Abstract
Background
Despite concerns about mineralocorticoid receptor antagonist therapies (MRAs) underuse and misuse in patients with heart failure, temporal and institutional variations of MRA prescription have not been reported.
Methods and Results
We studied a national sample of veterans hospitalized for heart failure between 2003 and 2009 and left ventricular ejection fraction <40%. We identified ideal and non‐ideal candidates for MRA therapy based on American College of Cardiology/American Heart Association guidelines. We measured temporal trends and hospital variation of MRA prescriptions within 90 days after discharge. We determined the median odds ratio (MOR), a measure of the relative odds of an MRA prescription for 2 individuals with similar characteristics discharged at 2 randomly selected hospitals. From 37 126 patients (n=131 hospitals), 9355 were ideal‐MRA candidates, and 4056 were non‐ideal candidates. Among ideal candidates, 36% received an MRA, but there was a decline in use (41% in 2003 to 31% in 2009, P<0.001). Of non‐ideal candidates, 27% received an MRA with a decline in use (34% in 2003 to 22% in 2009, P<0.001). Hospital MRA prescription ranged from 0% to 71% for ideal candidates and 0% to 100% for non‐ideal candidates. The median odds ratios of MRA prescription for ideal and non‐ideal candidates were 1.44 and 1.36, respectively; a median odds ratio >1.2 indicates significant practice‐level variation.
Conclusions
There was decreasing MRA use between 2003 and 2009 with wide institutional variation in MRA prescription, which suggests opportunities for improvement to stimulate MRA use in ideal candidates while further reducing use in those with contraindications.
Keywords: appropriateness criteria, quality of care, spironolactone, variation
Subject Categories: Heart Failure
Introduction
In 2011, experts estimated that ≈68 000 lives could be saved per year in the United States with optimal implementation of evidence‐based therapies in patients with heart failure with left ventricular reduced ejection fraction (HFrEF).1 Of 6 therapies, implementation of mineralocorticoid receptor antagonist therapy (MRA) was estimated to result in nearly a third of this potential benefit of improved care. Furthermore, 3 randomized trials have found MRAs to be a highly efficacious therapy with a low number needed to treat to save 1 life (number needed to treat to save 1 life for 1 year ≈18).2, 3 Unfortunately, only one third of eligible HF patients actually receive an MRA prescription at hospital discharge4 or in the outpatient setting,5 perhaps because MRAs have the capacity to induce potentially life‐threatening consequences by causing hyperkalemia. Furthermore, risk factors for hyperkalemia (eg, chronic kidney disease, elevated serum potassium level, potassium supplementation) are common in patients with HF, thus creating a need to balance use in ideal candidates with avoidance of use in those who are at risk for adverse consequences of therapy. Alongside the evidence of underuse, other studies have documented high rates of use (up to 1 in 6 patients) in non‐ideal patients who are at high risk6 for hyperkalemia.
Despite knowledge of underuse of MRA therapy, temporal trends in MRA use and variation of MRA prescribing between institutions are not well described. The assessment of temporal trends is important to understand the effectiveness of past and current efforts in narrowing the treatment gap for evidence‐based MRA use2, 3, 7 and therefore inform future resource allocation. Understanding institutional‐level variation is important for the design of quality improvement interventions and identification of best practices. For example, homogeneous underuse would signify that a physician‐level educational campaign is required to improve optimal MRA use, while heterogeneity in MRA prescription will indicate the need to evaluate local barriers and facilitators and possibly modeling best practices to narrow the MRA treatment gap. The aim of this study was to characterize temporal trends and hospital variation in MRA prescription for ideal and non‐ideal candidates in a nationwide cohort of veterans with HFrEF after HF hospitalization.
Methods
Human Subjects Protection
This study was approved by the Providence VA Medical Center Institutional Review Board with a waiver of informed consent. Centers for Medicare and Medicaid Services data were provided with permission from Veterans Health Administration Medicare and Medicaid Analysis Center.
Data Sources
The VA External Peer Review Program is a nationwide inpatient and outpatient random sample of veterans with at least 2 years of continuous enrollment who are evaluated for evidence‐based performance measures.8, 9 VA External Peer Review Program data were linked with other VA data sets (Patient Treatment File, VA‐Medicare and Decision Support System) to obtain demographic, clinical, laboratory, healthcare utilization (VA and Medicare), and prescription data.
Study Cohort
We used the VA External Peer Review Program data from fiscal year (FY) 2003–2009 (calendar year 10/2002 to 6/2009) to identify a cohort of veterans with HFrEF, defined as left ventricular ejection fraction (LVEF) <40% (n=37 126) (Figure 1). The samples from FY2003 and FY2004 were smaller than those of later years due the structure of the VA performance measurement audit system; however, the sampling strategy was unchanged. Our choice of LVEF <40% was due to the availability of VA External Peer Review Program HF performance measure data (ie, internal quality improvement data), which identified a sample of patients with LV systolic dysfunction, as defined by LVEF <40%. Precise quantitative information on LVEF is not available in the Veterans Health Care System to date. We restricted the study sample to veterans who were hospitalized with a principal discharge diagnosis of HF (International Classification of Diseases, Ninth Revision, Clinical Modification codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.93, or 428.x) within the study period and excluded patients who died before hospital discharge. We also excluded patients with no prior history of HF (because MRAs are not first‐line therapy in new diagnosis of HFrEF), length of stay <1 day duration or >365 days, transferred to another facility, left against medical advice, or missing serum potassium or creatinine values during index hospitalization. We also excluded patients not discharged with at least 1 of the following medications—angiotensin‐converting enzyme (ACE) inhibitor, angiotensin receptor blocker (ARB), or β‐blockers—as these are recommended first‐line agents before prescription of an MRA, resulting in a study cohort of 13 411.
