events, filled circles represent STEMI events, open circles represent non‐STEMI events, open triangles represent unstable angina events, and filled diamonds represent non–ST‐segment elevation ACS events. A, Results from nonthreshold models. B, Results from models with a PM 2.5 threshold of 25 μg/m3, except for 1 model with a threshold of 35 μg/m3, as labeled. ACS indicates acute coronary syndrome; PM 2.5, fine particulate matter ≤2.5 μm in aerodynamic diameter; Prev., previous; STEMI, ST‐segment elevation myocardial infarction; wks, weeks.
Figure 3 presents odds ratios for acute coronary events associated with a 10‐μg/m3 increase in PM2.5 for those with CAD, using both the nonthreshold and threshold models stratified by indicators of preexisting disease and patient characteristics. Although there was some evidence of differential effects across strata, only a few differences were statistically significant (P<0.05) based on formal statistical tests of effect modification, and even these significant differential effects were not consistent across types of events. Having hyperlipidemia, being aged <65 years, being male, and having a history of smoking were associated with greater estimated PM2.5 effects for STEMI but not for UA. Congestive heart failure and older age were associated with greater estimated PM2.5 effects for UA.
Figure 3.
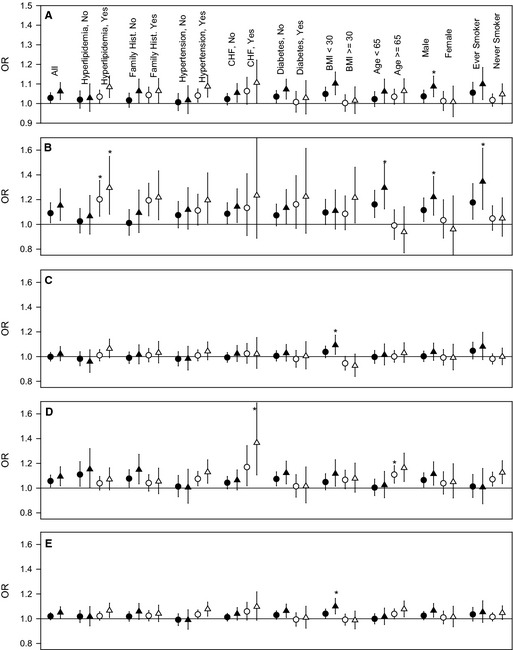
ORs (and 95% CIs) associated with a 10‐μg/m3 increase in PM 2.5 for all ACS (A), ST‐segment elevation myocardial infarction (B), non‐STEMI (C), unstable angina (D), and non–ST‐segment elevation ACS (E) events for those with coronary artery disease across various strata. Circles represent results from the standard nonthreshold models, and triangles represent results from models with a PM 2.5 threshold of 25 μg/m3. *Strata with a statistically significant (P<0.05) larger effect vs the alternate strata. ACS indicates acute coronary syndrome; BMI, body mass index; CHF, congestive heart failure; Hist., history; OR, odds ratio; PM 2.5, fine particulate matter ≤2.5 μm in aerodynamic diameter; STEMI, ST‐segment elevation myocardial infarction.
Discussion
The results of this analysis indicate that elevated short‐term exposures of PM2.5 are associated with greater risk of an acute coronary event and that the effect of exposures is substantially dependent on the type of event and whether or not the patient has preexisting coronary disease. Significant PM2.5 effects were observed only for patients with existing CAD. These results are largely consistent with previously reported results16, 17 and suggest that elevated PM2.5 exposures can contribute to triggering of acute coronary events, acutely destabilizing and rupturing atherosclerotic plaque, in those with existing clinically significant CAD but not in those with clean coronary arteries.
