Abstract
Background
Heart retransplant (HRT) recipients represent a growing number of transplant patients. The impact of concurrent kidney transplants (KTs) in this population has not been well studied. We tested the hypothesis that recipients of HRT with concurrent KT (HRT‐KT) would have worse survival than recipients of HRT alone.
Methods and Results
A retrospective analysis of the United Network of Organ Sharing database was performed for all patients undergoing HRT from 1987 to 2011. There were 1660 HRT patients, of which 116 (7%) received concurrent KT. Those who received HRT‐KT had older age, longer wait‐list time, worse kidney function, and more known diabetes. Survival among recipients of HRT‐KT was significantly better than that of recipients of HRT alone (P=0.005). A subgroup of 323 HRT patients with severe kidney dysfunction (estimated glomerular filtration rate <30 mL/min per 1.73 m2 or on dialysis) was studied in more detail, and 76 (24%) received concurrent KT. Those on dialysis at the time of HRT had better survival with versus without concurrent KT (P<0.0001). On multivariable analysis, concurrent KT was independently associated with better outcomes for all patients with HRT and for the subgroup of patients with severe kidney dysfunction.
Conclusions
Recipients of HRT‐KT have better survival than recipients of HRT alone. Further research is needed to determine which HRT patients may benefit the most from concurrent KT.
Keywords: heart, kidney, survival, transplantation
Subject Categories: Transplantation, Cardiorenal Syndrome, Cardiovascular Surgery
Introduction
Heart retransplant (HRT) recipients represent a small but growing proportion of heart transplant recipients. The number of total primary heart transplants in adult and pediatric patients has remained relatively stable in recent years, with 4079 primary heart transplants reported in the year 2000 and 4096 primary heart transplants reported in 2011.1 In adults, HRT represented 2.6% of total adult heart transplants in 2000 and 3.7% of total adult heart transplants in 2011. In children, HRT represented 5.8% of total pediatric heart transplants in the year 2011.2
In general, HRT recipients are known to have worse outcomes after repeat transplantation, possibly due to their prior history of surgery, risk of sensitization with elevated panel reactive antibody levels, side effects from chronic immunosuppressive therapy, and other increased comorbidities.3, 4 Some risk factors that consistently have been found to increase mortality in HRT recipients are the indication of allograft dysfunction (acute rejection) from primary graft failure and a shorter interval from primary to repeat transplant (intertransplant time either <6 or <12 months).5, 6, 7, 8, 9
Abnormal kidney function is a known risk factor for both early and late heart transplant mortality in all patients.10, 11, 12 Posttransplant renal dysfunction is a significant cause of morbidity that continues to increase in prevalence over time.13, 14 According to the International Society for Heart and Lung Transplantation (ISHLT) transplant registry, some degree of renal dysfunction is present in 26% of patients within 1 year after adult heart transplantation, in 52% within 5 years, and in 68% within 10 years. Severe renal dysfunction (creatinine >2.5 mg/dL) occurs in 6% of patients within 1 year, in 15% within 5 years, and in 20% within 10 years.1 Several research studies have also reported a similar progressive decline in renal function after pediatric heart transplantation.15, 16 Renal dysfunction may be more significant in HRT recipients due to their prolonged exposure to nephrotoxic drugs such as calcineurin inhibitors (cyclosporine and tacrolimus).17, 18, 19, 20, 21
Several studies in the literature suggest that multiorgan transplant recipients may fare as well as or even better than single‐organ transplant recipients22, 23; however, there is a paucity of published data on the impact of concurrent kidney transplant (KT) in the HRT population. It is unknown whether the theoretical advantages of multiorgan transplantation would be enough to overcome the comorbidities in this high‐risk population. In a recent minireview published by a working group on HRT, this area was specifically identified as one in which further information was needed.24 Consequently, we tested the hypothesis that patients undergoing HRT with concurrent KT (HRT‐KT) would have worse survival than patients undergoing HRT alone.
Methods
A retrospective analysis of the United Network of Organ Sharing (UNOS) thoracic transplant database was performed to assess the effect of concurrent KT on patient survival after HRT. Because this study included only deidentified information, it was not considered human subjects research and thus was exempt from review by our institutional review board. Inclusion criteria were patients of all ages who received HRT from 1987 to 2011. Data collection included several recipient and donor baseline characteristics. The primary end point of the study was patient survival.
A subgroup of patients with severely decreased renal function was also studied in more detail. An estimated glomerular filtration rate (eGFR) was calculated using the patient's creatinine level at the time of transplantation, the Modification of Diet in Renal Disease formula in participants aged ≥18 years, and the Schwartz formula in participants aged <18 years.25, 26 Subgroup inclusion criteria were eGFR <30 mL/min per 1.73 m2 or need for dialysis at the time of retransplantation. Although a single creatinine value is not necessarily representative of a patient's overall kidney function and does not differentiate between acute kidney injury and chronic kidney disease, this value was the most consistent measurement of renal function available for analysis in the thoracic transplant database. If patients were on dialysis at the time of retransplantation but not all of the information required for calculating eGFR was available in the database, they were still considered for the subgroup analysis because their need for dialysis was indicative of severely decreased renal function. If patients were not on dialysis and not all of the information required for calculating eGFR was available in the database, then they were excluded from consideration for the subgroup analysis because there was insufficient information to determine their renal function. This subgroup of patients with severe renal dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis) was then subdivided further into HRT patients on dialysis versus not on dialysis at the time of retransplantation.
Statistical analyses were performed using SAS version 9.2 (SAS Institute). The baseline characteristics of each group were reported as median values with 25% to 75% interquartile ranges or number values with percentages. Continuous variables were compared between the HRT‐KT and HRT‐alone groups using the Wilcoxon rank sum test, and categorical variables were compared using the chi‐square or Fisher exact test.
Kaplan–Meier survival curves were constructed, and the log‐rank test was used to compare patient survival among the different groups. Cox regression models were used to calculate hazard ratios and to assess the impact of concurrent KT on patient survival after HRT. All variables from the recipient and donor baseline characteristics were included in the univariate Cox regression models. A preliminary multivariable model was fit to include the covariates determined to be significant with P<0.10 in the univariate models. After backward stepwise regression was performed, all factors associated with survival with P<0.05 were included in the final multivariable model.
Multivariable Cox regression models were followed by a test of the proportional hazards assumption in which we generated time‐dependent covariates by creating interactions of the predictors and a function of survival time and included them in the model. If any of the time‐dependent covariates were significant, then those predictors were not proportional. For the final multivariable model using the entire cohort, concurrent KT satisfied the proportional hazards assumption, but 5 of the 7 remaining covariates did not. Consequently, we analyzed the multivariable model by stratifying for the covariates that did not satisfy the criteria. For the subgroup of patients with severe renal dysfunction, the proportional hazards assumption was met for all covariates in the final multivariable model.