Figure 1.

Proportion of MRA use in ideal candidates=patients with MRA prescription fill (within 90 days)/patients in “Ideal Cohort.” Proportion of MRA use in non‐ideal candidates=patients with MRA prescription fill (within 90 days)/patients in “Non‐Ideal Cohort.” ACE indicates angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; BB, β‐blocker; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist.
Ideal and Non‐Ideal Cohorts
American College of Cardiology/American Heart Association guidelines recommend careful patient selection for MRA therapy, specifically noting that HFrEF patients should only be considered if they have serum creatinine ≤2.5 (men) or estimated glomerular filtration rate >30 mL/min per 1.73 m2, and serum potassium <5.0 mEq/L. Of the study cohort of 13 411, 4096 patients had a contraindication to MRA therapy or were guideline ineligible; this was the “Non‐Ideal Cohort.” These factors included a history of hyperkalemia (ICD‐9 276.7 or serum potassium ≥6.0), pre‐discharge (within 2 days) serum potassium ≥5.0 or serum creatinine >2.5 in men and >2.0 in women, gynecomastia (ICD9 611.1) or mastodynia (ICD9 611.71), dialysis treatment (585.6 or V45.11), or concomitant prescription of both ACE inhibitor and ARB within 90 days of discharge. The remaining 9355 patients constituted the “Ideal Cohort” who met all criteria for MRA therapy.
Outcomes Measures and Definitions
The primary outcome measure was prescription (≥14 pills) of an MRA within 90 days of hospital discharge in Ideal and Non‐Ideal candidates. Because VA hospitals typically fill new patient prescriptions prior to hospital discharge, we included any inpatient prescriptions (≥14 pills) within 48 hours preceding discharge. A 14‐pill count was chosen because it represented a minimum 2‐week supply. All discharge medications were determined in the same manner. The 90‐day period was chosen because this period reflects the treatment strategy after HF hospitalization. Furthermore, a prior study found that some MRA‐eligible patients who were not prescribed an MRA at discharge subsequently filled a prescription within 90 days.10
Covariates
The following covariates were included as potential correlates of prescription of MRA: demographic characteristics (age, sex, race), vital signs (pulse, systolic blood pressure), medical history (all 29 comorbidities as defined by Elixhauser et al,11 pacemaker [v45.01], defibrillator [v45.02], noncompliance [ICD‐9 v15.81]), results of laboratory testing at discharge (hemoglobin, potassium, sodium, creatinine, blood urea nitrogen, and digoxin level), and medication prescription within 90 days of discharge (potassium supplement, loop‐ and thiazide‐type diuretics, β‐blocker, ACE inhibitor or ARB, lipid‐lowering agent, warfarin, and digoxin).
Statistical Analysis
Patient‐level analyses
We calculated the proportions of patients who received an MRA prescription within 90 days of discharge in the Ideal and Contraindication cohorts. We then applied the Cochran‐Armitage trend test to assess temporal trends across the study period (FY2003–2009). Next, we assessed the correlates of MRA use in the Ideal cohort by comparing patient demographics, vital signs, medications, lab values, and existing comorbidities among veterans who received a MRA prescription within 90 days of discharge compared to those who did not, using χ2 tests for categorical covariates and 1‐way ANOVAs for continuous variables. We included noncompliance (ICD‐9 v15.81) with medical treatment as a covariate for exploratory purposes. This was not intended to be a surrogate for patients who would not be expected to comply with MRA prescription, since a provider's assessment of patient's capacity for medication adherence cannot be assessed by administrative code alone. We utilized logistic regression with MRA prescription at discharge as the dependent variable and a backward selection procedure was used for covariate selection with a criterion of P<0.10 for model entry and termination. To determine the effects of practice variation in MRA prescription (in Ideal and Non‐Ideal cohorts), we used generalized estimating equations to calculate the median odds ratio12 (MOR). Models were adjusted for patient‐level clinical and demographic characteristics as well as fiscal year to account for time trends and VA facility to account for clustering by the institution. A MOR of 1.0 suggests no meaningful variation in the odds of 2 individuals with similar characteristics receiving a MRA prescription at different, randomly selected hospitals, whereas an increase of MOR >1.0 (eg, MOR of 2.0) indicates that the odds of receiving a MRA prescription would be 2‐fold higher for 2 patients with identical characteristics discharged from randomly selected hospitals. Based on previous literature, a MOR >1.2 indicates significant practice‐level variation.13
Hospital‐level analyses
To describe institutional‐level variation in MRA prescription among Ideal and Non‐Ideal patients, we assessed the distributions and interquartile ranges of MRA prescription by institution. In this analysis, we excluded hospitals with <2 eligible patients per fiscal year. We then conducted a sensitivity analysis in which we excluded hospitals with <20 candidates for MRA therapy from FY2003 to 2009 to determine whether volume of eligible patients may contribute to institutional‐level variation in MRA prescription practices. We then used Spearman correlation to estimate the correlation in hospital‐level MRA prescription between Ideal and Non‐Ideal candidates.
