Abstract
Background
Acute kidney injury (AKI) occurs frequently after cardiac catheterization and percutaneous coronary intervention. Although a clinical risk model exists for percutaneous coronary intervention, no models exist for both procedures, nor do existing models account for risk factors prior to the index admission. We aimed to develop such a model for use in prospective automated surveillance programs in the Veterans Health Administration.
Methods and Results
We collected data on all patients undergoing cardiac catheterization or percutaneous coronary intervention in the Veterans Health Administration from January 01, 2009 to September 30, 2013, excluding patients with chronic dialysis, end‐stage renal disease, renal transplant, and missing pre‐ and postprocedural creatinine measurement. We used 4 AKI definitions in model development and included risk factors from up to 1 year prior to the procedure and at presentation. We developed our prediction models for postprocedural AKI using the least absolute shrinkage and selection operator (LASSO) and internally validated using bootstrapping. We developed models using 115 633 angiogram procedures and externally validated using 27 905 procedures from a New England cohort. Models had cross‐validated C‐statistics of 0.74 (95% CI: 0.74–0.75) for AKI, 0.83 (95% CI: 0.82–0.84) for AKIN2, 0.74 (95% CI: 0.74–0.75) for contrast‐induced nephropathy, and 0.89 (95% CI: 0.87–0.90) for dialysis.
Conclusions
We developed a robust, externally validated clinical prediction model for AKI following cardiac catheterization or percutaneous coronary intervention to automatically identify high‐risk patients before and immediately after a procedure in the Veterans Health Administration. Work is ongoing to incorporate these models into routine clinical practice.
Keywords: angiography, angioplasty, catheterization, contrast media, kidney
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Percutaneous Coronary Intervention, Quality and Outcomes, Epidemiology, Imaging
Introduction
Acute kidney injury (AKI) occurs frequently after cardiac catheterization or percutaneous coronary intervention (PCI)1 with more than 5‐fold variability in incidence across hospitals.2 Patients developing AKI have an increased risk of short‐ and long‐term mortality3, 4 and progression of renal disease.5, 6
A number of preprocedural and periprocedural protocols have been developed to minimize the occurrence of AKI in these patients.2, 7, 8, 9 Current risk models have been developed largely identifying patient risk factors at the time of the PCI10, 11 and others including procedural risk factors.12, 13, 14 However, current models have not evaluated the predictive ability of patient factors and clinical biomarkers from the year prior to the procedure, nor with external validation. As more healthcare systems participate in health information exchanges, such as the integrated Veterans Administration healthcare system, and provide increasing levels of integrated outpatient and inpatient management to address quality metrics and systems‐based care requirements, more clinical data are becoming available and should be considered in prediction rules.
There are a number of postcatheterization AKI risk prediction models published outside of the Veterans Health Administration (VA).10, 12, 15, 16, 17, 18, 19, 20, 21, 22 A national association study was done using the American College of Cardiology's National Cardiovascular Data Registry (ACC‐NCDR) for predicting AKI, but these data have not yet been developed into a model and internally validated.13 Additionally, no model for prospectively predicting risk of AKI for patients undergoing either cardiac catheterization or PCI in the VA has been developed for routine clinical use. There is a growing body of literature that supports the need for each healthcare system to perform recalibration and remodeling on a regular basis within their own healthcare data in order to ensure sustained high performance for individual patient prediction. In addition, the comprehensive nature of the VA electronic health record (EHR) data sources allows novel risk factors to be explored and tested for importance in risk prediction.
Therefore, we sought to develop an externally validated national prediction model for AKI occurring after cardiac catheterization or PCI for prospective automated surveillance. We hypothesized that risk factors from 1 year prior to, immediately before, and during the procedure would predict AKI in the VA.
Methods
Study Setting and Design
We developed and internally validated a set of risk prediction models for postprocedural AKI following cardiac catheterization within a national VA cohort. This cohort included 222 669 catheterization procedures among 70 centers between January 1, 2009 and October 1, 2013. The Institutional Review Board approved this study with a waiver of patient consent.
The Clinical Assessment, Reporting and Tracking Program (CART) is a national clinical quality initiative for VA cardiac catheterization laboratories that began in 2005 and was in use in all of these laboratories by the beginning of 2008.23 The CART program includes a clinical software application designed to collect standardized data on all coronary angiograms and PCI. This software was designed to support clinical workflow and documentation. All data elements are mapped to the definitions and standards of the ACC‐NCDR.24, 25
The VA is an integrated care network that includes acute inpatient hospitals, outpatient primary care and subspecialty clinics, outpatient pharmacies, rehabilitation facilities, and long‐term care facilities and domiciliaries. All VA clinical providers and allied health personnel are required to use the same EHR for documentation and execution of all clinical care. The VA Central IRB and site VA R&D committees at VA Tennessee Valley Healthcare System, VA Eastern Colorado Healthcare System, and White River Junction, Vermont VA approved this study.
The external validation cohort was provided by the Northern New England Cardiovascular Disease Study Group (NNECDSG), which includes participating medical centers in New Hampshire, Maine, and Vermont. At total of 27 905 patients were included in the external validation cohort.
Data Collection
Data were collected from a retrospective VA cohort of adult patients with a cardiac catheterization, with or without intervention. All data for these patients were retrieved beginning 1 year prior to the first cardiac catheterization in the cohort (January 1, 2008). Each patient coronary angiogram or PCI procedure that occurred within the same day as cardiac catheterization was aggregated into a single procedure for the study. The data were collected from 2 national VA sources: (1) the CART program, and (2) the EHR data available from the Corporate Data Warehouse. The CART program was used to identify procedures and to collect contrast administration data. EHR data from the Corporate Data Warehouse was used for inpatient admission and administrative data, chemistry and hematology laboratory data, outpatient pharmacy fill records, and inpatient bar‐coded medication administration. Both domains were merged for patient demographics.
Data Definitions
A complete description of data definitions is included in Data S1. Estimated glomerular filtration rates were calculated for all serum creatinine measures using the readily available abbreviated Modification of Diet and Renal Disease equation. Chronic kidney disease was defined as a baseline estimated glomerular filtration rate <60 mL/min per 1.73 m2.26, 27 Chronic comorbidities were defined using administrative Current Procedural Terminology and International Statistical Classification of Diseases, 9th Revision (ICD‐9) diagnostic codes collected from data prior to hospital admission. Substantial ICD‐9 validation work has been performed previously in the Veterans Affairs and in general populations.28, 29 Additional related ICD‐9 codes used were aimed at increasing sensitivity to these validated codes.
Cohort Inclusion and Exclusion Criteria
Procedures were included if they had at least 1 serum creatinine measurement within 1 day to 365 days prior to cardiac catheterization and at least 1 serum creatinine measurement from 0 to 7 days following the procedure. Procedures were excluded if they had a renal transplant, or if they had end‐stage renal disease, which is defined as requiring chronic dialysis or having a baseline estimated glomerular filtration rate of <15 mL/min per 1.73 m2 (n=6998) at the time of the procedure. Procedures with missing pre‐ (21 014) or postprocedure serum creatinine (79 024) were excluded, which left a total of 115 633 patient procedures (Figure 1). A comparison of procedures included in the cohort with procedures excluded due to missing serum creatinine is reported in Table S1.
Figure 1.

Study flow diagram. Summary of the inclusion and exclusion criteria for the procedure‐based cohort. ESRD indicates end‐stage renal disease.
Outcome Definitions
AKI was defined using the Kidney Disease Improving Global Outcomes Guidelines definition: ≥0.3 mg/dL within 48 hours of the procedure or ≥50% increase in serum creatinine from baseline to the post–cardiac catheterization peak serum creatinine at any time during the hospitalization or up to 7 days following the procedure.30 Baseline creatinine was defined as the most recent serum creatinine between 365 and 7 days prior to the procedure, which is a method previously validated in the literature.31 Acute Kidney Injury Network Stage 2 (AKIN2) was defined as a doubling in serum creatinine from baseline or developing new dialysis‐dependent renal failure. Contrast‐induced nephropathy (CIN) was defined by ≥0.5 mg/dL within 7 days following the procedure. Dialysis was identified by any new dialysis‐dependent renal failure within 7 days following the procedure. The timing of the post–cardiac catheterization creatinine values is +1 to +7 days with mean of 2 days and a SD of 5 days.