To address the issue of missing data in the multivariable models, we completed a second multivariable regression using multiple imputation. We used imputation by chained equations to impute missing values of the covariates in the regression models with missing values. This multivariate approach uses the conditional distribution of each covariate, given other predictor variables, to cycle between filling the missing values for each covariate. We implemented the default approach, which repeats the imputation process 5 times, to create 5 data sets with complete data. We then conducted Cox regression on each imputed data set and combined the results using Proc MI in SAS.
Results
A total of 1660 HRT recipients were included in the study, and 116 (7%) received concurrent KT. The most common indication for HRT was coronary artery disease (cardiac allograft vasculopathy). Compared with recipients of HRT alone, those who received HRT‐KT had older age, longer wait‐list time, worse renal function, and more known diabetes. The degree of sensitization with elevated panel reactive antibodies was similar between the groups (Table 1).
Table 1.
Recipient and Donor Characteristics for All HRT Recipients (n=1660), With or Without Concurrent KT
| HRT‐KT (n=116) | HRT Alone (n=1544) | P Value | Missing Data | |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Recipient age, y | 47 (34–57) | 43 (19–55) | 0.002 | 0 (0%) |
| Recipient weight, kg | 75 (63–87) | 72 (54–85) | 0.028 | 176 (11%) |
| Recipient female sex | 28 (24%) | 487 (32%) | 0.096 | 0 (0%) |
| Status 1A | 35 (30%) | 459 (33%) | 0.591 | 136 (8%) |
| Days spent on wait‐list | 89 (41–268) | 64 (13–197) | 0.001 | 51 (3%) |
| Years since first transplant | 9.3 (4.1–12.7) | 5.6 (1.7–9.6) | <0.001 | 78 (5%) |
| Indication for heart retransplant | 0.090 | 0 (0%) | ||
| Acute rejection | 6 (5%) | 104 (7%) | ||
| Chronic rejection | 9 (8%) | 110 (7%) | ||
| Coronary artery disease | 71 (61%) | 843 (55%) | ||
| Primary graft failure | 7 (6%) | 117 (8%) | ||
| Nonspecific graft failure | 4 (3%) | 179 (12%) | ||
| Other | 19 (16%) | 191 (12%) | ||
| eGFR <30 or on dialysis | 76 (71%) | 247 (21%) | <0.001 | 325 (20%) |
| Dialysis | 42 (36%) | 104 (7%) | <0.0001 | 410 (25%) |
| Hypertension (data only available from 1994 to 2007) | 36 (67%) | 368 (47%) | 0.004 | 815 (49%) |
| Total bilirubin, mg/dL | 0.7 (0.6–1.2) | 0.9 (0.6–1.3) | 0.181 | 469 (28%) |
| Diabetes | 20 (20%) | 86 (8%) | <0.001 | 509 (31%) |
| Recipient CMV positive | 56 (60%) | 555 (72%) | 0.010 | 799 (48%) |
| Recipient inotrope use | 57 (49%) | 603 (39%) | 0.032 | 0 (0%) |
| ECMO support | 1 (1%) | 56 (4%) | 0.179 | 0 (0%) |
| Mechanical ventilation | 3 (3%) | 198 (13%) | 0.001 | 0 (0%) |
| Recipient PRA level >10% | 28 (27%) | 339 (24%) | 0.468 | 130 (8%) |
| Recipient PRA levels | 0.710 | 130 (8%) | ||
| ≤10% | 76 (73%) | 1087 (70%) | ||
| 11% to 50% | 14 (14%) | 182 (12%) | ||
| >50% | 14 (14%) | 157 (10%) | ||
| Donor characteristics | ||||
| Donor age, y | 26 (18–39) | 24 (17–37) | 0.018 | 0 (0%) |
| Donor weight, kg | 77 (63–87) | 72 (55–86) | 0.017 | 46 (3%) |
| Donor female sex | 39 (34%) | 502 (33%) | 0.806 | 0 (0%) |
| Donor CMV positive | 65 (56%) | 905 (59%) | 0.464 | 23 (1%) |
| Donor CPR support | 7 (7%) | 39 (4%) | 0.232 | 685 (41%) |
| Donor inotrope use | 52 (45%) | 496 (32%) | 0.016 | 5 (1%) |
| Heart ischemic time, hours | 3.4 (2.7–3.9) | 3.2 (2.4–3.9) | 0.169 | 125 (8%) |
| Era of heart retransplant | <0.001 | 0 (0%) | ||
| 1987–1995 | 11 (10%) | 452 (29%) | ||
| 1996–2000 | 16 (14%) | 340 (22%) | ||
| 2001–2005 | 34 (29%) | 337 (22%) | ||
| 2006–2011 | 55 (47%) | 415 (27%) | ||
CMV indicates cytomegalovirus; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate (mL/min per 1.73 m2); HRT, heart retransplant; KT, kidney transplant; PRA, panel reactive antibody; Status 1A, highest wait‐list urgency status.
All information required to calculate an eGFR was available in the database for 1236 (74%) HRT recipients, who were considered for inclusion in the subgroup analysis. A total of 323 HRT recipients met inclusion criteria for the subgroup with severe renal dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis at the time of transplantation), and 76 (24%) received concurrent KT (Table 2).
Table 2.