All analyses were performed using the SAS statistical package version 9.3 (Cary, NC) and approved by the Providence VAMC Institutional Review Board. Tests were 2‐tailed and P<0.05 was considered statistically significant.
Results
Baseline Characteristics
The characteristics of the overall, Ideal, and Non‐Ideal cohorts are presented in Table 1. For the overall cohort, only 2% had filled a MRA prescription prior to hospitalization, but >90% of patients filled prescriptions for a β‐blocker or ACE inhibitor (or ARB) at discharge. Mean serum potassium at discharge was 4.1 mEq/L (SD=0.5), and serum creatinine was 1.6 mg/dL (SD=1.2). Patients had a mean of 4.8 (SD=2.6) comorbidities (defined by Elixhauser11) in the prior 2 years. One in 6 patients had a history of noncompliance (ICD‐9 v15.81) with medical treatment.
Table 1.
Baseline Characteristics of Cohort
| Overall | Ideal MRA Candidates | Non‐Ideal MRA Candidates | |
|---|---|---|---|
| N=13 411 | n=9355 | n=4056 | |
| Demographics | |||
| Year | |||
| 2003, n (%) | 185 (1) | 111 (1) | 74 (2) |
| 2004, n (%) | 130 (1) | 92 (1) | 38 (1) |
| 2005, n (%) | 2334 (17) | 1552 (17) | 782 (19) |
| 2006, n (%) | 3488 (26) | 2434 (26) | 1054 (26) |
| 2007, n (%) | 3923 (29) | 2696 (29) | 1227 (30) |
| 2008, n (%) | 1307 (10) | 985 (11) | 322 (8) |
| 2009, n (%) | 2044 (15) | 1485 (16) | 559 (14) |
| Age at index date, mean (SD) | 71 (11) | 71 (11) | 71 (11) |
| Female, n (%) | 131 (1) | 88 (1) | 43 (1) |
| Race | |||
| White | 8783 (69%) | 6154 (70%) | 2629 (68%) |
| African American | 3373 (27%) | 2313 (26%) | 1060 (27%) |
| Other | 504 (4%) | 323 (4%) | 181 (5%) |
| Medications prior to index admission (<90 days) | |||
| MRA (spironolactone, eplerenone) | 306 (2%) | 178 (2%) | 128 (3%) |
| Medications at discharge/within 90 days | |||
| Loop diuretics, % | 12 739 (95%) | 9010 (96%) | 3729 (92%) |
| Thiazide diuretics, % | 2325 (17%) | 1453 (16%) | 872 (22%) |
| β‐Blocker (all), % | 12 386 (92%) | 8740 (93%) | 3646 (90%) |
| ACEI or ARB, % | 12 025 (90%) | 8811 (94%) | 3214 (79%) |
| Warfarin | 4380 (33%) | 3135 (34%) | 1245 (31%) |
| Digoxin | 5611 (42%) | 3984 (43%) | 1626 (40%) |
| Admission vitals | |||
| Systolic BP, mm Hg, mean (SD) | 130 (26) | 130 (25) | 129 (27) |
| Pulse, beats/min, mean (SD) | 83 (19) | 84 (19) | 82 (19) |
| BMI (kg/m2), mean (SD) | 29 (7) | 29 (7) | 29 (7) |
| Discharge lab values | |||
| Sodium, mEq/L, mean (SD) | 138 (5) | 138 (4) | 137 (6.58) |
| Potassium, mEq/L, mean (SD) | 4.1 (0.5) | 4.1 (0.4) | 4.3 (0.6) |
| Serum creatinine, mg/dL, mean (SD) | 1.6 (1.2) | 1.4 (0.4) | 2.3 (1.9) |
| Blood urea nitrogen, mg/dL, mean (SD) | 32 (20) | 28 (14) | 44 (27) |
| Hemoglobin, g/dL, mean (SD) | 12.3 (1.9) | 12.6 (1.9) | 11.7 (1.9) |
| Comorbid conditions | |||
| Total comorbidities (Elixhauser11), mean (SD) | 4.8 (2.6) | 4.3 (2.4) | 6.0 (2.6) |
| Peripheral vascular disease | 2531 (19%) | 1554 (17%) | 977 (24%) |
| Hypertension, n (%) | 11 510 (86) | 7878 (84) | 3632 (90) |
| Chronic pulmonary disease | 6216 (46%) | 4176 (45%) | 2040 (50%) |
| Diabetes (uncomplicated) | 6792 (51%) | 4452 (48%) | 2340 (58%) |
| Diabetes (complicated) | 2544 (19%) | 1328 (14%) | 1216 (30%) |
| Hypothyroidism | 1576 (12%) | 1032 (11%) | 544 (13%) |
| Liver disease | 858 (6%) | 489 (5%) | 369 (9%) |
| Metastatic cancer | 147 (1%) | 98 (1%) | 49 (1%) |
| Fluid and electrolyte disorders | 5043 (38%) | 2621 (28%) | 136 (3%) |
| Deficiency anemias | 4775 (36%) | 2782 (30%) | 1993 (49%) |
| Drug abuse | 940 (7%) | 644 (7%) | 296 (7%) |
| Depression | 1853 (14%) | 1230 (13%) | 623 (15%) |
| Additional comorbid conditions | |||
| Cardiac arrhythmias (from Quan14) | 8551 (64%) | 5877 (63%) | 2674 (66%) |
| Ischemic heart disease | 10 501 (78%) | 7153 (76%) | 3348 (83%) |
| Pulmonary circulation disorders | 1979 (15%) | 1267 (14%) | 712 (18%) |
| Valvular disease | 3828 (29%) | 2564 (27%) | 1264 (31%) |
| Noncompliancea | 2314 (17%) | 1562 (17%) | 752 (19%) |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; BMI, body mass index; BP, blood pressure; MRA, mineralocorticoid receptor antagonist.