Statistical Analysis
Procedures with incomplete pre‐ and postprocedure serum creatinine were excluded from the analysis. Dichotomous variables reflecting the presence of medical conditions were set to zero if they were coded as missing (eg, absent). Dummy variables were created for variables with greater proportions of missing values (eg, intravenous fluids and laboratory values) and modeled against dummy categories (eg, missing values dummy modeled against known intravenous fluid status prior to the procedure dummy variables). Predicted values were imputed for missing contrast volume using case complexity, number of vessels receiving direct intervention, and number of stents deployed. We developed prediction rules using logistic regression with variable selection by using the least absolute shrinkage and selection operator (LASSO). LASSO obtains coefficient estimates by maximizing a likelihood with a L1 penalty on coefficient size, for a sequence of penalty parameters.32 LASSO has the property that only a subset of variables in the model will have nonzero coefficients, which makes it a variable selection tool. We report the LASSO models for which the penalty term resulted in a maximized cross‐validated likelihood. The predictive ability of the model is reported using the C‐statistic (area under the Receiver Operating Characteristic (ROC) curve, AUC) corrected for overfitting using bootstrap validation by sampling the dataset with 200 iterations, and by plotting calibration of the model using (observed versus predicted rates of AKI after binning of predicted values). The bootstrap distribution of C‐statistic values was used to obtain 95% CIs around the AUC. Sensitivity analyses were conducted re‐running the models based on a 48‐hour definition of AKI end points and again on unique patients. We considered all pairwise interactions, but excluded all from the model because none increased the C‐statistic by more than 0.001. Analyses were conducted using R (library glmnet (http://cran.r-project.org/web/packages/glmnet/glmnet.pdf) and Stata.
External validation was conducted as follows: First, as the NNE cohort does not collect EHR variables from preceding outpatient encounters, each of the 4 models was reduced to only include variables also available in the NNE external validation cohort. Second, LASSO was performed again on the common variables of the VA and NNE cohorts. Third, the model development and bootstrapping methods were repeated in the VA cohort for the reduced VA models. Last, the coefficients from the VA reduced models were then used to calculate the NNE external validation ROC and 95% CI for each of the 4 models.
Results
Table 1 shows the characteristics of the cohort for the AKI and non‐AKI groups, including demographics, prior comorbid events, comorbidities at presentation, and procedural aspects. The univariate association (odds ratio) of each characteristic with AKI occurrence is also presented in Table 1. Out of a total of 115 633 coronary angiography procedures, AKI developed in 16 036 (13.9%), while the number with AKIN Stage 2 was 2017 (1.7%), the number with CIN was 13 763 (14.4%), and 476 (0.4%) received dialysis treatment for AKI.
Table 1.
Patient and Procedural Characteristics
| No AKI | AKI | OR (95% CI) | P Value | |
|---|---|---|---|---|
| Procedures (n=115 633) | 99 596 | 16 037 | ||
| Comorbidities | ||||
| Age | 65.2±9.5 | 67.2±10.0 | 1.02 (1.02–1.02) | <0.001 |
| Female | 2.2 | 2.1 | 0.93 (0.83–1.05) | 0.233 |
| Nonwhite race | 22.8 | 26.7 | 1.23 (1.19–1.28) | <0.001 |
| Tobacco use (any) | 38.7 | 34.1 | 0.82 (0.79–0.85) | <0.001 |
| Prior tobacco use | 52.7 | 47.5 | 0.81 (0.79–0.84) | <0.001 |
| Prior comorbidities | ||||
| Prior catheterization | 43.9 | 24.3 | 0.41 (0.40–0.43) | <0.001 |
| 0 to 1 days from catheterization | 33.4 | 19.1 | 0.47 (0.45–0.49) | <0.001 |
| 0 to 2 days from catheterization | 34.2 | 19.9 | 0.48 (0.46–0.50) | <0.001 |
| Days from catheterization | 15.5±87.5 | 12.8±77.4 | 1.00 (1.00–1.00) | 0.064 |
| Prior PCI | 33.5 | 25.5 | 0.68 (0.66–0.71) | <0.001 |
| Prior CABG | 12.9 | 11.1 | 0.85 (0.80–0.89) | <0.001 |
| Prior MI | 28.1 | 30.2 | 1.11 (1.07–1.15) | <0.001 |
| Prior stroke | 7.9 | 8.5 | 1.08 (1.02–1.14) | 0.014 |
| Diabetes | 48.5 | 51.8 | 1.14 (1.10–1.18) | <0.001 |
| Dyslipidemia | 74.4 | 65.9 | 0.66 (0.64–0.69) | <0.001 |
| Hypertension | 78.6 | 76.2 | 0.87 (0.84–0.91) | <0.001 |
| Hypotension | 8.8 | 11.3 | 1.33 (1.26–1.40) | <0.001 |
| Mitral regurgitation | 0.5 | 0.5 | 0.99 (0.77–1.26) | 0.911 |
| Peripheral vascular disease | 19.2 | 22.8 | 1.24 (1.19–1.29) | <0.001 |
| Number of prior comorbid events | ||||
| Number of prior admissions | 0.7±1.2 | 0.9±1.6 | 1.12 (1.11–1.14) | <0.001 |
| CHF | 0.02±0.14 | 0.02±0.14 | 1.06 (0.95–1.20) | 0.286 |
| CHF 7 to 365 days | 24.1 | 35.8 | 1.76 (1.70–1.82) | <0.001 |
| CKD | 0.4±1.9 | 1.1±3.3 | 1.11 (1.10–1.11) | <0.001 |
| Diabetes | 4.1±7.7 | 5.1±9.1 | 1.01 (1.01–1.02) | <0.001 |
| Dyslipidemia | 2.6±3.0 | 2.4±3.1 | 0.97 (0.97–0.98) | <0.001 |
| Hypertension | 4.1±5.6 | 4.5±6.4 | 1.01 (1.01–1.02) | <0.001 |
| Hypoalbuminemia 7 to 30 days | 5.5 | 13.9 | 2.77 (2.62–2.91) | <0.001 |
| Hypoalbuminemia 7 to 90 days | 9.6 | 19.91 | 2.34 (2.24–2.45) | <0.001 |
| Hypotension | 0.1±0.8 | 0.2±0.9 | 1.07 (1.05–1.09) | <0.001 |
| Peripheral vascular disease | 0.8±2.9 | 1.0±3.4 | 1.02 (1.02–1.03) | <0.001 |
| Anemia 3‐days | 32.6 | 52.6 | 2.29 (2.22–2.37) | <0.001 |
| Anemia 3 to 90 days | 33.1 | 46.5 | 1.76 (1.71–1.82) | <0.001 |
| Anemia 3 to 365 days | 46.4 | 58.6 | 1.64 (1.58–1.69) | <0.001 |
| Shock events | 0.0007±0.0454 | 0.0043±0.0943 | 2.22 (1.70–2.90) | <0.001 |
| CHF events | 0.3±1.0 | 0.6±1.5 | 1.23 (1.21–1.24) | <0.001 |
| CKD events | 0.05±0.37 | 0.13±0.63 | 1.37 (1.33–1.41) | <0.001 |
| Dyslipidemia events | 0.25±0.66 | 0.21±0.64 | 0.90 (0.87–0.92) | <0.001 |
| Prior renal complications and function | ||||
| Prior CKD | 13.1 | 24.4 | 2.14 (2.05–2.23) | <0.001 |
| Prior AKI (KDIGO) | 11.0 | 29.8 | 3.45 (3.32–3.59) | <0.001 |
| Prior highest AKIN Stage | 1.83 (1.78–1.87) | <0.001 | ||
| AKIN Stage 1 | 17.9 | 27.4 | ||
| AKIN Stage 2 | 2.5 | 7.7 | ||
| AKIN Stage 3 | 0.6 | 1.7 | ||
| Prior CIN (>0.5) | 15.2 | 30.1 | 2.41 (2.32–2.50) | <0.001 |
| Prior ARF (ICD9) | 6.5 | 15.5 | 2.64 (2.52–2.78) | <0.001 |
| Number of prior AKI admissions | 0.0003±0.0164 | 0.0006±0.0249 | 2.35 (1.15–4.79) | 0.019 |
| Number of prior CKD admissions | 0.0025±0.0547 | 0.0082±0.1097 | 2.48 (2.05–3.02) | <0.001 |
| Change in eGFR prior year | 0.9±13.7 | 1.3±17.4 | 1.00 (1.00–1.00) | 0.001 |