Recipient and Donor Characteristics for HRT Recipients in the Subgroup With Severe Renal Dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis; n=323), With or Without Concurrent KT
| HRT‐KT (n=76) | HRT Alone (n=247) | P Value | Missing Data | |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Recipient age, y | 46 (33–56) | 41 (25–56) | 0.689 | 0 (0%) |
| Recipient weight, kg | 75 (63–88) | 72 (58–88) | 0.567 | 6 (2%) |
| Recipient female sex | 16 (21%) | 86 (35%) | 0.024 | 0 (0%) |
| Status 1A | 22 (29%) | 108 (46%) | 0.010 | 11 (3%) |
| Days spent on wait‐list | 84 (35–212) | 28 (5–140) | <0.001 | 4 (1%) |
| Years since first transplant | 10 (4–13) | 6 (0 to 10) | <0.001 | 6 (2%) |
| Indication for heart retransplant | 0.051 | 0 (0%) | ||
| Acute rejection | 3 (4%) | 18 (7%) | ||
| Chronic rejection | 6 (8%) | 16 (6%) | ||
| Coronary artery disease | 43 (57%) | 96 (39%) | ||
| Primary graft failure | 5 (7%) | 43 (17%) | ||
| Nonspecific graft failure | 4 (5%) | 22 (9%) | ||
| Other | 15 (20%) | 52 (21%) | ||
| Dialysis | 42 (58%) | 104 (46%) | 0.081 | 23 (7%) |
| Hypertension (data only available from 1994 to 2007) | 25 (63%) | 77 (44%) | 0.053 | 109 (34%) |
| Total bilirubin, mg/dL | 1.2 (0.6–1.4) | 2.6 (0.7–2.0) | 0.004 | 31 (10%) |
| Diabetes | 14 (20%) | 22 (11%) | 0.067 | 58 (18%) |
| Recipient CMV positive | 37 (55%) | 115 (74%) | 0.007 | 100 (40%) |
| Recipient inotrope use | 39 (51%) | 158 (64%) | 0.048 | 0 (0%) |
| ECMO support | 1 (1%) | 18 (7%) | 0.054 | 0 (0%) |
| Mechanical ventilation | 3 (4%) | 73 (30%) | <0.001 | 0 (0%) |
| Recipient PRA level >10% | 15 (22%) | 60 (27%) | 0.517 | 29 (9%) |
| Recipient PRA levels | 0.668 | 28 (9%) | ||
| ≤10% | 53 (78%) | 161 (73%) | ||
| 11% to 50% | 7 (10%) | 31 (14%) | ||
| >50% | 8 (12%) | 29 (13%) | ||
| Donor characteristics | ||||
| Donor age, y | 30 (20–41) | 28 (18–38) | 0.102 | 0 (0%) |
| Donor weight, kg | 78 (65–92) | 73 (61–89) | 0.118 | 3 (1%) |
| Donor female sex | 30 (39%) | 73 (30%) | 0.105 | 0 (0%) |
| Donor CMV positive | 43 (57%) | 151 (61%) | 0.455 | 1 (1%) |
| Donor CPR support | 6 (9%) | 6 (4%) | 0.115 | 84 (26%) |
| Donor inotrope use | 42 (55%) | 96 (39%) | 0.025 | 5 (2%) |
| Heart ischemic time, hours | 3.3 (2.6–3.8) | 3.3 (2.5–4.0) | 0.937 | 27 (8%) |
| Era of heart retransplant | <0.001 | 0 (0%) | ||
| 1987–1995 | 4 (5%) | 36 (15%) | ||
| 1996–2000 | 9 (12%) | 69 (28%) | ||
| 2001–2005 | 27 (36%) | 79 (32%) | ||
| 2006–2011 | 36 (47%) | 63 (26%) | ||
CMV indicates cytomegalovirus; CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; HRT, heart retransplant; KT, kidney transplant; PRA, panel reactive antibody level; Status 1A, highest wait‐list urgency status.
This subgroup of 323 patients with severe renal dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis) was subdivided further. A total of 146 HRT patients were on dialysis at the time of retransplantation, and of those, 42 (29%) received concurrent KT and 104 (71%) received HRT alone. Another 177 HRT patients had eGFR <30 mL/min per 1.73 m2 (and were not on dialysis) at the time of retransplantation, and 34 (19%) received concurrent KT and 143 (81%) received HRT alone (Figure 1).
Figure 1.
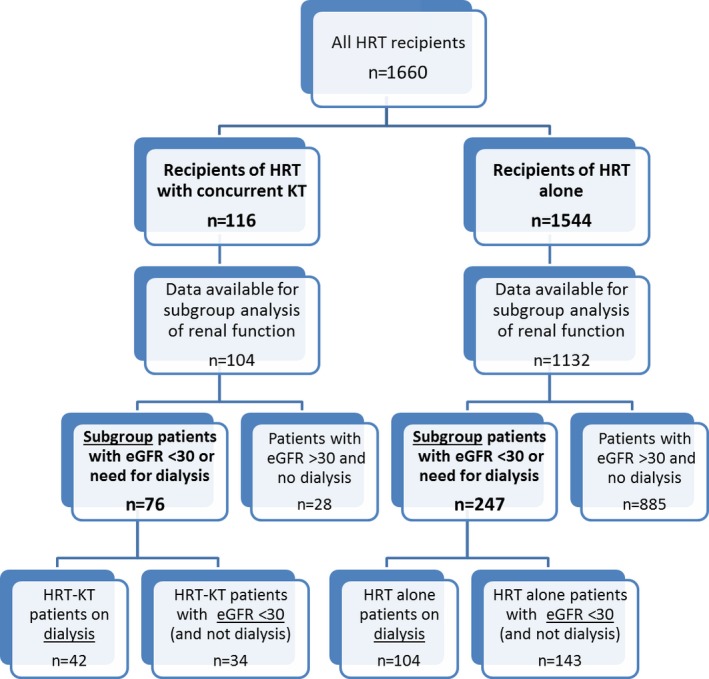
Flowchart of all patients included in the study. eGFR indicates estimated glomerular filtration rate (mL/min per 1.73 m2); HRT, heart retransplant; KT, kidney transplant.
In the overall cohort of all HRT recipients (n=1660), survival among recipients of HRT‐KT was significantly better than that of recipients of HRT alone (P=0.005) (Figure 2). In the subgroup analysis of HRT recipients with severe renal dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis, n=323), survival among recipients of HRT‐KT was also significantly better than that of recipients of HRT alone (P<0.001) (Figure 3).
Figure 2.
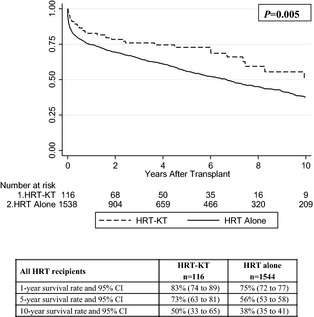
Kaplan–Meier survival curves, risk tables, and survival analysis for all HRT recipients in the entire cohort (n=1660), with or without concurrent KT. HRT indicates heart retransplant; KT, kidney transplant.
Figure 3.
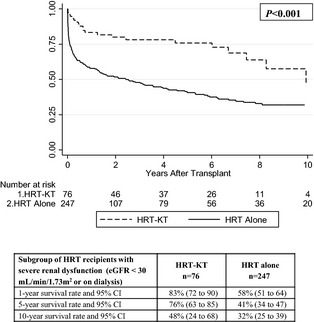
Kaplan–Meier survival curves, risk tables, and survival analysis for HRT recipients in the subgroup with severe renal dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis; n=323), with or without concurrent KT. eGFR indicates estimated glomerular filtration rate; HRT, heart retransplant; KT, kidney transplant.