History of past noncompliance (ICD‐9, International Statistical Classification of Diseases, 9th Revision, v15.81).
Proportion and Trends in MRA Use in Ideal Candidates
Thirty‐six percent (n=3408) of Ideal candidates (n=9355) were prescribed a MRA within 90 days of discharge (Table 2), of whom virtually all (99%) prescriptions were spironolactone. From 2003 to 2009, there was a significant decrease in the proportion of MRA prescriptions within 90 days of discharge from 41% (46/111) in 2003 to 31% in 2009 (465/1485) (P<0.001 for trend) (Figure 2). This trend persisted when restricting analysis to patients (n=8196) who received both a β‐blocker and ACE inhibitor (or ARB) within 90 days of discharge (from 45% to 31%, P<0.0001).
Table 2.
Correlates of MRA Prescription in Ideal Candidates Within 90 Days of Hospital Discharge
| Received MRA (n=3408) | Did Not Receive MRA (n=5947) | Unadjusted P Value | |
|---|---|---|---|
| Demographics | |||
| Year | Overall trend P<0.001 | ||
| 2003, (%) | 41% | 59% | |
| 2004, (%) | 39% | 61% | |
| 2005, (%) | 41% | 60% | |
| 2006, (%) | 36% | 64% | |
| 2007, (%) | 38% | 62% | |
| 2008, (%) | 34% | 67% | |
| 2009, (%) | 31% | 69% | |
| Age at index date, mean (SD) | 67 (12) | 71 (12) | <0.001 |
| Female, (%) | 1.1% | 0.9% | 0.38 |
| Race | <0.001 | ||
| White | 68% | 71% | |
| African American | 29% | 25% | |
| Other | 4% | 4% | |
| Medications at discharge/within 90 days | |||
| Loop diuretics, % | 97% | 96% | <0.001 |
| Thiazide diuretics, % | 19% | 14% | <0.001 |
| β‐Blocker (all), % | 95% | 93% | <0.001 |
| ACEI or ARB, % | 95% | 94% | <0.001 |
| Warfarin | 36% | 32% | <0.001 |
| Digoxin | 52% | 37% | <0.001 |
| Admission vitals | |||
| Systolic BP, mm Hg | 128 (25) | 132 (25) | <0.001 |
| Pulse, beats/min | 84 (20) | 83 (19) | 0.009 |
| BMI (kg/m2) | 30 (7) | 29 (7) | <0.001 |
| Discharge lab values | |||
| Sodium, mEq/L, mean (SD) | 137 (4) | 138 (4) | <0.001 |
| Potassium, mEq/L, mean (SD) | 4.1 (0.4) | 4.0 (0.4) | 0.23 |
| Serum creatinine, mg/dL, mean (SD) | 1.3 (0.4) | 1.4 (0.4) | <0.001 |
| Blood urea nitrogen, mg/dL, mean (SD) | 27 (14) | 28 (14) | 0.001 |
| Hemoglobin, g/dL, mean (SD) | 12.7 (1.8) | 12.5 (1.9) | <0.001 |
| Comorbid conditions | |||
| Total comorbidities (Elixhauser11), mean (SD) | 4.4 (2.4) | 4.3 (2.3) | 0.31 |
| Peripheral vascular disease | 15% | 17% | 0.02 |
| Hypertension, n (%) | 85% | 84% | 0.41 |
| Chronic pulmonary disease | 44% | 45% | 0.27 |
| Diabetes (uncomplicated) | 51% | 46% | <0.001 |
| Diabetes (complicated) | 15% | 14% | 0.047 |
| Hypothyroidism | 11% | 11% | 0.63 |
| Liver disease | 7% | 4% | <0.001 |
| Fluid and electrolyte disorders | 30% | 27% | 0.005 |
| Deficiency anemias | 29% | 30% | 0.47 |
| Depression | 15% | 12% | <0.001 |
| Additional comorbid conditions | |||
| Cardiac arrhythmias (from Quan14) | 63% | 63% | 0.42 |
| Ischemic heart disease | 76% | 77% | 0.97 |
| Pulmonary circulation disorders | 15% | 13% | 0.02 |
| Valvular disease | 27% | 28% | 0.41 |
| Noncompliancea | 18% | 16% | 0.03 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; BMI, body mass index; BP, blood pressure; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonist.
History of past noncompliance (ICD‐9, International Statistical Classification of Diseases, 9th Revision, v15.81).