Another key finding of this analysis is that PM2.5‐related effects on MI were observed for STEMI events and not for NSTEMI events. These results are somewhat consistent with a recent study using data from the University of Rochester Medical Center Cardiac Catheterization Laboratory18 that observed that increased PM2.5 concentrations in the hour prior to ACS onset were associated with STEMI events but not with NSTEMI events. They reported that each 7.1‐μg/m3 increase in previous‐hour PM2.5 was associated with an 18% greater risk of STEMI, which would be an ≈26% increase per 10‐μg/m3 increase in previous‐hour PM2.5. In our present analysis, a 10‐μg/m3 increase in concurrent‐day PM2.5 was associated with an estimated 8% to 15% greater risk of STEMI.
Because STEMI typically involves plaque rupture, thrombus formation, and coronary artery occlusion, the differential effect of PM2.5 on STEMI versus NSTEMI may be suggestive of important mechanisms.18 In fact, there is evidence that adverse mechanisms associated with PM2.5 exposure include elevated inflammation and enhanced thrombosis as well as vascular dysfunction and autonomic imbalance.1, 2 A recent randomized, double‐blind, crossover intervention study of healthy adults from Shanghai, China, for example, reported that substantial reductions in PM2.5 exposures for 48 hours from air purification resulted in cardiopulmonary benefits, including decreases in several circulating inflammatory and thrombogenic biomarkers and lower systolic and diastolic blood pressure.27 Given these implied adverse mechanisms, in the context of universal definition and clinical classification of MI,28 these results suggest that there are differential effects of PM2.5 exposures on different types of MI. It is likely, for example, that MI events that occur in those with no CAD by angiogram would be classified as type 2 MI, and these events would be less likely to be associated with short‐term elevated exposure to PM2.5 air pollution than type 1 (or even possibly type 3) MI events.
The discussion of mechanisms is somewhat complicated by another finding of the present study: PM2.5 effects were not observed with NSTEMIs but were observed with UA events. Furthermore, the PM2.5 effect on STEMI risk tended to be larger in those who were younger and who had a history of smoking, existing CAD, and hyperlipidemia. These apparent risk modifiers were not the same for UA. In fact, the PM2.5 effect on UA tended to be larger for those who had congestive heart failure and who were older (aged >65 years). The relevance of these results is unclear regarding alternative susceptibility for STEMI versus UA events. When UA and NSTEMI events are combined to approximately represent NSTE‐ACS events,22 they are less strongly associated with short‐term increases in PM2.5 exposures than STEMI events; however, for those with CAD, NSTE‐ACS events were significantly associated with elevated PM2.5 concentrations >25 μg/m3. Given the difficulty of adequately characterizing these combined NSTE‐ACS events with overlapping diagnoses, these results are challenging to fully interpret, but they remain consistent with the finding that the excess risk associated with short‐term exposure to PM2.5 is observed primarily for those with existing diseased coronary arteries.
Another interesting finding from this study is evidence of a threshold level of exposure. Based on the Akaike information criterion, a threshold model with a threshold at 25 μg/m3 of PM2.5 and a linear effect thereafter fit the data better than a nonthreshold linear model. The results suggest that there is very little if any effect of PM2.5 concentrations at low levels and that the excess risk associated with PM2.5 air pollution is largely dependent on days with elevated pollution >25 μg/m3.
Important strengths of this study include the study size, namely, >16 000 events spanning a 20‐year time period. Study participants were well characterized, with existence of CAD determined by angiography. Throughout the full study period, the study area had daily monitoring of particulate matter air pollution (PM10 and/or PM2.5) from multiple monitoring sites, and this allowed for nearly complete pollution estimates of ambient PM2.5 concentrations (monitored plus imputed). There was substantial temporal variability in ambient PM2.5 concentrations. Furthermore, the estimates from the models were quite robust in relation to inclusion of the weather variables, the use of imputed versus unimputed pollution data, the selection of referent periods, and varying lagged structures.
A primary strength of the time‐stratified case‐crossover design used in this analysis is that there was near‐perfect matching for all participant‐specific characteristics that do not vary over time; therefore, there was likely no confounding by age, sex, smoking habits, underlying chronic disease, or other patient‐level characteristics. In addition, the analysis has been fully stratified by these characteristics. Furthermore, matching event periods with referent periods on the same day of the week before and after the event in the same month allowed for seasonality, long‐term time trends, and long‐term changes in individual characteristics to be controlled for by design.