The converse analysis was performed on patients without severe kidney dysfunction who had eGFR >30 mL/min per 1.73 m2 and did not need dialysis. Concurrent KT did not significantly affect survival in those without severe renal dysfunction and with KT versus those without KT (P=0.441). Concurrent KT also eliminated any detectable difference in survival between those with and without severe renal dysfunction (P=0.322). The median follow‐up time was 3.0 years (interquartile range 0.5 to 7.0 years) for the entire cohort of patients and 1.7 years (interquartile range 0.2 to 6.0 years) for patients in the severe renal dysfunction subgroup.
In HRT patients on dialysis (n=146), survival among recipients of HRT‐KT was significantly longer than that of recipients of HRT alone (P<0.0001) (Figure 4). In HRT patients with eGFR <30 mL/min per 1.73 m2 (and not dialysis, n=177), survival among recipients of HRT‐KT was also longer than that of recipients of HRT alone (P=0.014) (Figure 5). Due to the small sample size, no multivariable analysis was performed for these subdivided groups.
Figure 4.
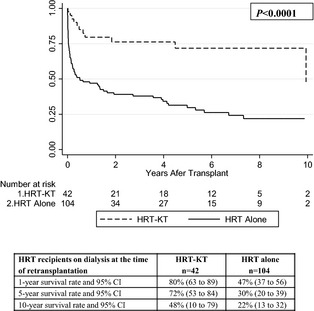
Kaplan–Meier survival curves, risk tables, and survival analysis for HRT recipients in the subdivided group on dialysis at the time of retransplantation (n=146), with or without concurrent KT. HRT indicates heart retransplant; KT, kidney transplant.
Figure 5.
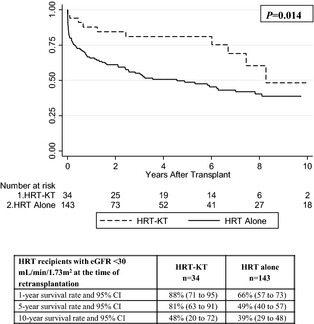
Kaplan–Meier survival curves, risk tables, and survival analysis for HRT recipients in the subdivided group with eGFR <30 mL/min per 1.73 m2 (and not on dialysis) at the time of retransplantation (n=177), with or without concurrent KT. eGFR indicates estimated glomerular filtration rate; HRT, heart retransplant; KT, kidney transplant.
In the univariate models, concurrent KT was independently associated with the primary outcome of patient survival for all patients with HRT in the overall cohort (Table 3) and for subgroup patients with severe renal dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis) (Table 4). Recipients of HRT‐KT had a decreased hazard ratio and thus a lower incidence of death compared with recipients of HRT alone. In the final multivariable model, concurrent KT remained significantly associated with decreased risk of death (compared with HRT alone) while adjusting for other significant variables. HRT patients with concurrent KT had half the mortality of those without KT during the study period, a benefit that was even more pronounced in the subgroup with severe renal dysfunction.
Table 3.
Univariate and Multivariable Cox Regression Models for All HRT Recipients
| Univariate Cox Models | Final Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| Heart retransplant | ||||||
| With concurrent KT (HRT‐KT) | 0.63 | 0.45–0.87 | 0.006 | 0.50 | 0.33–0.76 | 0.001 |
| Without KT (HRT alone) | 1.00 | — | — | 1.00 | — | — |
| Recipient age | 1.01 | 1.00–1.01 | <0.001 | |||
| Status 1A | 1.61 | 1.39–1.87 | <0.001 | 1.28 | 1.04–1.58 | 0.021 |
| Days spent on wait‐list | 1.00 | 1.00–1.00 | 0.036 | |||
| Years since first transplant | 0.94 | 0.92–0.95 | <0.001 | 0.98 | 0.95–1.00 | 0.032 |
| eGFR <30 or on dialysis | 1.69 | 1.41–2.02 | <0.001 | 1.87 | 1.53–2.30 | <0.001 |
| ECMO support | 2.62 | 1.87–3.66 | <0.001 | 1.89 | 1.15–3.12 | 0.013 |
| Mechanical ventilation | 1.87 | 1.55–2.25 | <0.001 | |||
| Donor age | 1.01 | 1.00–1.01 | 0.001 | 1.01 | 1.01–1.02 | 0.001 |
| Donor inotrope use | 0.70 | 0.59–0.82 | <0.001 | |||
| Heart ischemic time | 1.07 | 1.01–1.14 | 0.032 | 1.18 | 1.09–1.28 | <0.001 |
| Era of heart retransplant | ||||||
| 1987–1995 | 1.00 | — | — | 1.00 | — | — |
| 1996–2000 | 0.65 | 0.55–0.78 | <0.001 | 0.68 | 0.53–0.88 | 0.003 |
| 2001–2005 | 0.51 | 0.42–0.62 | <0.001 | 0.53 | 0.40–0.70 | <0.001 |
| 2006–2011 | 0.47 | 0.38–0.59 | <0.001 | 0.57 | 0.42–0.78 | 0.001 |
All variables included in this table had univariate P<0.10 and were considered for the preliminary multivariable model. The final multivariable model was reduced to all variables with P<0.05. ECMO indicates extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; HRT, heart retransplant; KT, kidney transplant; Status 1A, highest wait‐list urgency status.
Table 4.
Univariate and Multivariable Cox Regression Models for HRT Recipients in the Subgroup With Severe Renal Dysfunction (eGFR <30 mL/min per 1.73 m2 or on Dialysis)
| Univariate Cox Models | Final Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P Value | Hazard Ratio | 95% CI | P Value | |
| Heart retransplant | ||||||
| With concurrent KT (HRT‐KT) | 0.36 | 0.23–0.57 | <0.001 | 0.36 | 0.20–0.63 | <0.001 |
| Without KT (HRT alone) | 1.00 | — | — | 1.00 | — | — |
| Status 1A | 1.76 | 1.30–2.39 | <0.001 | |||
| Years since first transplant | 0.94 | 0.91–0.97 | <0.001 | |||
| Total bilirubin | 1.05 | 1.03–1.07 | <0.001 | 1.03 | 1.01–1.06 | 0.007 |
| ECMO support | 4.41 | 2.61–7.44 | <0.001 | 4.02 | 2.02–8.03 | <0.001 |
| Mechanical ventilation | 2.28 | 1.65–3.14 | <0.001 | |||
| Heart ischemic time | 1.22 | 1.05–1.42 | 0.009 | 1.21 | 1.02–1.43 | 0.024 |
| Era of heart retransplant | ||||||
| 1987–1995 | 1.00 | — | — | 1.00 | — | — |
| 1996–2000 | 0.78 | 0.50–1.21 | 0.270 | 0.73 | 0.42–1.28 | 0.274 |
| 2001–2005 | 0.46 | 0.29–0.73 | 0.001 | 0.46 | 0.26–0.81 | 0.007 |
| 2006–2011 | 0.55 | 0.34–0.90 | 0.018 | 0.52 | 0.28–0.96 | 0.037 |
All variables included in this table had univariate P<0.10 and were considered for the preliminary multivariable model. The final multivariable model was reduced to all variables with P<0.05. ECMO indicates extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; HRT, heart retransplant; KT, kidney transplant; Status 1A, highest wait‐list urgency status.