Figure 2.

Between FY2003 to 2009, there was a significant decrease in proportion of MRA prescriptions within 90 days from 41% (46/111) to 31% (465/1485) (P<0.001 for trend). This trend persisted when restricting analysis to patients (n=8196) who received both a β‐blocker and ACE inhibitor (or ARB) within 90 days of discharge (from 45% to 31%, P<0.0001). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; MRA, mineralocorticoid receptor antagonist.
Proportion and Trends in MRA Use in Non‐Ideal Candidates
Twenty‐seven percent (n=1096) of Non‐Ideal patients (n=4056) received a MRA prescription. From 2003 to 2009, there was a significant decrease in MRA prescriptions from 34% (25/74) to 22% (123/559) (P<0.001 for trend), respectively (Figure 3). This temporal trend was consistent for all 3 subcategories of contraindicated MRA prescription: documented MRA contraindication (P=0.0013), serum creatinine >2.5 mg/dL in men or >2.0 mg/dL in women (P=0.0004), and serum potassium >5.0 mEq/L (P=0.03).
Figure 3.

From FY2003 to 2009, there was a significant decrease in mineralocorticoid receptor antagonist (MRA) prescriptions from 34% (25/81) to 22% (158/720) (P<0.001 for trend), respectively. The number (n) of Non‐Ideal candidates is indicated below each fiscal year. This temporal trend was consistent for all 3 subcategories of contraindicated MRA prescription: documented MRA contraindication (P=0.0013), serum creatinine >2.5 mg/dL (or >2.0 mg/dL in women) (P=0.0004), and serum potassium >5.0 mEq/L (P=0.03). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker.
Hospital‐Level Variation
Among hospitals with at least 2 eligible candidates per fiscal year, the hospital‐level MRA prescribing rate (n=125 hospitals) for Ideal candidates ranged from 0% to 71% (median 36% [interquartile range 28, 45]) (Figure 4A). The corresponding rate for Non‐Ideal candidates (n=120 hospitals) ranged from 0% to 100% (median 26% [interquartile range 18, 34]) (Figure 4B). Sensitivity analysis restricting to hospitals with at least 20 Ideal and Non‐Ideal candidates for MRA therapy, respectively, during the study period demonstrated very similar results (Figure 5A and 5B). The hospital‐level MRA prescribing rate for Ideal Candidates (n=104 hospitals) ranged from 11% to 65% (median 37% [interquartile range 29, 44]) and for Non‐Ideal candidates (n=72 hospitals) ranged from 9% to 58% (median 24% [interquartile range 19, 31]). There was a positive correlation between MRA use in Ideal and Non‐Ideal candidates, with higher rates of use in Non‐Ideal candidates at institutions with higher rates of use in Ideal candidates (Figure 6A); the Spearman correlation coefficient was r=0.41 (P<0.0001). Restricting to institutions with at least 20 Ideal and Non‐Ideal candidates for MRA therapy during the study period, respectively, the correlation was similar (r=0.39, P<0.001, Figure 6B).
Figure 4.

A, Of VA hospitals with at least 2 Ideal candidates for MRA therapy (n=125 hospitals) in a given fiscal year, the hospital‐level MRA prescription rate is shown on the Y axis. The black bar indicates the median rate of 36% (interquartile range 28, 45). B, Of VA hospitals with at least 2 Non‐Ideal candidates for MRA therapy (n=120 hospitals) in a given fiscal year, the hospital‐level MRA prescription rate is shown on the Y axis. The black bar indicates the median rate of 26% (interquartile range 18, 34). MRA indicates mineralocorticoid receptor antagonist; VA, Veterans Affairs.
Figure 5.

A, Of VA hospitals with at least 20 Ideal candidates for MRA therapy (n=104 hospitals) during the study period, the hospital‐level MRA prescription rate is shown on the Y axis. The black bar indicates the median rate of 37% (interquartile range 29, 44). B, Of VA hospitals with at least 20 Non‐Ideal candidates for MRA therapy (n=72 hospitals) during the study period, the hospital‐level MRA prescription rate is shown on the Y axis. The black bar indicates the median rate of 24% (interquartile range 19, 31). MRA indicates mineralocorticoid receptor antagonist; VA, Veterans Affairs.
Figure 6.
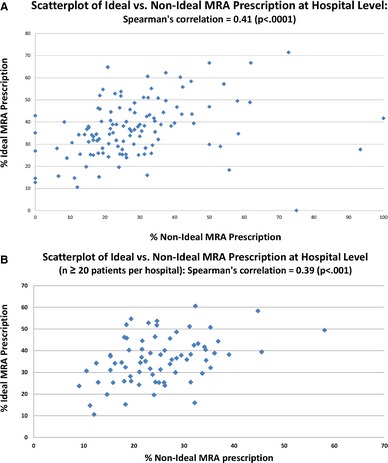
A, This scatterplot demonstrates a positive correlation between mineralocorticoid receptor antagonist (MRA) prescriptions in Ideal and Non‐Ideal candidates at the hospital level. B, This scatterplot demonstrates a positive correlation between MRA prescriptions in Ideal and Non‐Ideal candidates at the hospital level. Only hospitals with ≥20 Ideal and Non‐Ideal candidates, respectively, during the study period were included.