The present analysis has a number of limitations. The conditional logistic regression model assumes conditional independence across events. This assumption may be less valid for multiple events from the same patient that occurred in the same month or if the effect of PM2.5 exposures on following referent or control days was modified because of the event itself or the accompanying medical therapy. The results, however, were not sensitive to analysis that dropped all same‐month subsequent events or used only reference exposure periods that preceded the events. In this analysis, ≈560 subsequent events occurred in the same month as a previous MI or UA event. When same‐month subsequent events were excluded from the analysis, the results were nearly identical. The time‐stratified case‐crossover design with control or referent periods in the same month before and after the event period should provide unbiased effect estimates.25, 26 To check this assumption, a sensitivity analysis was conducted with control or referent periods matched on the same day of week for the 3 weeks prior to the event with no referent periods following the event. The results were similar with only slightly larger effect estimates, suggesting minimal potential bias from the referent selection strategy (Figure 2).
The results of this study are generalizable only to persons with ACS events that resulted in hospital admission and angiography. Some persons who had ACS events did not survive long enough to be hospitalized, thus the study may have a bias toward inclusion of those whose ACS event was less acute or who lived closer to health care facilities such that they were able to be transported and treated at the hospital. Furthermore, various other factors (eg, prior symptoms, higher medical usage, vigilance with symptoms) may affect the likelihood of being enrolled as a study participant.
Another limitation of the study is that we had only 24‐hour ambient PM2.5 concentrations using data from available monitoring sites without personal or in‐home monitoring, resulting in the potential for some exposure misclassification. Furthermore, without hourly concentrations, these results cannot be compared directly with the analyses that included hourly data,13, 18 and we were unable to explore lag structures with <24 hours.
The overall findings of the analysis are intriguing. They indicate that elevated exposures to ambient PM2.5 air pollution can contribute to triggering of acute coronary events, especially STEMI, in those with preexisting seriously diseased coronary arteries but not in those with clean coronary arteries.
Sources of Funding
Supported in part by National Institutes of Health (NIH ES019217) and by the Mary Lou Fulton Professorship at Brigham Young University.
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002506 doi: 10.1161/JAHA.115.002506)
References
- 1. Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong YL, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. [DOI] [PubMed] [Google Scholar]
- 2. Franklin BA, Brook R, Pope CA III. Air pollution and cardiovascular disease. Curr Probl Cardiol. 2015;40:207–238. [DOI] [PubMed] [Google Scholar]
- 3. Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long‐term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. [DOI] [PubMed] [Google Scholar]
- 4. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long‐term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. [DOI] [PubMed] [Google Scholar]
- 5. Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000–2005). Environ Health Perspect. 2008;116:1614–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow‐up of the Harvard Six Cities Study from 1974 to 2009. Environ Health Perspect. 2012;120:965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, Khan S, Atari DO, Jerrett M, Pope CA III, Brauer M, Brook JR, Martin RV, Stieb D, Burnett RT. Risk of non accidental and cardiovascular mortality in relation to long‐term exposure to low concentrations of fine particulate matter: a Canadian national‐level cohort study. Environ Health Perspect. 2012;120:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long‐term air pollution exposure and cardio‐respiratory mortality: a review. Environ Health. 2013;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beelen R, Raaschou‐Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M, Vineis P, Xun WW, Katsouyanni K, Dimakopoulou K, Oudin A, Forsberg B, Modig L, Havulinna AS, Lanki T, Turunen A, Oftedal B, Nystad W, Nafstad P, De Faire U, Pedersen NL, Östenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno‐de‐Mesquita B, Sugiri D, Krämer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Ineichen A, Schaffner E, Probst‐Hensch N, Künzli N, Schindler C, Schikowski T, Adam M, Phuleria H, Vilier A, Clavel‐Chapelon F, Declercq C, Grioni S, Krogh V, Tsai MY, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Brunekreef B, Hoek G. Effects of long‐term exposure to air pollution on natural‐cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383:785–795. [DOI] [PubMed] [Google Scholar]