| Decline in eGFR prior year | 3.5±8.2 | 3.8±11.5 | 0.99 (0.99–0.99) | <0.001 |
| CKD | 6.9 | 13.4 | 2.07 (1.96–2.18) | <0.001 |
| eGFR <60, mL/min per m2 | 14.8 | 33.5 | 2.90 (2.79–3.01) | <0.001 |
| eGFR <45, mL/min per m2 | 5.0 | 18.1 | 4.17 (3.96–4.38) | <0.001 |
| eGFR <30, mL/min per m2 | 0.9 | 6.7 | 8.14 (7.43–8.92) | <0.001 |
| Medications | ||||
| ARB | 9.56 | 9.6 | 1 (0.95–1.06) | 0.893 |
| ACE | 41.78 | 38.9 | 0.888 (0.86–0.92) | <0.001 |
| Loop diuretic | 18.99 | 28.4 | 1.69 (1.63–1.76) | <0.001 |
| K‐sparing diuretic | 5.77 | 7.7 | 1.37 (1.29–1.46) | <0.001 |
| Statins | 62.03 | 54.2 | 0.72 (0.70–0.75) | <0.001 |
| Aminoglycosides | 0.1 | 0.1 | 0.79 (0.47–1.34) | 0.387 |
| Cimetidine | 0.1 | 0.1 | 0.47 (0.24–0.93) | 0.031 |
| Cyclosporine | 0.3 | 0.4 | 1.46 (1.11–1.90) | 0.006 |
| N‐acetylcysteine | 2.6 | 1.7 | 0.67 (0.59–0.75) | <0.001 |
| NSAIDS | 11.2 | 7.8 | 0.67 (0.63–0.71) | <0.001 |
| Trimethoprim | 0.9 | 1.1 | 1.21 (1.03–1.42) | 0.024 |
| Thrombolytic use | 0.03 | 0.02 | 0.89 (0.31–2.53) | 0.823 |
| At presentation | ||||
| Hypertension | 43.6 | 45.7 | 1.09 (1.05–1.12) | <0.001 |
| Hypotension | 22.2 | 32.4 | 1.68 (1.62–1.75) | <0.001 |
| Prepresent MI | 15.1 | 17.9 | 1.23 (1.18–1.28) | <0.001 |
| Acute coronary syndrome | 23.9 | 32.1 | 1.51 (1.46–1.56) | <0.001 |
| Anemia | 32.6 | 52.6 | 2.29 (2.22–2.37) | <0.001 |
| Shock | 0.4 | 4.3 | 10.22 (9.06–11.55) | <0.001 |
| CHF | 0.4 | 4.3 | 10.23 (9.06–11.55) | <0.001 |
| Dyslipidemia | 34.2 | 29.6 | 0.81 (0.78–0.84) | <0.001 |
| Unstable angina | 9.2 | 4.1 | 0.42 (0.39–0.46) | <0.001 |
| Ejection fraction ≤40% | 4.05 | 5.58 | 1.40 (1.30–1.51) | <0.001 |
| Pre creatine‐kinase ≥100 | 6.9 | 7.7 | 1.11 (1.05–1.19) | 0.001 |
| Pre CKMB ≥2.66 | 4.2 | 6.1 | 1.49 (1.38–1.60) | <0.001 |
| Preprocedural volume supplementation | ||||
| Pre IV normal saline, mL | 95.3±373.9 | 100.2±386.1 | 1.00 (1.00–1.00) | 0.177 |
| Pre IV total fluids, mL | 176.0±593.2 | 176.1±560.3 | 1.00 (1.00–1.00) | 0.983 |
| Pre IV category | ||||
| Missing IV fluids | 21.5 | 20.5 | 0.94 (0.90–0.98) | 0.005 |
| No IV fluids | 64.5 | 65.3 | 1.03 (1.00–1.07) | 0.059 |
| 1 to 999 (mL) IV fluids | 6.4 | 6.6 | 1.04 (0.97–1.11) | 0.267 |
| 1000+ (mL) IV fluids | 7.6 | 7.6 | 1.00 (0.94–1.06) | 0.946 |
| Procedural characteristics | ||||
| Priority | ||||
| Elective | 62.7 | 47.6 | 0.55 (0.53–0.57) | <0.001 |
| Urgent | 33.5 | 42.6 | 1.46 (1.41–1.51) | <0.001 |
| Emergent | 3.7 | 9.4 | 2.65 (2.49–2.83) | <0.001 |
| Salvage | 0.1 | 0.4 | 8.73 (6.07–12.55) | <0.001 |
| Diagnostic cardiac catheterization | 86.1 | 92.3 | 1.94 (1.82–2.06) | <0.001 |
| Percutaneous coronary intervention | 45.7 | 25.6 | 0.41 (0.39–0.42) | <0.001 |
| Ad hoc PCI | 31.8 | 17.9 | 0.47 (0.45–0.49) | <0.001 |
| Multivessel disease | 4.4 | 3.3 | 0.75 (0.68–0.82) | <0.001 |
| Number of diseased vessels | 0.4±0.6 | 0.2±0.5 | 0.53 (0.51–0.55) | <0.001 |
| None | 63.5 | 80.0 | ||
| Single | 32.1 | 16.7 | ||
| Double | 4.1 | 2.9 | ||
| Triple | 0.3 | 0.4 | ||
| Number of diseased lesions | 0.5±0.8 | 0.3±0.7 | 0.65 (0.63–0.67) | <0.001 |
| None | 63.5 | 80.0 | ||
| One | 24.2 | 12.6 | ||
| Two | 9.0 | 5.3 | ||
| Three | 2.6 | 1.6 | ||
| Four | 0.6 | 0.4 | ||
| Five | 0.2 | 0.2 | ||
| Stent procedure | 38.6 | 20.4 | 0.41 (0.39–0.42) | <0.001 |
| Number of interventions | 1.7±2.6 | 1.0±2.3 | 0.88 (0.87–0.89) | <0.001 |
| Number of stents | 0.6±0.9 | 0.4±0.9 | 0.69 (0.67–0.71) | <0.001 |
| Intra‐aortic balloon pump | 0.8 | 5.7 | 7.21 (6.56–7.93) | <0.001 |
| Contrast type | ||||
| Hexabrix | 0.4 | 0.9 | 2.59 (2.14–3.13) | <0.001 |
| Isovue | 15.1 | 11.1 | 0.70 (0.67–0.74) | <0.001 |
| Omnipaque | 8.0 | 6.8 | 0.84 (0.79–0.90) | <0.001 |
| Other contrast | 2.1 | 1.9 | 0.88 (0.78–0.99) | 0.037 |
| Ultravist | 5.0 | 5.1 | 1.02 (0.95–1.10) | 0.553 |
| Unknown contrast | 31.3 | 32.9 | 1.08 (1.04–1.11) | <0.001 |
| Visipaque270 | 5.5 | 6.3 | 1.16 (1.08–1.24) | <0.001 |
| Visipaque320 | 24.4 | 23.0 | 0.93 (0.89–0.97) | <0.001 |
| Contrast volume, mL | 155.2±104.1 | 125.8±103.4 | 1.00 (1.00–1.00) | <0.001 |
| Contrast: GFR ratio33 | 2.1±1.7 | 2.1±2.5 | 1.01 (1.00–1.02) | 0.013 |
| Contrast GFR ratio >3 | 12.5 | 14.9 | 1.22 (1.16–1.28) | <0.001 |
ACE indicates angiotensin‐converting enzyme; AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; ARB, angiotensin receptor blockers; ARF, acute renal failure; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CIN, contrast‐induced nephropathy; CKD, chronic kidney disease; CKMB, creatinine kinase, muscle and brain subunits; eGFR, estimated glomerular filtration rate; ICD9, Current Procedural Terminology and International Statistical Classification of Diseases, 9th Revision; IV, intravenous; KDIGO, kidney disease improving global outcomes; MI, myocardial infarction; NSAIDS, nonsteroidal anti‐inflammatory drugs; OR, odds ratio; PCI, percutaneous coronary intervention.
Using the variables included in Table 1, we built a logistic regression model for prediction of AKI occurrence using LASSO. Table 2 contains the odds ratios we obtained from fitting this model to our data. First from the left is the LASSO‐derived model for patients developing AKI. The overfitting‐corrected C‐statistic for the AKI prediction model was 0.742 (95% CI: 0.738–0.747). The second column contains the LASSO‐derived model results for patients developing AKIN Stage 2, for which the bias‐corrected C‐statistic was 0.826 (95% CI: 0.816–0.836). The third column contains our results from the model for patients developing ≥0.5 (mg/dL) increase in serum creatinine (CIN), with a bias‐corrected C‐statistic of 0.741 (95% CI: 0.737–0.746). The last column shows the results from our LASSO‐derived model for dialysis with a bias‐corrected C‐statistic of 0.885 (95% CI: 0.870–0.902). We present observed‐versus‐expected plots showing calibration for each model in Figure 2. Each plot indicates strong agreement between the observed and predicted values, although for all 4 outcomes the observed odds are below our predictions for subjects with the lowest expected odds. We conducted sensitivity analyses for AKI at 48 hours and by limiting the cohort to unique patient procedures as opposed to a unique procedure analysis. All ROCs were robust for AKI, AKIN2, CIN, and dialysis end points: 0.768, 0.855, 0.736, and 0.895, respectively. When limiting to unique patients (n=90 105), the ROCs for AKI, AKIN2, CIN, and dialysis end points were 0.738, 0.826, 0.739, and 0.883, respectively. Both sensitivity analyses proved the model to perform well on a 48‐hour definition for AKI end points and restricting to unique patient procedures.
Table 2.