Effect of Hospital Variation on the Individual Patient: MOR of MRA Prescription in Ideal and Non‐Ideal Cohorts
Univariate predictors of MRA prescription are listed in Table 2. Compared with patients who did not receive a MRA prescription, MRA‐treated patients had slightly better renal function (serum creatinine and blood urea nitrogen), but there was no difference in serum potassium between the 2 groups. Multivariable predictors of MRA prescription (P<0.05) are listed in Table 3. Of these predictors, prescription of digoxin, lower age, lower systolic blood pressure, and presence of liver disease accounted for the greatest percentage of total variability in MRA use. Importantly, a coded diagnosis of noncompliance was not predictive of MRA prescription. After adjustment for patient predictors and clustering within hospitals, the MORs of MRA prescription for Ideal and Non‐Ideal candidates were 1.44 and 1.36, respectively, suggesting significant practice variation. In other words, for 2 individuals discharged from 2 randomly selected hospitals, the odds of 1 of them being prescribed with an MRA would be 44% higher (for Ideal candidates) and 36% higher (for Non‐Ideal candidates) than for the other individual, despite similar characteristics and indications/contraindications for therapy.
Table 3.
Patient‐Level Predictors of MRA Prescription in Ideal Candidatesa
| Comparison | Odds Ratio (95% CI) | F Value | P Value |
|---|---|---|---|
| Race | 7.67 | <0.001 | |
| Black vs white | 1.28 (1.13, 1.44) | ||
| Hospitalization year | 3.80 | <0.001 | |
| 2003 vs 2009 | 1.22 (0.79, 1.88) | ||
| 2004 vs 2009 | 1.30 (0.81, 2.09) | ||
| 2005 vs 2009 | 1.43 (1.21, 1.69) | ||
| 2006 vs 2009 | 1.12 (0.96, 1.31) | ||
| 2007 vs 2009 | 1.28 (1.10, 1.49) | ||
| 2008 vs 2009 | 1.12 (0.93, 1.36) | ||
| Age (per 1 year from mean) | 0.98 (0.98, 0.99) | 60.42 | <0.001 |
| Total comorbidities (per comorbidity)11 | 0.93 (0.90, 0.96) | 19.10 | <0.001 |
| Serum sodium, per mEq/L | 0.97 (0.96, 0.99) | 18.28 | <0.001 |
| Serum creatinine, per mg/dL | 0.81 (0.71, 0.92) | 10.93 | 0.001 |
| Body mass index, per kg/m2 | 1.01 (1.01, 1.02) | 11.42 | <0.001 |
| Systolic BP, admission (per mm Hg) | 0.993 (0.991, 0.995) | 43.84 | <0.001 |
| Hypertension (complicated) | 1.22 (1.06, 1.41) | 7.57 | 0.006 |
| Diabetes (uncomplicated) | 1.28 (1.15, 1.42) | 19.80 | <0.001 |
| Diabetes (complicated) | 1.17 (1.01, 1.36) | 4.51 | 0.03 |
| Liver disease | 1.86 (1.49, 2.31) | 30.40 | <0.001 |
| Metastatic cancer | 0.29 (0.15, 0.54) | 15.02 | <0.001 |
| Fluid and electrolyte disorders | 1.26 (1.12, 1.43) | 14.09 | <0.001 |
| Deficiency anemias | 1.18 (1.05, 1.33) | 7.60 | 0.006 |
| Drug abuse | 0.73 (0.59, 0.91) | 7.60 | 0.006 |
| Depression | 1.31 (1.13, 1.52) | 12.92 | <0.001 |
| Loop diuretic | 1.69 (1.29, 2.21) | 14.47 | <0.001 |
| Thiazide diuretic | 1.24 (1.09, 1.41) | 10.36 | 0.001 |
| Digoxin | 1.65 (1.50, 1.82) | 99.36 | <0.001 |
BP indicates blood pressure; MRA, mineralocorticoid receptor antagonist.
Adjusted for clustering within hospital.
Discussion
In a national cohort of veterans discharged for HF between 2003 and 2009, MRA prescription decreased over time. MRA prescription fell not only in Non‐Ideal candidates, but unexpectedly fell in Ideal candidates. The MORs of MRA prescription for both Ideal and Non‐Ideal candidates indicate significant institutional‐level variation. These findings suggest that many patients who could benefit from MRAs are still not receiving them and that there is a wide gap between high‐ and low‐performing institutions.
This is to our knowledge the first study to report hospital‐level variation in MRA prescribing. The MOR for both Ideal and Non‐Ideal candidates were above the threshold required to indicate significant practice‐level variation, defined as MOR >1.2.13 Indeed, the degree of variation in MRA prescription was comparable with practice‐level variation in warfarin prescribing (MOR 1.3) in patients with atrial fibrillation.13 However, the practice variation was lower when compared to variation of other performance measures such as primary percutaneous intervention within 90 minutes (MOR 2.2), smoking cessation instructions (MOR 2.7), and cardiac rehabilitation referral (MOR 4.8).15 This hospital‐level heterogeneity in MRA prescribing suggests there are significant opportunities to improve MRA utilization by assessing the local barriers and intervening on hospitals with lower MRA use in ideal candidates while learning from hospitals that have higher MRA use.