Following each multivariable Cox regression, a test of the proportional hazards assumption was completed. For the final multivariable model of the entire cohort, the results were consistent and showed that concurrent KT was significantly associated with a decreased risk of death compared with HRT alone (hazard ratio 0.53 [95% CI 0.34 to 0.81], P=0.004).
Multiple imputations were completed to generate multiple data sets without missing data. In the combined multivariable adjusted result with all imputed data sets, concurrent KT remained significantly associated with decreased risk of death compared with HRT alone (hazard ratio 0.64 [95% CI 0.45 to 0.92], P=0.015). Results were similar in the subgroup of patients with severe renal dysfunction, for which concurrent KT was significantly associated with decreased risk of death compared with HRT alone (hazard ratio 0.43 [95% CI 0.27 to 0.68], P<0.001).
An analysis of recipient cause of death was performed for all HRT recipients in the entire cohort (844 deaths) (Table 5) and for subgroup patients with severe renal dysfunction (173 deaths) (Table 6). The cardiovascular category includes myocardial infarction, arrhythmia, and cardiac arrest. The graft failure category includes acute rejection, chronic rejection, and primary graft failure. The infection category includes bacterial pneumonia, viral hepatitis, and sepsis. The “other” category includes respiratory failure, stroke, and hemorrhage. Patients with HRT‐KT had fewer deaths due to cardiovascular diagnoses and more deaths due to infection compared with patients with HRT alone. More important, there was a significant difference in graft failure as a cause of death between concurrent HRT‐KT versus HRT alone in the entire cohort of HRT recipients (6% versus 23%, P=0.002).
Table 5.
Recipient Cause of Death for All HRT Recipients With or Without Concurrent KT (844 Deaths)
| Recipient Cause of Death | HRT‐KT (n=36) | HRT Alone (n=808) | P Value | Missing Data |
|---|---|---|---|---|
| Cardiovascular | 2 (6%) | 161 (22%) | 0.002 | 86 (10%) |
| Graft failure | 2 (6%) | 166 (23%) | ||
| Infection | 12 (38%) | 110 (15%) | ||
| Multiorgan failure | 4 (13%) | 84 (12%) | ||
| Malignancy | 3 (9%) | 41 (6%) | ||
| Other | 9 (28%) | 164 (23%) |
HRT indicates heart retransplant; KT, kidney transplant.
Table 6.
Recipient Cause of Death for HRT Recipients in the Subgroup With Severe Renal Dysfunction (eGFR <30 mL/min per 1.73 m2 or on Dialysis) With or Without Concurrent KT (173 Deaths)
| Recipient Cause of Death | HRT‐KT (n=21) | HRT Alone (n=152) | P Value | Missing Data |
|---|---|---|---|---|
| Cardiovascular | 1 (5%) | 23 (16%) | 0.75 | 10 (6%) |
| Graft failure | 1 (5%) | 24 (17%) | ||
| Infection | 8 (38%) | 26 (18%) | ||
| Multiorgan failure | 3 (14%) | 24 (17%) | ||
| Malignancy | 3 (14%) | 6 (4%) | ||
| Other | 5 (24%) | 39 (27%) |
eGFR indicates estimated glomerular filtration rate; HRT, heart retransplant; KT, kidney transplant.
Discussion
This study is the first, to our knowledge, to compare survival outcomes in HRT recipients with or without concurrent KT. In all patients undergoing HRT, recipients of concurrent KT had significantly better survival than recipients of HRT alone. Perhaps not surprisingly, this effect was most pronounced among HRT recipients with the worst renal function. Importantly, this observation was independent of the baseline characteristics of the recipient and the donor.
Median patient survival in this study (10.5 years for recipients of HRT‐KT and 6.7 years for recipients of HRT alone) is comparable with current outcomes published in the literature. According to the ISHLT transplant registry, median survival for all primary heart transplants performed from 1982 to 2011 was 11 years. Median survival for all HRTs during that same time period was 6.3 years.1 Consequently, the patients in our study who received HRT‐KT had outcomes similar to primary heart transplant recipients. Conversely, the patients in our study who received HRT alone had outcomes similar to those reported elsewhere in the HRT literature.
Previous studies support the notion that there are potential benefits to performing combined primary heart transplant and KT.27 Primary heart transplants with concurrent KTs have been increasing in prevalence, with 34 reported in 2000 (≈1% of total adult heart transplants that year) and 94 in 2011 (≈3% of total adult heart transplants that year).1 Multiple studies found that recipients of combined primary heart transplant and KT have less acute cardiac rejection and cardiac allograft vasculopathy than recipients of primary heart transplant alone; however, many of these studies did not find any additional survival benefit.28, 29, 30, 31, 32 This supports the notion that there may be benefits to performing combined multiorgan transplantation, such as a decrease in rejection and graft failure.
Russo et al33 reported a significant survival benefit in a specific cohort of heart transplant recipients with decreased renal function undergoing combined heart transplant and KT. Low‐risk patients with an eGFR of <33 mL/min had significantly better survival compared with similar patients (with eGFRs and risk scores in the same range) undergoing heart transplantation alone (P=0.006). When outcomes of combined heart transplant and KT were compared with heart transplantation alone among patients with eGFR of ≥33 mL/min, there was no statistical difference in survival within the low‐risk (P=0.31), moderate‐risk (P=0.61), or high‐risk (P=0.72) groups. This study was very informative in its stratification of patients by eGFR, demonstrating that low‐risk patients with severe renal dysfunction may benefit the most from combined heart transplant and KT. This is consistent with some of the findings of our study, and this survival benefit may be even more pronounced in our heart retransplant population. Contrary to our findings, in their analysis of pretransplant patient characteristics, the factors associated with worse survival following combined heart transplant and KT included dialysis dependence at the time of transplantation.