Multivariable Logistic Regression Models for Predicting AKI, AKIN Stage 2, CIN, and Dialysis Selected Using LASSO With Maximumized Cross‐Validation
| Risk Factor | AKI | AKIN 2 | CIN | Dialysis |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Comorbidities | ||||
| Age | 1.01 (1.01–1.01)a | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) | 1.00 (1.00–1.00) |
| Nonwhite race | 1.10 (1.06–1.15)a | 1.14 (1.09–1.18)a | 1.01 (0.98–1.05) | 1.26 (1.22–1.30)a |
| Tobacco use (any) | 0.90 (0.86–0.95)a | 0.92 (0.88–0.97)a | 0.98 (0.93–1.03) | 0.82 (0.79–0.86)a |
| Prior tobacco use | 0.99 (0.94–1.04) | 1.00 (0.95–1.05) | 1.00 (0.95–1.05) | 1.00 (0.96–1.04) |
| Prior comorbidities | ||||
| 0 to 1 days from catheterization | 0.60 (0.50–0.72)a | 0.87 (0.74–1.03) | 0.89 (0.77–1.03) | 0.70 (0.61–0.81)a |
| 0 to 2 days from catheterization | 0.88 (0.73–1.05) | 0.90 (0.76–1.05) | 0.61 (0.53–0.71)a | 1.00 (0.87–1.15) |
| Prior PCI | 0.79 (0.76–0.82)a | 0.79 (0.76–0.82)a | 0.83 (0.80–0.86)a | 0.89 (0.87–0.92)a |
| Prior CABG | 0.82 (0.77–0.86)a | 0.87 (0.83–0.92)a | 0.86 (0.82–0.90)a | 0.84 (0.80–0.88)a |
| Prior MI | 1.00 (0.95–1.06) | 0.97 (0.92–1.02) | 1.00 (0.95–1.05) | 1.00 (0.96–1.05) |
| Prior stroke | 1.06 (1.00–1.13) | 0.88 (0.83–0.93)a | 1.02 (0.96–1.08) | 0.93 (0.88–0.98)a |
| Diabetes | 1.10 (1.06–1.15)a | 1.30 (1.25–1.36)a | 1.25 (1.20–1.29)a | 1.35 (1.30–1.39)a |
| Dyslipidemia | 0.78 (0.74–0.82)a | 0.95 (0.91–1.00)a | 0.87 (0.83–0.91)a | 0.73 (0.70–0.75)a |
| Hypertension | 0.90 (0.86–0.94)a | 1.21 (1.15–1.26)a | 1.04 (0.99–1.08) | 0.91 (0.88–0.95)a |
| Hypotension | 0.96 (0.89–1.04) | 0.99 (0.92–1.07) | 0.88 (0.83–0.94)a | 1.00 (0.94–1.07) |
| Mitral regurgitation | 0.89 (0.71–1.12) | 0.81 (0.65–1.01) | 1.03 (0.83–1.28) | 0.34 (0.29–0.41)a |
| Peripheral vascular disease | 1.09 (1.03–1.15)a | 1.11 (1.06–1.17)a | 1.00 (0.95–1.05) | 1.00 (0.96–1.04) |
| Number of prior comorbid events | ||||
| Number of prior admissions | 0.98 (0.96–1.00)a | 0.98 (0.96–1.00)a | 1.00 (0.99–1.02) | 0.95 (0.93–0.96)a |
| CHF | 1.17 (1.04–1.33)a | 1.14 (1.02–1.29)a | 1.04 (0.94–1.16) | 0.85 (0.78–0.93)a |
| CHF 7 to 365 days | 1.13 (1.07–1.18)a | 1.23 (1.17–1.29)a | 1.08 (1.04–1.13)a | 1.08 (1.04–1.12)a |
| CKD | 1.00 (0.99–1.02) | 1.00 (0.98–1.02) | 0.99 (0.98–1.01) | 1.04 (1.02–1.07)a |
| Diabetes | 1.00 (1.00–1.01)a | 1.00 (1.00–1.00) | 1.00 (1.00–1.01) | 1.00 (1.00–1.00) |
| Dyslipidemia | 1.00 (0.99–1.01) | 0.99 (0.98–0.99)a | 0.99 (0.98–1.00)a | 1.00 (0.99–1.01) |
| Hypertension | 0.99 (0.99–1.00)a | 1.00 (0.99–1.00) | 1.00 (1.00–1.00) | 0.99 (0.98–0.99)a |
| Hypoalbuminemia 7 to 30 days | 1.26 (1.10–1.44)a | 1.09 (0.95–1.25) | 1.19 (1.06–1.33)a | 1.19 (1.07–1.31)a |
| Hypoalbuminemia 7 to 90 days | 1.05 (0.95–1.15) | 1.33 (1.20–1.47)a | 1.14 (1.05–1.24)a | 1.00 (0.93–1.08) |
| Hypotension | 0.97 (0.95–1.00) | 0.99 (0.96–1.02) | 0.98 (0.96–1.01) | 0.96 (0.93–0.98)a |
| Peripheral vascular disease | 1.00 (0.99–1.01) | 1.00 (0.99–1.01) | 1.00 (1.00–1.01) | 1.02 (1.01–1.02)a |
| Shock events | 0.66 (0.37–1.16) | 0.65 (0.37–1.15) | 0.55 (0.31–0.95)a | 0.76 (0.47–1.24) |
| CHF Events | 1.06 (1.03–1.09)a | 1.03 (1.00–1.05)a | 1.09 (1.07–1.12)a | 1.00 (0.98–1.02) |
| CKD events | 0.95 (0.88–1.04) | 0.94 (0.87–1.02) | 0.92 (0.87–0.97)a | 0.93 (0.86–1.01) |
| Dyslipidemia events | 0.96 (0.93–0.99)a | 1.00 (0.97–1.03) | 0.98 (0.95–1.01) | 1.00 (0.98–1.02) |
| Prior renal complications and function | ||||
| Prior CKD | 1.09 (1.01–1.18)a | 1.00 (0.93–1.08) | 0.83 (0.79–0.89)a | 1.73 (1.60–1.87)a |
| Prior AKI (KDIGO) | 1.88 (1.73–2.04)a | 1.72 (1.59–1.86)a | 1.58 (1.48–1.68)a | 1.44 (1.36–1.53)a |
| Prior highest AKIN Stage | 1.14 (1.08–1.21)a | 1.24 (1.17–1.31)a | 1.00 (0.96–1.05) | 1.35 (1.29–1.41)a |
| Prior CIN (>0.5) | 1.07 (0.99–1.15) | 1.13 (1.05–1.21)a | 1.35 (1.27–1.44)a | 0.88 (0.83–0.94)a |
| Prior ARF (ICD9) | 1.04 (0.94–1.16) | 1.02 (0.93–1.13) | 0.99 (0.91–1.07) | 1.11 (1.03–1.21)a |
| Number of prior AKI admissions | 0.68 (0.13–3.48) | 1.00 (0.10–9.78) | 0.94 (0.23–3.88) | 1.58 (0.07–36.35) |
| Number prior CKD admissions | 1.10 (0.70–1.70) | 1.28 (0.72–2.26) | 0.93 (0.69–1.25) | 1.41 (0.77–2.57) |
| Change in eGFR prior year | 1.00 (1.00–1.00)a | 0.99 (0.99–1.00)a | 0.98 (0.98–0.98)a | 1.00 (1.00–1.00) |
| Decline in eGFR prior year | 1.01 (1.00–1.01)a | 1.01 (1.01–1.02)a | 1.01 (1.00–1.01)a | 1.00 (1.00–1.00) |
| CKD | 1.13 (1.02–1.25)a | 1.00 (0.91–1.10) | 0.91 (0.84–0.99)a | 1.34 (1.22–1.46)a |
| eGFR <60, mL/min per m2 | 1.37 (1.28–1.47)a | 1.50 (1.41–1.60)a | 1.28 (1.21–1.35)a | 1.19 (1.14–1.25)a |
| eGFR <45, mL/min per m2 | 1.31 (1.14–1.52)a | 1.37 (1.20–1.56)a | 1.40 (1.27–1.55)a | 2.02 (1.80–2.27)a |
| eGFR <30, mL/min per m2 | 2.06 (1.29–3.27)a | 3.81 (1.82–8.00)a | 2.74 (2.06–3.64)a | 5.52 (1.76–17.29)a |
| Presenting medication use | ||||
| ARB | 0.94 (0.88–0.99)a | 0.93 (0.88–0.98)a | 0.93 (0.88–0.98)a | 0.89 (0.85–0.94)a |
| ACE | 0.99 (0.95–1.03) | 1.00 (0.97–1.04) | 1.00 (0.97–1.03) | 0.85 (0.82–0.87)a |
| Loop diuretic | 1.08 (1.03–1.14)a | 1.15 (1.09–1.21)a | 1.08 (1.03–1.13)a | 1.38 (1.32–1.44)a |