Another important finding is the declining MRA prescription in Ideal candidates during the study period. These findings in a broad national sample of hospitalized veterans who met criteria for MRA therapy confirm the findings of other registry4, 10and single‐center16 studies, which found that only approximately one quarter to one third of eligible HF patients receive a MRA. From the Get With The Guidelines‐Heart Failure (GWTG‐HF) national registry (2005–2007) of hospitalized patients with HF, Albert et al reported that 32.5% of the eligible cohort received a MRA prescription at discharge.4 However, in that study the proportion of patients who received a MRA prescription increased from 28% to 34% over the 3‐year study period, in contrast to the declining trend of 41% to 31% found in our study from 2003 to 2009. This declining trend persisted in a sensitivity analysis that included only those patients treated with both β‐blockers and ACE inhibitors, an approach advocated in the European Society of Cardiology Heart Failure guideline17 when selecting patients for MRA therapy. The reasons for these contrasting temporal trends between the 2 studies could be due to different duration of comparison (7 versus 3 years) or a greater MRA utilization at VA hospitals after the Randomized Aldactone Evaluation Study trial,3 which regressed to the national baseline over time. Another possibility is that non‐VA, Get With The Guidelines hospitals benefited from participating in a quality‐improvement registry that provides hospitals with performance data on their MRA utilization rates. In contrast, while VA hospitals did establish a HF Network (community of practitioners) and collect basic HF performance data (ACE/ARB for LVEF <40%, measurement of LVEF, smoking cessation, discharge instructions), they did not participate in the Get With The Guidelines registry and did not have specific quality‐improvement efforts targeting MRA utilization. Nonetheless, the widening MRA treatment gap in the VA over time suggests opportunities for improvement and merits additional investigation.
The proportion of Non‐Ideal candidates (27%) who were prescribed a MRA was much higher than that found in the Get With The Guidelines study of Albert et al4 (≈10%) in a 2005–2007 cohort or an earlier study in a post‐Randomized Aldactone Evaluation Study cohort6 (2000–2001), which found a proportion of 17%. Our observed proportion may have been higher because we utilized clinical and administrative data with a 2‐year “look‐back” regarding history of contraindications and included historical laboratory values of prior hyperkalemia (potassium ≥6.0 mEq/L); this approach was likely very sensitive to detect potential MRA prescribing in Non‐Ideal candidates. While there are differences in how studies defined inappropriate prescribing, our finding has 2 implications. First, though MRA prescribing in Non‐Ideal candidates substantially declined during the study period, it may still be a larger quality problem than has previously been acknowledged4 despite a 2004 publication on excess death and hyperkalemia hospitalizations associated with rising spironolactone use.18 Second, the positive correlation between hospital‐level use in Ideal and Non‐Ideal candidates supports concerns that efforts to increase appropriate use of MRAs could have the unintended consequence of increasing use in patients for whom therapy might be detrimental. Attempts to stimulate greater use of MRA should be accompanied by surveillance for potential overuse.
There were several methodological considerations in our study. First, we measured MRA exposure up to 90 days after hospitalization to capture a complete picture of HF pharmacotherapy in the transition from hospital to home. This is important because renal dysfunction typically worsens during and immediately after HF hospitalization19 and because 13% of Medicare patients with HF who are discharged without a MRA subsequently filled a new MRA prescription within 90 days.10 Therefore, we utilized serum potassium and creatinine values at time of hospital discharge to determine MRA eligibility rather than at admission,4 which mirrors the process of clinical decision making. Second, we carefully excluded patients who were not ideal for MRA therapy based on an elevated serum potassium level (≥5.0 mEq/L) prior to hospital discharge as well as those with a history of serious hyperkalemia, whether diagnosed by ICD‐9 code or evidence of an elevated potassium ≥6.0 mEq/L, as far back as 2 years. Incorporation of laboratory data is important because ICD‐9‐defined hyperkalemia underestimates the true incidence of laboratory‐defined serious hyperkalemia (serum potassium ≥6.0).20 Third, the study time period pre‐dated the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure trial (2011), which expanded the indication for MRAs (ie, eplerenone) in select patients with mild HF symptoms.7 Thus, we might not have captured the potential increase in MRA use after the publication. However, the 7‐year time window of our study provides an ample window to assess the impact on clinical practice before and after a 2004 publication on MRA‐related hyperkalemia. Fourth, our use of clinical and laboratory variables from electronic medical records in addition to standard administrative data may be more sensitive to identify patient‐specific contextual factors21 that would preclude use of a MRA than administrative data alone. Fifth, the VA provides a unique lens in which to examine quality of HF care. The VA is the largest integrated health system in the United States, with an electronic medical record system that allows for detailed clinical assessment of MRA indication as well as tracking of prescriptions for patients despite geographic mobility.22 Features common to VA hospitals such as a national formulary, electronic medical record, and academic affiliations may favor a homogeneous pattern of MRA utilization. Furthermore, veterans with HF are typically older, more symptomatic, and have more comorbidities than non‐VA patients yet have similar outcomes.23 Sixth, we utilized a LVEF cutoff of <40%, rather than ≤35%, due to limitations of the VA clinical data sets, though some studies on this topic have also utilized a cutoff of LVEF <40%.6
There was a temporal decline in MRA prescription among Ideal candidates from 2003 to 2009 as well as important hospital‐level variation in MRA use for Ideal and Non‐Ideal candidates in the VA system, suggesting the importance of system factors in MRA prescribing in addition to patient factors. System efforts identifying best practices while assessing local barriers to MRA prescription will be needed to target low use of MRA in eligible patients and to reduce use in patients who could potentially be harmed.