More recently, 2 studies have been published on the topic of combined heart transplant and KT and also analyzed the UNOS database; however, these recent studies were based on primary heart transplants, whereas our study focused on HRT. Schaffer et al34 found that 5‐year posttransplant survival was improved in recipients of combined heart transplant and KT compared with recipients of isolated heart transplant alone, for both patients with dialysis dependence (73% versus 51%, P<0.001) and non–dialysis‐dependent renal insufficiency (80% versus 69%, P=0.004). Karamlou et al35 found that patients in the lowest eGFR quintile (mean eGFR <37 mL/min) who received isolated heart transplants had significantly worse median survival compared with recipients of combined heart transplant and KT (7.1 versus 7.7 years, P<0.001). These results are consistent with the findings of our study, in which HRT‐KT patients on dialysis had significantly improved survival compared with dialysis patients who received HRT alone. Furthermore, HRT‐KT patients with eGFR <30 mL/min per 1.73 m2 and not on dialysis had a less dramatic but still significant survival advantage over patients with eGFR <30 mL/min per 1.73 m2 and not on dialysis who received HRT alone.
Patients undergoing heart retransplantation are a unique, high‐risk population with worse outcomes than recipients of primary heart transplants. This study addresses the lack of published data on the impact of concurrent KTs specifically in the HRT population to assist clinicians in their decision making at the time of listing for retransplantation. Given the significant issue of wait‐list mortality, it is conceivable that for those with severe renal dysfunction who underwent HRT alone, the perceived risk of a longer wait‐list time and more complicated surgery might not have outweighed the perceived benefits of multiorgan transplantation. Patients in our study who received HRT‐KT had wait‐list times that were significantly longer than patients who received HRT alone, a discrepancy that was even more evident in the subgroup of patients with severe renal dysfunction (eGFR <30 mL/min per 1.73 m2 or on dialysis). Those patients with severe renal dysfunction who received HRT alone appeared to be more ill, with higher rates of status 1A urgency classification, extracorporeal membrane oxygenation support, and mechanical ventilation; however, all of these variables were controlled for in the multivariable analysis, which still showed benefit for those who received concurrent KT. The results of this study clearly demonstrate that very careful patient selection is warranted, especially for patients with severe renal dysfunction who may be under consideration to receive HRT alone.
Other studies have suggested that recipients of multiorgan transplants from the same donor tend to fare better than single‐organ transplant recipients. Pinderski et al found that acute cardiac rejection within the first 3 months was higher in recipients of heart transplant alone (81%) than in heart–kidney recipients (12%) or heart–lung recipients (22%). Survival at 3 years also differed among the groups after heart–kidney (100%), heart alone (82%), heart–lung (74%), and lung alone (70%) transplantation.36 Rana et al reported that rejection rates for allografts cotransplanted with liver, kidney, or heart allografts were significantly lower than rejection rates for those allografts transplanted alone. Heart–kidney recipients, for example, experienced only half as many cardiac rejection episodes compared with recipients of heart transplant alone (26% versus 52%). Those same heart–kidney recipients also had less kidney allograft rejection compared with recipients of KT alone (17% versus 24%).37 Consequently, the simultaneous transplantation of different organs from the same donor potentially offers significant protection from rejection.
There is no clear explanation for this observed phenomenon. Several potential mechanisms have been proposed. Immune modulation of the recipient by the simultaneous transplant of disparate tissues or the introduction of additional hematopoietic elements may contribute.38 Previous studies considered the possibility of simultaneous donor hematopoietic cell infusions with heart transplantation to decrease the rate of rejection.39, 40 Donor‐derived hematopoietic cells can migrate into recipient lymphoid and nonlymphoid tissue, resulting in microchimerism.37, 41 In addition, the persistence of donor major histocompatibility complex class II cells in the allografts of combined organ transplant recipients has been linked to decreased chronic rejection in animal models.42 It is also possible that the detrimental effects of perioperative renal insufficiency on heart transplantation are at least partially overcome by KT.1, 43 Further study is needed to understand the magnitude and mechanisms of benefit observed with combined solid organ transplantation.
An essential aspect of any discussion about retransplantation is the ongoing ethical debate about organ allocation and utilization.24, 44 Due to a shortage of donors and the disparity between supply and demand, organs for transplantation are a limited resource. Some may contend that it is unethical for any patient to receive a second heart transplant while other patients die still waiting for their first, a notion supported by the superior outcomes of primary heart transplant recipients relative to HRT recipients.45 Adding a concurrent KT magnifies this dilemma in terms of how many organs 1 person should be eligible to receive in a lifetime. The counternotion that may be proposed is that once a patient receives any transplanted organ, the transplant team has made a commitment and has a sense of obligation toward the patient to pursue all available options to improve survival.45, 46 Given this ethical consideration, it is imperative that evidence guides clinical decision making to justify listing or not listing a patient for retransplantation with a second or third organ.
One of the many important factors in determining transplant candidacy is the likelihood of success following transplantation. This study supports the notion that concurrent KT is associated with improved survival in the HRT population and is comparable to survival among primary heart transplant recipients. Conversely, HRT alone in patients with severe renal dysfunction should be considered with caution.
Limitations
Several limitations in this study deserve mention. First, as a retrospective analysis, the results may differ from those of a prospectively evaluated cohort. The database itself is subject to misclassification and selection bias. In addition, treatment standards of care often vary between different centers and over time. There were variables with missing data that required multiple imputation in the multivariable analyses, and for the subgroup analyses of patients with severe renal dysfunction, some patients were excluded because eGFR could not be calculated. These issues, however, likely did not introduce any systematic bias, and the robust statistical analyses performed accounted for these issues.
Finally, this study was limited to the use of a single creatinine value at the time of transplantation to calculate an eGFR. This eGFR and the need for dialysis provided the best characterization of renal function available within the constructs of the available data set; however, a single creatinine value is not necessarily representative of a patient's overall kidney function and does not differentiate between acute kidney injury and chronic kidney disease. Further research in this population will almost certainly require more granular assessment of pretransplant kidney function to better understand who is best suited to HRT, with or without concurrent KT.
Conclusion
In patients undergoing HRT, recipients of HRT‐KT had better survival than recipients of HRT alone. This survival benefit was most pronounced among those with severe renal dysfunction at the time of transplantation; therefore, concurrent KT should be considered for this population. Further research is needed to determine which HRT patients may benefit the most from concurrent KT to improve outcomes for this high‐risk population.