| K‐sparing diuretic | 1.02 (0.94–1.10) | 1.00 (0.93–1.08) | 0.97 (0.91–1.04) | 0.79 (0.74–0.84)a |
| Statins | 0.87 (0.84–0.91)a | 0.83 (0.80–0.86)a | 0.86 (0.84–0.90)a | 0.76 (0.74–0.79)a |
| Aminoglycosides | 0.73 (0.47–1.12) | 1.00 (0.64–1.57) | 1.00 (0.65–1.54) | 0.89 (0.62–1.28) |
| Cimetidine | 0.64 (0.43–0.94)a | 0.55 (0.39–0.77)a | 1.00 (0.65–1.53) | 0.78 (0.56–1.09) |
| Cyclosporine | 1.00 (0.72–1.40) | 1.00 (0.71–1.40) | 1.00 (0.74–1.36) | 1.22 (0.87–1.70) |
| N‐acetylcysteine | 0.80 (0.73–0.88)a | 0.78 (0.72–0.85)a | 0.74 (0.68–0.81)a | 0.84 (0.77–0.91)a |
| NSAIDS | 0.87 (0.83–0.91)a | 1.00 (0.96–1.05) | 0.93 (0.89–0.98)a | 0.96 (0.93–1.00)a |
| Trimethoprim | 0.88 (0.73–1.05) | 1.00 (0.83–1.21) | 1.00 (0.83–1.21) | 1.00 (0.86–1.17) |
| Thrombolytic | 0.94 (0.34–2.58) | 1.00 (0.33–3.02) | 1.00 (0.40–2.51) | 1.00 (0.46–2.17) |
| Clinical presentation | ||||
| Elective | 0.87 (0.79–0.95)a | 0.60 (0.55–0.65)a | 0.87 (0.80–0.94)a | 0.82 (0.76–0.87)a |
| Urgent | 1.21 (1.10–1.33)a | 1.00 (0.91–1.10) | 1.16 (1.06–1.26)a | 1.00 (0.93–1.08) |
| Emergent | 2.72 (2.32–3.18)a | 3.54 (2.96–4.24)a | 2.97 (2.59–3.40)a | 2.46 (2.19–2.75)a |
| Salvage | 4.87 (0.92–25.76) | 8.05 (0.34–191.31) | 4.16 (1.47–11.83)a | 1.78 (0.83–3.84) |
| Unstable angina | 0.73 (0.70–0.77)a | 0.61 (0.58–0.63)a | 0.76 (0.73–0.80)a | 1.00 (0.96–1.04) |
| Shock | 4.46 (2.30–8.67)a | 4.76 (1.99–11.39)a | 4.76 (2.97–7.65)a | 4.14 (2.69–6.40)a |
| Hypertension | 0.94 (0.91–0.98)a | 0.86 (0.83–0.89)a | 0.96 (0.93–1.00)a | 0.96 (0.94–0.99)a |
| Hypotension | 1.04 (0.99–1.08) | 1.30 (1.24–1.35)a | 1.16 (1.11–1.20)a | 1.00 (0.97–1.04) |
| Ejection fraction ≤40% | 1.13 (1.03–1.24)a | 0.96 (0.88–1.04) | 1.12 (1.03–1.21)a | 1.00 (0.93–1.07) |
| Acute coronary syndrome | 1.33 (1.26–1.39)a | 1.13 (1.08–1.18)a | 1.24 (1.19–1.29)a | 1.08 (1.04–1.12)a |
| Pre‐creatine‐kinase ≥100 | 0.88 (0.82–0.95)a | 1.00 (0.93–1.07) | 0.94 (0.88–1.00) | 1.00 (0.94–1.06) |
| Pre CKMB ≥2.66 | 1.18 (1.06–1.31)a | 1.00 (0.91–1.10) | 1.13 (1.04–1.24)a | 1.00 (0.93–1.08) |
| Prepresent MI | 1.06 (0.99–1.13) | 1.10 (1.03–1.18)a | 1.06 (1.00–1.13) | 1.04 (0.99–1.10) |
| Dyslipidemia | 0.96 (0.92–1.01) | 0.98 (0.94–1.03) | 1.00 (0.96–1.05) | 0.81 (0.79–0.84)a |
| Anemia | 1.30 (1.25–1.36)a | 1.23 (1.18–1.27)a | 1.28 (1.23–1.32)a | 1.16 (1.12–1.19)a |
| Preprocedural volume supplementation | ||||
| No IV fluids | 1.02 (0.98–1.06) | 1.00 (0.96–1.04) | 1.00 (0.96–1.04) | 0.96 (0.93–0.99)a |
| 1 to 999 (mL) IV fluids | 1.02 (0.95–1.09) | 1.00 (0.94–1.07) | 1.00 (0.94–1.07) | 1.00 (0.94–1.06) |
| 1000+ (mL) IV fluids | 1.02 (0.96–1.09) | 0.99 (0.93–1.06) | 1.02 (0.96–1.09) | 1.00 (0.95–1.05) |
ACE indicates angiotensin‐converting enzyme; AKI, acute kidney injury; AKIN, acute kidney injury network; ARB, angiotensin receptor blockers; ARF, acute renal failure; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CIN, contrast‐induced nephropathy; CKD, chronic kidney disease; CKMB, creatinine kinase, muscle and brain subunits; eGFR, estimated glomerular filtration rate; ICD9, Current Procedural Terminology and International Statistical Classification of Diseases, 9th Revision; IV, intravenous; KDIGO, kidney disease improving global outcomes; LASSO, least absolute shrinkage and selection operator; MI, myocardial infarction; NSAIDS, nonsteroidal anti‐inflammatory drugs; OR, odds ratio; PCI, percutaneous coronary intervention.
P<0.05.
Figure 2.
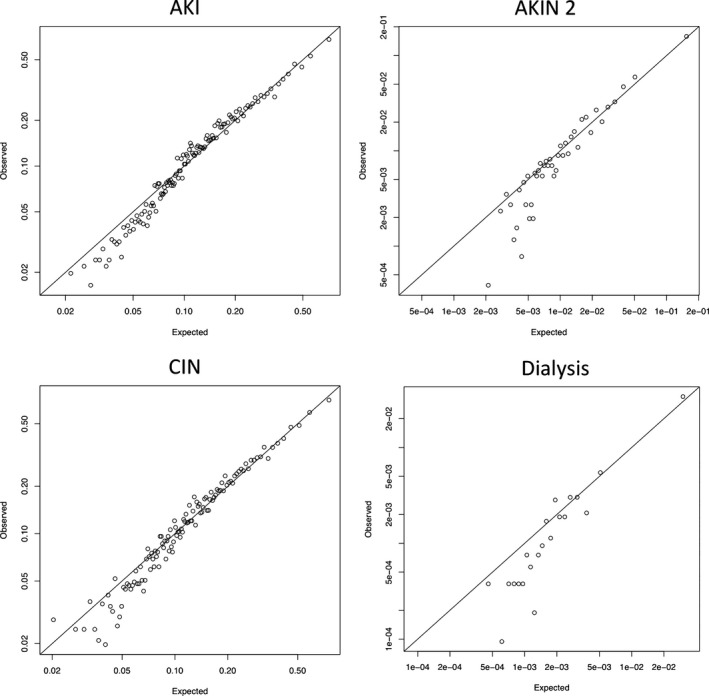
Observed vs expected calibration plots. This figure plots the observed vs expected calibration plots for AKI, AKIN 2, CIN, and dialysis. AKI indicates acute kidney injury; AKIN 2, Acute Kidney Injury Network 2; CIN, contrast‐induced nephropathy.
A small number of candidate variables were statistically significant and associated consistently with occurrence of AKI for each of our outcome definitions (Table 2). History of diabetes was a risk factor, while prior coronary artery bypass grafting and PCI were protective. Prior instances of congestive heart failure and low albumin were also risk factors, as were prior AKI by Kidney Disease Improving Global Outcomes definition and chronic kidney disease by observed estimated glomerular filtration rates (creatinine). Exposure to either N‐acetylcysteine, angiotensin receptor blockers, or hydroxymethyl glutaryl coenzyme A reductase inhibitors at presentation was protective, whereas exposure to loop diuretics was a risk factor. Clinical presentation risk factors were catheterization urgency status, shock, acute coronary syndrome, and anemia.
External validation limited to variables included in the NNECDSG registry showed results consistent with our VA AKI population findings (Table 3). The final VA reduced multivariate model results are shown in Table 4. The VA reduced models performed with high agreement in ROC statistics for all prediction end points, including any AKI occurrence (VA: 0.70, 95% CI 0.70, 0.71; NNECDSG: 0.69, 95% CI 0.68, 0.71), CIN (VA: 0.68, 95% CI 0.68, 0.69; NNECDSG: 0.72; 95% CI 0.70, 0.74), AKIN Stage 2 (VA: 0.80, 95% CI 0.79, 0.81; NNECDSG: 0.76, 95% CI 0.73, 0.79), and dialysis (VA: 0.85, 95% CI 0.82, 0.87; NNECDSG: 0.86, 95% CI 0.76, 0.96).