Sources of Funding
This study was supported by funding from the Veterans Administration Health Services Research and Development Quality Enhancement Research Initiative (RRP 12‐456, PI: Dev; RRP 09‐172, PI: Wu) and by the resources of the Phoenix and Providence VA Medical Centers. The views expressed do not represent the views of the Department of Veterans Affairs or the United States Government.
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002268 doi: 10.1161/JAHA.115.002268)
References
- 1. Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence‐based heart failure therapies on mortality. Am Heart J. 2011;161:1024–1030. [DOI] [PubMed] [Google Scholar]
- 2. Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. [DOI] [PubMed] [Google Scholar]
- 3. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 4. Albert NM, Yancy CW, Liang L, Zhao X, Hernandez AF, Peterson ED, Cannon CP, Fonarow GC. Use of aldosterone antagonists in heart failure. JAMA. 2009;302:1658–1665. [DOI] [PubMed] [Google Scholar]
- 5. Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O'Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence‐based care for heart failure in outpatient cardiology practices: primary results of the registry to improve the use of evidence‐based heart failure therapies in the outpatient setting (IMPROVE HF). Circulation. 2010;122:585–596. [DOI] [PubMed] [Google Scholar]
- 6. Masoudi FA, Gross CP, Wang Y, Rathore SS, Havranek EP, Foody JM, Krumholz HM. Adoption of spironolactone therapy for older patients with heart failure and left ventricular systolic dysfunction in the United States, 1998–2001. Circulation. 2005;112:39–47. [DOI] [PubMed] [Google Scholar]
- 7. Zannad F, McMurray JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. [DOI] [PubMed] [Google Scholar]
- 8. Ather S, Chan W, Chillar A, Aguilar D, Pritchett AM, Ramasubbu K, Wehrens XHT, Deswal A, Bozkurt B. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J. 2011;161:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu WC, Jiang L, Friedmann PD, Trivedi A. Association between process quality measures for heart failure and mortality among US veterans. Am Heart J. 2014;168:713–720.e713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Curtis LH, Mi X, Qualls LG, Check DK, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Setoguchi S, Hernandez AF, Fonarow GC. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986. [DOI] [PubMed] [Google Scholar]
- 11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 12. Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Rastam L, Larsen K. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice‐level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol. 2011;108:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 15. Vigen R, Spertus JA, Maddox TM, Ho PM, Jones PG, Arnold SV, Masoudi FA, Bradley SM. Hospital‐level variation in angina and mortality at 1 year after myocardial infarction: insights from the translational research investigating underlying disparities in acute myocardial infarction patients™ health status (TRIUMPH) registry. Circ Cardiovasc Qual Outcomes. 2014;7:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chamsi‐Pasha MA, Dupont M, Al Jaroudi WA, Tang WH. Utilization pattern of mineralocorticoid receptor antagonists in contemporary patients hospitalized with acute decompensated heart failure: a single‐center experience. J Card Fail. 2014;20:229–235. [DOI] [PubMed] [Google Scholar]
- 17. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. [DOI] [PubMed] [Google Scholar]
- 18. Juurlink DN, Mamdani MM, Lee DS, Kopp A, Austin PC, Laupacis A, Redelmeier DA. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. [DOI] [PubMed] [Google Scholar]
- 19. Givertz MM, Postmus D, Hillege HL, Mansoor GA, Massie BM, Davison BA, Ponikowski P, Metra M, Teerlink JR, Cleland JG, Dittrich HC, O'Connor CM, Cotter G, Voors AA. Renal function trajectories and clinical outcomes in acute heart failure. Circ Heart Fail. 2014;7:59–67. [DOI] [PubMed] [Google Scholar]
- 20. Urbine TF, Schwenke DC, Wu W‐C, Dev S. ICD9 coding of hyperkalemia greatly underestimates incidence of lab‐defined hyperkalemia in veterans with heart failure (abstr). J Cardiac Fail. 2013;19:S32. [Google Scholar]
- 21. Steinman MA, Dimaano L, Peterson CA, Heidenreich PA, Knight SJ, Fung KZ, Kaboli PJ. Reasons for not prescribing guideline‐recommended medications to adults with heart failure. Med Care. 2013;51:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. U.S. Department of Veterans Affairs: Veterans Health Administration . Available at: http://www.Va.Gov/health/aboutvha.Asp.2014. Accessed December 10, 2014.
- 23. Jones LG, Sin MK, Hage FG, Kheirbek RE, Morgan CJ, Zile MR, Wu WC, Deedwania P, Fonarow GC, Aronow WS, Prabhu SD, Fletcher RD, Ahmed A, Allman RM. Characteristics and outcomes of patients with advanced chronic systolic heart failure receiving care at the Veterans Affairs versus other hospitals: insights from the Beta‐Blocker Evaluation of Survival Trial (BEST). Circ Heart Fail. 2015;8:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]