Sources of Funding
This work was supported in part by Health Resources and Services Administration contract 234‐2005‐37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002435 doi: 10.1161/JAHA.115.002435)
This study was presented as an oral abstract at the Scientific Sessions of the American Heart Association meeting in Los Angeles, CA on November 5, 2012.
References
- 1. Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, Dobbels F, Kirk R, Rahmel AO, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:951–964. [DOI] [PubMed] [Google Scholar]
- 2. Dipchand AI, Kirk R, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, Lund LH, Rahmel AO, Yusen RD, Stehlik J. The Registry of the International Society for Heart and Lung Transplantation: sixteenth official pediatric heart transplantation report–2013; focus theme: age. J Heart Lung Transplant. 2013;32:979–988. [DOI] [PubMed] [Google Scholar]
- 3. Tsao L, Uriel N, Leitz K, Naka Y, Mancini D. Higher rate of comorbidities after cardiac retransplantation contributes to decreased survival. J Heart Lung Transplant. 2009;28:1072–1074. [DOI] [PubMed] [Google Scholar]
- 4. Mahle WT, Vincent RN, Kanter KR. Cardiac retransplantation in childhood: analysis of data from the United Network for Organ Sharing. J Thorac Cardiovasc Surg. 2005;130:542–546. [DOI] [PubMed] [Google Scholar]
- 5. Srivastava R, Keck BM, Bennett LE, Hosenpud JD. The results of cardiac retransplantation: an analysis of the Joint International Society for Heart and Lung Transplantation/United Network for Organ Sharing Thoracic Registry. Transplantation. 2000;70:606–612. [DOI] [PubMed] [Google Scholar]
- 6. Radovancevic B, McGiffin DC, Kobashigawa JA, Cintron GB, Mullen GM, Pitts DE, O'Donnell J, Thomas C, Bourge RC, Naftel DC. Retransplantation in 7,290 primary transplant patients: a 10‐year multi‐institutional study. J Heart Lung Transplant. 2003;22:862–868. [DOI] [PubMed] [Google Scholar]
- 7. Kanter KR, Vincent RN, Berg AM, Mahle WT, Forbess JM, Kirshbom PM. Cardiac retransplantation in children. Ann Thorac Surg. 2004;78:644–649; discussion 644–649. [DOI] [PubMed] [Google Scholar]
- 8. Topkara VK, Dang NC, John R, Cheema FH, Barbato R, Cavallo M, Liu JF, Liang LM, Liberman EA, Argenziano M, Oz MC, Naka Y. A decade experience of cardiac retransplantation in adult recipients. J Heart Lung Transplant. 2005;24:1745–1750. [DOI] [PubMed] [Google Scholar]
- 9. Chin C, Naftel D, Pahl E, Shankel T, Clark ML, Gamberg P, Kirklin J, Webber S. Cardiac re‐transplantation in pediatrics: a multi‐institutional study. J Heart Lung Transplant. 2006;25:1420–1424. [DOI] [PubMed] [Google Scholar]
- 10. Vossler MR, Ni H, Toy W, Hershberger RE. Pre‐operative renal function predicts development of chronic renal insufficiency after orthotopic heart transplantation. J Heart Lung Transplant. 2002;21:874–881. [DOI] [PubMed] [Google Scholar]
- 11. Ostermann ME, Rogers CA, Saeed I, Nelson SR, Murday AJ. Pre‐existing renal failure doubles 30‐day mortality after heart transplantation. J Heart Lung Transplant. 2004;23:1231–1237. [DOI] [PubMed] [Google Scholar]
- 12. Rubel JR, Milford EL, McKay DB, Jarcho JA. Renal insufficiency and end‐stage renal disease in the heart transplant population. J Heart Lung Transplant. 2004;23:289–300. [DOI] [PubMed] [Google Scholar]
- 13. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 14. Al Aly Z, Abbas S, Moore E, Diallo O, Hauptman PJ, Bastani B. The natural history of renal function following orthotopic heart transplant. Clin Transplant. 2005;19:683–689. [DOI] [PubMed] [Google Scholar]
- 15. Pradhan M, Leonard MB, Bridges ND, Jabs KL. Decline in renal function following thoracic organ transplantation in children. Am J Transplant. 2002;2:652–657. [DOI] [PubMed] [Google Scholar]
- 16. Lee CK, Christensen LL, Magee JC, Ojo AO, Harmon WE, Bridges ND. Pre‐transplant risk factors for chronic renal dysfunction after pediatric heart transplantation: a 10‐year national cohort study. J Heart Lung Transplant. 2007;26:458–465. [DOI] [PubMed] [Google Scholar]
- 17. Goldstein DJ, Zuech N, Sehgal V, Weinberg AD, Drusin R, Cohen D. Cyclosporine‐associated end‐stage nephropathy after cardiac transplantation: incidence and progression. Transplantation. 1997;63:664–668. [DOI] [PubMed] [Google Scholar]
- 18. Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine‐associated chronic nephropathy. N Engl J Med. 1984;311:699–705. [DOI] [PubMed] [Google Scholar]
- 19. Reichenspurner H. Overview of tacrolimus‐based immunosuppression after heart or lung transplantation. J Heart Lung Transplant. 2005;24:119–130. [DOI] [PubMed] [Google Scholar]
- 20. Smith JA, Ribakove GH, Hunt SA, Miller J, Stinson EB, Oyer PE, Robbins RC, Shumway NE, Reitz BA. Heart retransplantation: the 25‐year experience at a single institution. J Heart Lung Transplant. 1995;14:832–839. [PubMed] [Google Scholar]
- 21. Kilic A, Weiss ES, Arnaoutakis GJ, George TJ, Conte JV, Shah AS, Yuh DD. Identifying recipients at high risk for graft failure after heart retransplantation. Ann Thorac Surg. 2012;93:712–716. [DOI] [PubMed] [Google Scholar]
- 22. Rasmussen A, Davies HF, Jamieson NV, Evans DB, Calne RY. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 23. Simpson N, Cho YW, Cicciarelli JC, Selby RR, Fong TL. Comparison of renal allograft outcomes in combined liver‐kidney transplantation versus subsequent kidney transplantation in liver transplant recipients: analysis of UNOS database. Transplantation. 2006;82:1298–1303. [DOI] [PubMed] [Google Scholar]
- 24. Johnson MR, Aaronson KD, Canter CE, Kirklin JK, Mancini DM, Mehra MR, Radovancevic B, Taylor DO, Webber SA. Heart retransplantation. Am J Transplant. 2007;7:2075–2081. [DOI] [PubMed] [Google Scholar]