Table 3.
Northern New England Cardiovascular Disease Study Group External Validation Patient and Disease Characteristics
| No AKI | AKI | P Value | |
|---|---|---|---|
| Procedures (n=27 905) | 26 375 | 1530 | |
| Comorbidities | |||
| Age | 63.79±11.93 | 69.58±12.40 | <0.001 |
| Female | 28.69 | 38.17 | <0.001 |
| Nonwhite race | 0.15 | 0.20 | 0.667 |
| Tobacco use (any) | 27.81 | 20.39 | <0.001 |
| Prior comorbidities | |||
| Prior PCI | 34.13 | 31.70 | 0.051 |
| Prior CABG | 16.05 | 19.74 | <0.001 |
| Prior MI | 26.96 | 30.07 | 0.008 |
| Prior stroke | 11.22 | 20.13 | <0.001 |
| Diabetes | 30.51 | 47.12 | <0.001 |
| Hypertension | 74.23 | 81.76 | <0.001 |
| Peripheral vascular disease | 17.52 | 29.48 | <0.001 |
| Number of prior comorbid events | |||
| CHF | 10.45 | 30.52 | <0.001 |
| Shock within prior 24 hours | 0.66 | 4.51 | <0.001 |
| Prior renal complications and function | |||
| Prior CKD | 18.67 | 43.53 | <0.001 |
| eGFR <60, mL/min per m2 | 17.77 | 36.01 | <0.001 |
| eGFR <45, mL/min per m2 | 0.77 | 6.01 | <0.001 |
| eGFR <30, mL/min per m2 | 0.13 | 1.50 | <0.001 |
| Medications | |||
| Thrombolytic use | 5.04 | 5.75 | 0.214 |
| At presentation | |||
| Prepresent MI | 18.89 | 25.82 | <0.001 |
| Anemia | 14.35 | 23.33 | <0.001 |
| Shock | 0.79 | 5.42 | <0.001 |
| Unstable angina | 0.79 | 5.42 | <0.001 |
| Ejection fraction ≤40% | 6.93 | 15.03 | <0.001 |
| Procedural characteristics | |||
| Priority | |||
| Elective | 26.96 | 11.24 | <0.001 |
| Urgent | 51.80 | 53.14 | 0.31 |
| Emergent | 21.20 | 34.97 | <0.001 |
| Salvage | 0.03 | 0.65 | <0.001 |
| Ad hoc PCI | 83.05 | 82.36 | 0.716 |
| Multivessel disease | |||
| Number of diseased vessels | |||
| Single | 58.15 | 45.56 | <0.001 |
| Double | 27.21 | 31.83 | |
| Triple | 14.64 | 22.61 | |
| Number of stents | 1.56±1.04 | 1.60±1.18 | 0.1827 |
| Intra‐aortic balloon pump | 1.69 | 11.08 | <0.001 |
AKI indicates acute kidney injury; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Table 4.
VA Reduced Multivariate Models for External Validation
| Risk Factor | Any AKI | AKIN 2+ | CIN | Dialysis |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Comorbidities | ||||
| Age | 1.01 (1.01–1.01)a | 1.00 (0.99–1.01) | ||
| Nonwhite race | 1.18 (1.13–1.23)a | 1.24 (1.12–1.38)a | 1.08 (1.03–1.13)a | 1.44 (1.17–1.77)a |
| Tobacco use (any) | 0.89 (0.85–0.92)a | 0.95 (0.86–1.05) | 1.01 (0.97–1.05) | 0.76 (0.61–0.94)a |
| Prior comorbidities | ||||
| Prior PCI | 0.68 (0.65–0.72)a | 0.73 (0.65–0.82)a | 0.74 (0.71–0.78)a | 0.74 (0.58–0.94)a |
| Prior CABG | 0.80 (0.76–0.86)a | 0.84 (0.72–0.99)a | 0.82 (0.77–0.88)a | 0.69 (0.50–0.97)a |
| CHF 7 to 365 days | 1.45 (1.39–1.51)a | 1.75 (1.57–1.94)a | 1.37 (1.31–1.43)a | 1.51 (1.22–1.87)a |
| Diabetes | 1.05 (1.01–1.09)a | 1.43 (1.29–1.58)a | 1.22 (1.17–1.27)a | 1.37 (1.12–1.68)a |
| Prior MI | 1.05 (0.99–1.12) | 0.93 (0.80–1.09) | 1.01 (0.95–1.08) | 1.04 (0.76–1.42) |
| Peripheral vascular disease | 1.13 (1.08–1.19)a | 1.24 (1.11–1.39)a | 1.07 (1.02–1.12)a | 1.21 (0.97–1.52) |
| Prior stroke | 1.06 (0.99–1.13) | 0.86 (0.71–1.04) | 1.05 (0.97–1.12) | 0.80 (0.55–1.17) |
| Shock events | 0.79 (0.62–1.01) | 0.68 (0.43–1.08) | 0.68 (0.51–0.91)a | 0.67 (0.27–1.68) |
| Prior renal complications and function | ||||
| CKD | 1.12 (1.05–1.20)a | 0.90 (0.78–1.05) | 0.79 (0.73–0.85)a | 1.54 (1.21–1.97)a |
| eGFR <60, mL/min per m2 | 1.57 (1.49–1.66)a | 1.73 (1.50–2.01)a | 1.31 (1.23–1.39)a | 1.57 (1.10–2.25)a |
| eGFR <45, mL/min per m2 | 1.49 (1.38–1.60)a | 1.52 (1.27–1.81)a | 1.41 (1.30–1.53)a | 2.54 (1.72–3.76)a |
| eGFR <30, mL/min per m2 | 2.44 (2.18–2.73)a | 4.54 (3.79–5.44)a | 2.81 (2.49–3.16)a | 7.43 (5.54–9.96)a |
| Presentation | ||||
| Urgent | 1.53 (1.47–1.59)a | 1.83 (1.64–2.03)a | 1.45 (1.39–1.51)a | 1.29 (1.04–1.59)a |
| Emergent | 3.24 (3.01–3.48)a | 6.21 (5.33–7.23)a | 3.34 (3.09–3.61)a | 3.35 (2.40–4.67)a |
| Salvage | 5.54 (3.64–8.42)a | 13.82 (8.45–22.61)a | 4.43 (2.88–6.84)a | 2.76 (0.90–8.50)a |
| Hypertension | 0.89 (0.86–0.93)a | 0.78 (0.71–0.87)a | 0.95 (0.91–0.99)a | 0.84 (0.68–1.03) |
| Unstable angina | 0.49 (0.45–0.53)a | 0.45 (0.35–0.59)a | 0.53 (0.49–0.58)a | 0.65 (0.39–1.08) |
| Ejection fraction ≤40% | 1.12 (1.03–1.21)a | 0.91 (0.74–1.13) | 1.11 (1.02–1.21)a | 0.91 (0.60–1.37) |
| Prepresent MI | 1.05 (0.98–1.13) | 1.23 (1.03–1.47)a | 1.12 (1.04–1.20)a | 1.03 (0.72–1.47) |
| Shock | 4.87 (4.23–5.60)a | 5.34 (4.43–6.45)a | 5.00 (4.31–5.81)a | 4.09 (2.83–5.92)a |
| Anemia | 1.62 (1.55–1.68)a | 1.68 (1.50–1.88)a | 1.65 (1.58–1.72)a | 1.41 (1.10–1.81)a |
| Thrombolytic use | 0.71 (0.24–2.14) | 1.39 (0.18–10.98) | 0.75 (0.25–2.23) | 0.00 (0.00–0.00) |
AKI indicates acute kidney injury; AKIN, acute kidney injury network; CABG, coronary artery bypass grafting; CHF, congestive heart failure; CIN, contrast‐induced nephropathy; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; VA, Veterans Health Administration.
P<0.05.
Discussion
We developed a comprehensive and externally validated clinical prediction rule for AKI among cardiac catheterization and/or PCI procedures in the VA. This is the first such tool developed from national VA CART and EHR data, which explores novel outpatient and periprocedural risk factors for AKI. All of the models developed for different severities of AKI had good discrimination performance (0.741–0.885), and maintained adequate calibration across the full spectrum of risk in each model. Reduced VA models using routine NCDR variables and paired with the external validation cohort demonstrated the generalizability of our AKI and dialysis prediction tool to non‐VA catheterization laboratories.