- 10. Brook RD, Cakmak S, Turner MC, Brook JR, Crouse DL, Peters PA, van Donkelaar A, Villeneuve PJ, Brion O, Jerrett M, Martin RV, Rajaqopalan S, Goldberg MS, Pope CA III, Burnett RT. Long‐term fine particulate matter exposure and mortality from diabetes in Canada. Diabetes Care. 2013;36:3313–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pope CA III, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relatioships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–115. [DOI] [PubMed] [Google Scholar]
- 12. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair‐Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker‐Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan‐Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen HL, Chen JS, Cheng ATA, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FGR, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD III, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan HD, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah KM, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CDH, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA III, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez‐Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez‐Riera L, Sanman E, Sapkota A, Seedat S, Shi PH, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJC, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJL, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peters A, Von Klot S, Heier M, Trentinaglia I, Cyrys J, Hormann A, Hauptmann M, Wichmann HI, Lowell H. Particulate air pollution and nonfatal cardiac events. Part I. Air pollution, personal activities, and onset of myocardial infarction in a case‐crossover study. Res Rep Health Eff Inst. 2005;124:1–66. [PubMed] [Google Scholar]
- 14. Mustafić H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Périer MC, Marijon E, Vernerey D, Empana JP, Jouven X. Main air pollutants and myocardial infarction: a systematic review and meta‐analysis. JAMA. 2012;307:713–721. [DOI] [PubMed] [Google Scholar]
- 15. Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case‐crossover analysis. Environ Health Perspect. 2005;113:978–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pope CA III, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short‐term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. [DOI] [PubMed] [Google Scholar]
- 17. Rich DQ, Kipen HM, Zhang J, Kamat L, Wilson AC, Kostis JB. Triggering of transmural infarctions, but not nontransmural infarctions, by ambient fine particles. Environ Health Perspect. 2010;118:1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gardner B, Ling F, Hopke PK, Frampton MW, Utell MJ, Zareba W, Cameron SJ, Chalupa D, Kane C, Kulandhaisamy S, Topf MC, Rich DQ. Ambient fine particulate air pollution triggers ST‐elevation myocardial infarction, but not non‐ST elevation myocardial infarction: a case‐crossover study. Part Fibre Toxicol. 2014;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, Renlund DG, Muhlestein J. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. [DOI] [PubMed] [Google Scholar]
- 20. Horne BD, May HT, Kfoury AG, Renlund DG, Muhlestein JB, Lappé DL, Rasmusson KD, Bunch TJ, Carlquist JF, Bair TL, Jensen KR, Ronnow BS, Anderson JL. The Intermountain Risk Score (including the red cell distribution width) predicts heart failure and other morbidity endpoints. Eur J Heart Fail. 2010;12:1203–1213. [DOI] [PubMed] [Google Scholar]
- 21. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 22. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 23. Ambient air monitoring reference and equivalent methods , 40 CFR Part 53 . (2012). [Google Scholar]
- 24. Maclure M. The case‐crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. [DOI] [PubMed] [Google Scholar]
- 25. Janes H, Sheppard L, Lumley T. Overlap bias in the case‐crossover design, with application to air pollution exposures. Stat Med. 2005;24:285–300. [DOI] [PubMed] [Google Scholar]
- 26. Janes H, Sheppard L, Lumley T. Case‐crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005;16:717–726. [DOI] [PubMed] [Google Scholar]
- 27. Chen R, Zhao A, Chen H, Zhao Z, Cai J, Wang C, Yang C, Li H, Xu X, Ha S, Li T, Kan H. Cardiopulmonary benefits of reducing indoor particles of outdoor origin: a randomized, double‐blind crossover trial of air purifiers. J Am Coll Cardiol. 2015;65:2279–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD; the Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035.22923432 [Google Scholar]