- 25. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz GJ, Haycock GB, Edelmann CM Jr, Spitzer A. A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics. 1976;58:259–263. [PubMed] [Google Scholar]
- 27. Narula J, Bennett LE, DiSalvo T, Hosenpud JD, Semigran MJ, Dec GW. Outcomes in recipients of combined heart‐kidney transplantation: multiorgan, same‐donor transplant study of the International Society of Heart and Lung Transplantation/United Network for Organ Sharing Scientific Registry. Transplantation. 1997;63:861–867. [DOI] [PubMed] [Google Scholar]
- 28. Vermes E, Kirsch M, Houel R, Legouvelo S, Benvenuti C, Aptecar E, Le Besnerais P, Lang P, Abbou C, Loisance D. Immunologic events and long‐term survival after combined heart and kidney transplantation: a 12‐year single‐center experience. J Heart Lung Transplant. 2001;20:1084–1091. [DOI] [PubMed] [Google Scholar]
- 29. Luckraz H, Parameshwar J, Charman SC, Firth J, Wallwork J, Large S. Short‐ and long‐term outcomes of combined cardiac and renal transplantation with allografts from a single donor. J Heart Lung Transplant. 2003;22:1318–1322. [DOI] [PubMed] [Google Scholar]
- 30. Hermsen JL, Nath DS, del Rio AM, Eickstaedt JB, Wigfield C, Lindsey JD, Edwards NM. Combined heart‐kidney transplantation: the University of Wisconsin experience. J Heart Lung Transplant. 2007;26:1119–1126. [DOI] [PubMed] [Google Scholar]
- 31. Vermes E, Grimbert P, Sebbag L, Barrou B, Pouteil‐Noble C, Pavie A, Obadia JF, Loisance D, Lang P, Kirsch M. Long‐term results of combined heart and kidney transplantation: a French multicenter study. J Heart Lung Transplant. 2009;28:440–445. [DOI] [PubMed] [Google Scholar]
- 32. Raichlin E, Kushwaha SS, Daly RC, Kremers WK, Frantz RP, Clavell AL, Rodeheffer RJ, Larson TS, Stegall MD, McGregor C, Pereira NL, Edwards BS. Combined heart and kidney transplantation provides an excellent survival and decreases risk of cardiac cellular rejection and coronary allograft vasculopathy. Transplant Proc. 2011;43:1871–1876. [DOI] [PubMed] [Google Scholar]
- 33. Russo MJ, Rana A, Chen JM, Hong KN, Gelijns A, Moskowitz A, Widmann WD, Ratner L, Naka Y, Hardy MA. Pretransplantation patient characteristics and survival following combined heart and kidney transplantation: an analysis of the United Network for Organ Sharing Database. Arch Surg. 2009;144:241–246. [DOI] [PubMed] [Google Scholar]
- 34. Schaffer JM, Chiu P, Singh SK, Oyer PE, Reitz BA, Mallidi HR. Heart and combined heart‐kidney transplantation in patients with concomitant renal insufficiency and end‐stage heart failure. Am J Transplant. 2014;14:384–396. [DOI] [PubMed] [Google Scholar]
- 35. Karamlou T, Welke KF, McMullan DM, Cohen GA, Gelow J, Tibayan FA, Mudd JM, Slater MS, Song HK. Combined heart‐kidney transplant improves post‐transplant survival compared with isolated heart transplant in recipients with reduced glomerular filtration rate: analysis of 593 combined heart‐kidney transplants from the United Network Organ Sharing Database. J Thorac Cardiovasc Surg. 2014;147:456–461.e451. [DOI] [PubMed] [Google Scholar]
- 36. Pinderski LJ, Kirklin JK, McGiffin D, Brown R, Naftel DC, Young KR Jr, Smith K, Bourge RC, Tallaj JA, Rayburn BK, Benza R, Zorn G, Leon K, Wille K, Deierhoi M, George JF. Multi‐organ transplantation: is there a protective effect against acute and chronic rejection? J Heart Lung Transplant. 2005;24:1828–1833. [DOI] [PubMed] [Google Scholar]
- 37. Rana A, Robles S, Russo MJ, Halazun KJ, Woodland DC, Witkowski P, Ratner LE, Hardy MA. The combined organ effect: protection against rejection? Ann Surg. 2008;248:871–879. [DOI] [PubMed] [Google Scholar]
- 38. Szabolcs P, Burlingham WJ, Thomson AW. Tolerance after solid organ and hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:S193–S200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fontes P, Rao AS, Demetris AJ, Zeevi A, Trucco M, Carroll P, Rybka W, Rudert WA, Ricordi C, Dodson F, Shapiro R, Tzakis A, Todo S, Abu‐Elmagd K, Jordan M, Fung JJ, Starzl TE. Bone marrow augmentation of donor‐cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gammie JS, Pham SM. Simultaneous donor bone marrow and cardiac transplantation: can tolerance be induced with the development of chimerism? Curr Opin Cardiol. 1999;14:126–132. [DOI] [PubMed] [Google Scholar]
- 41. Wood KJ. Passenger leukocytes and microchimerism: what role in tolerance induction? Transplantation. 2003;75:17S–20S. [DOI] [PubMed] [Google Scholar]
- 42. Demetris AJ, Murase N, Ye Q, Galvao FH, Richert C, Saad R, Pham S, Duquesnoy RJ, Zeevi A, Fung JJ, Starzl TE. Analysis of chronic rejection and obliterative arteriopathy. Possible contributions of donor antigen‐presenting cells and lymphatic disruption. Am J Pathol. 1997;150:563–578. [PMC free article] [PubMed] [Google Scholar]
- 43. Janus N, Launay‐Vacher V, Sebbag L, Despins P, Epailly E, Pavie A, Obadia JF, Pattier S, Varnous S, Pezzella V, Trillaud L, Deray G, Guillemain R. Renal insufficiency, mortality, and drug management in heart transplant. Results of the CARIN study. Transpl Int. 2014;27:931–938. [DOI] [PubMed] [Google Scholar]
- 44. Kaufman BD, Jessup M. Adult and pediatric perspectives on heart retransplant. World J Pediatr Congenit Heart Surg. 2013;4:75–79. [DOI] [PubMed] [Google Scholar]
- 45. Ubel PA, Arnold RM, Caplan AL. Rationing failure. The ethical lessons of the retransplantation of scarce vital organs. JAMA. 1993;270:2469–2474. [DOI] [PubMed] [Google Scholar]
- 46. Griffin L. Retransplantation of multiple organs: how many organs should one individual receive? Prog Transplant. 2002;12:92–96. [DOI] [PubMed] [Google Scholar]