There are a number of risk prediction models for AKI following PCI, but most were developed from data prior to 2005 with a variety of outcome definitions.34 The 2 most modern coronary angiography AKI models were developed by Tsai et al and Gurm et al. Both focused only on preprocedural risk estimation with a wider array of candidate variables than previously evaluated, including preprocedural inpatient medication exposures, clinical history and demographics, patient presentation characteristics, and laboratory assessments.11, 13
This study extends prior research by exploring additional risk factors available within a comprehensive EHR for use within AKI risk prediction models. Only 1 study developed a model using both coronary angiography and PCI procedures from 1218 patients at a single center and did not find a multivariate association between intervention status and AKI.35 No prior modeling study has incorporated inpatient bar‐coded records of intravenous fluid administration, which have been shown in numerous studies to be protective of postprocedural AKI. No prior study has included prevalent use of outpatient medications, such nonsteroidal anti‐inflammatory drugs, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, or diuretics, all of which have been shown to be associated with AKI.
In this study, risk factors at the time of presentation as well as renal function or prior renal events were key variables for predicting AKI and dialysis. Within these categories, the strongest risk factors included urgency of the procedure, shock, chronic kidney disease stage, and development of AKI prior to the procedure.
We previously developed a single‐center risk model to predict hospital‐acquired AKI for use in an EHR; this effort extends that model to coronary angiography within a national VA cohort for use in the VA EHR.36 Because we intend these models to be used in a clinical setting, the potential predictors are limited to those available in real‐time from the EHR and the CART clinical applications. However, because these models provide an automated way to predict AKI risk, they allow clinicians to account for a larger number of variables while reducing data entry time. Integration of these models into EHRs has not yet been met with wide acceptance and success for several reasons. First, they have not yet been consistently and completely adapted for clinical practice. Second, it is difficult to update the models regularly for maintenance of calibration. Third, we need to expand our capacity to automate data collection in real time, and last, we need better ways to visualize the clinical decision process.37 The CART program and the Office of Analytics and Business Intelligence have prioritized the implementation of a real‐time high risk AKI clinical reminder using these models to be delivered during preprocedural assessment.
Since the development of the Mehran CIN risk score,12 new investigations have led to the creation of new CIN and AKI risk models with variable AKI definitions and levels of discrimination.10, 11, 13, 14 A recent study by the NCDR Cath‐PCI registry including 954 729 PCI patients examined the association of common AKI risk factors known at presentation, in addition to contrast use and length of hospitalization.13 Unlike the NCDR investigators, who did not focus on the development of a risk model, the Blue Cross Blue Shield of Michigan Cardiovascular Consortium constructed a model for CIN (≥0.5 mg/dL increase in serum creatinine from baseline to hospital discharge) using 48 001 PCI patients and found an ROC of 0.85 in the study cohort and an ROC of 0.84 in the 20 572‐patient internal validation cohort.11 Similarly to the NCDR study, the Blue Cross Blue Shield of Michigan Cardiovascular Consortium model incorporated common AKI risk factors at presentation, including patient characteristics (age, weight, height, and heart failure), coronary disease, PCI indication, and priority.11, 13
Nevertheless, all of these modeling approaches have been limited only to PCI patients and have broadly excluded diagnostic cardiac catheterization patients. In addition, past studies have limited the definition of AKI or CIN to in‐hospital serum creatinine measurement alone, which falls short of meeting the current Kidney Disease Improving Global Outcomes guidelines for defining AKI.30 Our models advance the state of AKI clinical risk modeling in several ways. First, we include both cardiac catheterizations and PCI procedures. Second, we use a sample of consecutive patients in a large, national set of VA catheterization laboratories. Third, we investigate novel risk factors by leveraging a robust electronic medical record rich in clinical patient characteristics including both outpatient and perioperative factors not available in the NCDR or Blue Cross Blue Shield of Michigan Cardiovascular Consortium clinical registries. Fourth, we used LASSO to aid in variable selection in place of the conventional stepwise regression approach. Fifth, implementation of our model in electronic medical recordkeeping would eliminate the need for data entry at the time of use and reduces the need for model parsimony. Sixth, we used a cross‐validated model fit selection to eliminate overfitting. Seventh, we incorporated the new Kidney Disease Outcomes Quality Initiative AKI guidelines to include serum creatinine measures up to 7 days postprocedure from inpatient or outpatient blood draws.30
Limitations
We developed our prediction model based on a sample of VA users, in which females are under‐represented and low‐income and complex patients are over‐represented. Because some patients receive some of their care outside of the VA system, we might have been unable to identify all prior events. Despite searching multiple data sets in the VA, postprocedure serum creatinine was missing for a large proportion of the population. However, this issue is common across all cardiac catheterization populations for coronary angiography because patients are released the same day of the procedure without creatinine measurement follow‐up. Even though we need to account for this limitation, we elected to retain angiography patients in order to be able to assess risk on a larger patient population. Baseline serum creatinine was defined as the most recent serum creatinine measurement taken between 365 and 7 days prior to the procedure, a method previously validated by Siew and colleagues.31 Next, the observed odds were lower than the predicted odds among the lowest risk of AKI and therefore the models we derived may slightly overestimate risk for those whose risk is in the lower tercile of risk. The prediction model coefficients (and odds ratios) corresponding to collinear (correlated) groups of variables should be interpreted cautiously, as they correspond to unit changes in the variable keeping the correlated variables constant, which may be unrealistic. Last, because external validation of the VA models was performed using the NNECDSG registry, we could only use variables present in the NNECDSG registry to build our model. The only variables excluded were those created by the VA EHR from the year prior to the angiography procedure. Although the inclusion of these variables in an AKI risk model is one of the innovations of our study, the external validation of the VA reduced models proves that the standard variables in registries such as the NNECDSG, which harmonizes variables with the NCDR data collection, are generalizable largely to NCDR participating centers. Therefore, the external validation of the VA AKI risk models in our investigation is valid and generalizable to non‐VA cardiac catheterization laboratories in the United States.
We must also be mindful about the uncertain implications of AKI risk modeling for patients and providers. We propose our preprocedural predictive tool will be used by providers to assess patients at high risk of AKI and therefore implement necessary prophylactic strategies to minimize the risk of AKI.38 Second, providers will need to utilize this tool along with other tools to determine a safety threshold of contrast use during the procedure,8, 33 and therefore use judgment regarding duration of case, ad hoc PCI, and number of interventions. The unintended consequences of identifying high risk patients for AKI may be delayed or postponed procedures. Providers will need to balance the tradeoffs of readiness for cardiac catheterization for patients at high risk of AKI and potential delays in the procedure with the other clinical needs and timing of revascularization.
Conclusions
We developed a robust clinical prediction model for AKI following cardiac catheterization or PCI to be used in a prospective automated surveillance program in the VA. These models both explore novel variables that may be useful in future prediction modeling and risk adjustment, and we have confirmed external validity in a reduced model within the NNECDSG registry. This model has been operationally prioritized by the VA to be incorporated into routine clinical practice, with the intention to automatically identify patients at risk of AKI before and immediately following a cardiac catheterization or PCI procedure. It is our hope that automated surveillance of AKI will help clinicians reduce the incidence of AKI and incorporate protocols to prevent AKI urgently among those patients identified as high risk.
Sources of Funding
This project was supported in part by Veterans Health Administration Health Services Research and Development (HSR&D) CDA‐08‐020 (Matheny), HSR&D IIR‐11‐292 (Matheny, Tsai, Fly, Plomondon, Resnic, Brown, MacKenzie), and HS018443 (Brown) from the Agency for Healthcare Research and Quality. Dr Siew was supported by K23 DK088964‐01A1 and K24 DK62849 grants from the National Institute of Diabetes and Digestive and Kidney Diseases. This project was executed in collaboration with the national CART Program in collaboration with John Rumsfeld and the Predictive Analytics group of the VA Office of Analytics and Business Intelligence in collaboration with Christopher Nielson, Clifton Baker, and Stephan Fihn.
Disclosures
None.
Supporting information
Data S1. CART‐CL variable data definitions. Table S1. Comparison of Study Cohort with Missing Patient Characteristics
(J Am Heart Assoc. 2015;4:e002136 doi: 10.1161/JAHA.115.002136)
Accompanying Data S1 and Table S1 are available at http://jaha.ahajournals.org/content/4/12/e002136/suppl/DC1
References
- 1. McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, Tumlin J; Panel CINCW . Epidemiology and prognostic implications of contrast‐induced nephropathy. Am J Cardiol. 2006;98:5K–13K. [DOI] [PubMed] [Google Scholar]
- 2. Brown JR, McCullough PA, Splaine ME, Davies L, Ross CS, Dauerman HL, Robb JF, Boss R, Goldberg DJ, Fedele FA, Kellett MA, Phillips WJ, Ver Lee PN, Nelson EC, Mackenzie TA, O'Connor GT, Sarnak MJ, Malenka DJ. How do centres begin the process to prevent contrast‐induced acute kidney injury: a report from a new regional collaborative. BMJ Qual Saf. 2012;21:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown JR, Malenka DJ, DeVries JT, Robb JF, Jayne JE, Friedman BJ, Hettleman BD, Niles NW, Kaplan AV, Schoolwerth AC, Thompson CA. Transient and persistent renal dysfunction are predictors of survival after percutaneous coronary intervention: insights from the Dartmouth Dynamic Registry. Catheter Cardiovasc Interv. 2008;72:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Becker CR, Davidson C, Lameire N, McCullough PA, Stacul F, Tumlin J, Adam A. High‐risk situations and procedures. Am J Cardiol. 2006;98:37K–41K. [DOI] [PubMed] [Google Scholar]
- 5. James MT, Ghali WA, Knudtson ML, Ravani P, Tonelli M, Faris P, Pannu N, Manns BJ, Klarenbach SW, Hemmelgarn BR. Associations between acute kidney injury and cardiovascular and renal outcomes after coronary angiography. Circulation. 2011;123:409–416. [DOI] [PubMed] [Google Scholar]
- 6. Maioli M, Toso A, Leoncini M, Gallopin M, Musilli N, Bellandi F. Persistent renal damage after contrast‐induced acute kidney injury: incidence, evolution, risk factors, and prognosis. Circulation. 2012;125:3099–3107. [DOI] [PubMed] [Google Scholar]
- 7. Brown JR, Block CA, Malenka DJ, O'Connor GT, Schoolwerth AC, Thompson CA. Sodium bicarbonate plus N‐acetylcysteine prophylaxis: a meta‐analysis. JACC Cardiovasc Interv. 2009;2:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown JR, Robb JF, Block CA, Schoolwerth AC, Kaplan AV, O'Connor GT, Solomon RJ, Malenka DJ. Does safe dosing of iodinated contrast prevent contrast‐induced acute kidney injury? Circ Cardiovasc Interv. 2010;3:346–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minsinger KD, Kassis HM, Block CA, Sidhu M, Brown JR. Meta‐analysis of the effect of automated contrast injection devices versus manual injection and contrast volume on risk of contrast‐induced nephropathy. Am J Cardiol. 2014;113:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brown JR, DeVries JT, Piper WD, Robb JF, Hearne MJ, Ver Lee PM, Kellet MA, Watkins MW, Ryan TJ, Silver MT, Ross CS, MacKenzie TA, O'Connor GT, Malenka DJ; Northern New England Cardiovascular Disease Study G . Serious renal dysfunction after percutaneous coronary interventions can be predicted. Am Heart J. 2008;155:260–266. [DOI] [PubMed] [Google Scholar]
- 11. Gurm HS, Seth M, Kooiman J, Share D. A novel tool for reliable and accurate prediction of renal complications in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2013;61:2242–2248. [DOI] [PubMed] [Google Scholar]
- 12. Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast‐induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. [DOI] [PubMed] [Google Scholar]
- 13. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Messenger JC, Rumsfeld JS, Spertus JA. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath‐PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tziakas D, Chalikias G, Stakos D, Altun A, Sivri N, Yetkin E, Gur M, Stankovic G, Mehmedbegovic Z, Voudris V, Chatzikyriakou S, Garcia‐Moll X, Serra A, Passadakis P, Thodis E, Vargemezis V, Kaski JC, Konstantinides S. Validation of a new risk score to predict contrast‐induced nephropathy after percutaneous coronary intervention. Am J Cardiol. 2014;113:1487–1493. [DOI] [PubMed] [Google Scholar]
- 15. McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, Tumlin J. Risk prediction of contrast‐induced nephropathy. Am J Cardiol. 2006;98:27K–36K. [DOI] [PubMed] [Google Scholar]
- 16. Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O'Neill WW. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–1519. [DOI] [PubMed] [Google Scholar]
- 17. Freeman RV, O'Donnell M, Share D, Meengs WL, Kline‐Rogers E, Clark VL, DeFranco AC, Eagle KA, McGinnity JG, Patel K, Maxwell‐Eward A, Bondie D, Moscucci M; Blue Cross‐Blue Shield of Michigan Cardiovascular C . Nephropathy requiring dialysis after percutaneous coronary intervention and the critical role of an adjusted contrast dose. Am J Cardiol. 2002;90:1068–1073. [DOI] [PubMed] [Google Scholar]
- 18. Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 19. Lindsay J, Apple S, Pinnow EE, Gevorkian N, Gruberg L, Satler LF, Pichard AD, Kent KM, Suddath W, Waksman R. Percutaneous coronary intervention‐associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv. 2003;59:338–343. [DOI] [PubMed] [Google Scholar]
- 20. Gruberg L, Mehran R, Dangas G, Mintz GS, Waksman R, Kent KM, Pichard AD, Satler LF, Wu H, Leon MB. Acute renal failure requiring dialysis after percutaneous coronary interventions. Catheter Cardiovasc Interv. 2001;52:409–416. [DOI] [PubMed] [Google Scholar]
- 21. Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv. 2005;64:442–448. [DOI] [PubMed] [Google Scholar]
- 22. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, Garratt KN, Holmes DR Jr. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 23. Maddox TM, Plomondon ME, Petrich M, Tsai TT, Gethoffer H, Noonan G, Gillespie B, Box T, Fihn SD, Jesse RL, Rumsfeld JS. A national clinical quality program for Veterans Affairs catheterization laboratories (from the Veterans Affairs clinical assessment, reporting, and tracking program). Am J Cardiol. 2014;114:1750–1757. [DOI] [PubMed] [Google Scholar]
- 24. Byrd JB, Vigen R, Plomondon ME, Rumsfeld JS, Box TL, Fihn SD, Maddox TM. Data quality of an electronic health record tool to support VA cardiac catheterization laboratory quality improvement: the VA Clinical Assessment, Reporting, and Tracking System for Cath Labs (CART) program. Am Heart J. 2013;165:434–440. [DOI] [PubMed] [Google Scholar]
- 25. Box TL, McDonell M, Helfrich CD, Jesse RL, Fihn SD, Rumsfeld JS. Strategies from a nationwide health information technology implementation: the VA CART story. J Gen Intern Med. 2010;25(suppl 1):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 27. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 28. Grams ME, Plantinga LC, Hedgeman E, Saran R, Myers GL, Williams DE, Powe NR. Validation of CKD and related conditions in existing data sets: a systematic review. Am J Kidney Dis. 2011;57:44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh JA. Accuracy of Veterans Affairs databases for diagnoses of chronic diseases. Prev Chronic Dis. 2009;6:A126. [PMC free article] [PubMed] [Google Scholar]
- 30. Lameire N, Kellum JA. Contrast‐induced acute kidney injury and renal support for acute kidney injury: a KDIGO summary (Part 2). Crit Care. 2013;17:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, Dwyer JP, Srichai M, Hung AM, Smith JP, Peterson JF. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol. 2012;7:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tibshirani R. Regression shrinkage and selection via the lasso: a retrospective. J R Stat Soc Series B Stat Methodol. 2011;73:273–282. [Google Scholar]
- 33. Gurm HS, Dixon SR, Smith DE, Share D, Lalonde T, Greenbaum A, Moscucci M , Registry BMC. Renal function‐based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2011;58:907–914. [DOI] [PubMed] [Google Scholar]
- 34. Matheny ME, Siew ED, Resnic FS, Speroff T, Peterson JF, Brown JR. Risk factors for acute kidney injury following cardiac catheterization. US Nephrol. 2011;6:95–99. [Google Scholar]
- 35. Maioli M, Toso A, Gallopin M, Leoncini M, Tedeschi D, Micheletti C, Bellandi F. Preprocedural score for risk of contrast‐induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown). 2010;11:444–449. [DOI] [PubMed] [Google Scholar]
- 36. Matheny ME, Miller RA, Ikizler TA, Waitman LR, Denny JC, Schildcrout JS, Dittus RS, Peterson JF. Development of inpatient risk stratification models of acute kidney injury for use in electronic health records. Med Decis Making. 2010;30:639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amarasingham R, Patzer RE, Huesch M, Nguyen NQ, Xie B. Implementing electronic health care predictive analytics: considerations and challenges. Health Aff (Millwood). 2014;33:1148–1154. [DOI] [PubMed] [Google Scholar]
- 38. Brown JR, Solomon RJ, Sarnak MJ, McCullough PA, Splaine ME, Davies L, Ross CS, Dauerman HL, Stender JL, Conley SM, Robb JF, Chaisson K, Boss R, Lambert P, Goldberg DJ, Lucier D, Fedele FA, Kellett MA, Horton S, Phillips WJ, Downs C, Wiseman A, MacKenzie TA, Malenka DJ. Reducing Contrast‐Induced Acute Kidney Injury Using a Regional Multicenter Quality Improvement Intervention. Circulation Quality and Outcomes. 2014;7:693‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. CART‐CL variable data definitions. Table S1. Comparison of Study Cohort with Missing Patient Characteristics


