Abstract
Background
Renal denervation represents an emerging treatment for resistant hypertension in patients with end‐stage renal disease, but data about the anatomic substrate of this treatment are lacking. Therefore, the aim of this study was to investigate the morphological basis of sympathetic hyperactivity in the setting of hemodialysis patients to identify an anatomical substrate that could warrant the use of this new therapeutic approach.
Methods and Results
The distribution of sympathetic nerves was evaluated in the adventitia of 38 renal arteries that were collected at autopsy or during surgery from 25 patients: 9 with end‐stage renal disease on dialysis (DIAL group) and 16 age‐matched control nondialysis patients (CTRL group). Patients in the DIAL group showed a significant increase in nerve density in the internal area of the peri‐adventitial tissue (within the first 0.5 mm of the beginning of the adventitia) compared with the CTRL group (4.01±0.30 versus 2.87±0.28×mm2, P=0.01). Regardless of dialysis, hypertensive patients with signs of severe arteriolar damage had a greater number of nerve endings in the most internal adventitia, and this number was significantly higher than in patients without hypertensive arteriolar damage (3.90±0.36 versus 2.87±0.41×mm2, P=0.04), showing a correlation with hypertensive arteriolar damage rather than with hypertensive clinical history.
Conclusions
The findings from this study provide a morphological basis underlying sympathetic hyperactivity in patients with end‐stage renal disease and might offer useful information to improve the use of renal denervation in this group of patients.
Keywords: end‐stage renal disease, hemodialysis, histology, sympathetic renal innervation
Subject Categories: Hypertension, Catheter Ablation and Implantable Cardioverter-Defibrillator
Introduction
Sympathetic nerve activity associated with hypertension and cardiovascular events is markedly increased in patients with chronic kidney failure.1, 2, 3, 4 Hypertension is observed in >80% of patients with end‐stage renal disease (ESRD).5, 6, 7 Several studies have demonstrated that current hemodialysis procedures and antihypertensive drugs normalize blood pressure in only a small percentage of these patients.8 Although hypertension in hemodialysis patients has traditionally been thought of as being volume dependent, the results from different studies suggest that it should be considered a “neurogenic” hypertension that is sustained by overactivity of the sympathetic nervous system.2, 9, 10
Using microelectrodes to record action potentials from postganglionic sympathetic nerves in patients undergoing long‐term hemodialysis, Converse first documented evidence of sympathetic hyperactivity in hemodialyzed patients.1 Additional studies have shown an exponential increase in sympathetic activity during the various stages of chronic renal failure, which suggested significant nervous hyperactivity in patients with hemodialysis that was greater than that observed in essential hypertension.11 The contribution of the sympathetic nervous system to the development of hypertension is well known; however, the exact mechanisms underlying the heightened sympathetic tone in patients with chronic kidney disease remain unclear. Several indirect pieces of evidence have demonstrated that the sympathetic overactivity in patients with ESRD is caused by neurogenic signals originating in the damaged kidneys.12
Currently, renal denervation represents an emerging treatment for resistant hypertension in patients with preserved renal function, as demonstrated by most randomized controlled trials.13, 14, 15, 16, 17, 18, 19 Recent promising studies have reported a significant reduction in blood pressure in patients with chronic kidney disease at different stages of chronic kidney disease up to ESRD after renal denervation,20, 21, 22, 23, 24 but data about the anatomic substrate of this surgical procedure are lacking.
The significance and variability of the distribution and density of the renal sympathetic nervous system in humans have been investigated in several cases of normotensive and hypertensive patients25, 26 but not in patients with ESRD who are on hemodialysis. Therefore, the aim of our study was to investigate the morphological basis of the sympathetic hyperactivity in hemodialysis patients and thereby to identify an anatomical substrate that could warrant the use of renal denervation in these patients.
Materials and Methods
Patient Selection
Thirty‐eight renal arteries with peri‐adventitial tissue from 25 patients were studied. These patients were divided into 2 treatment groups: the first group consisted of 9 patients with end‐stage renal disease (ESRD) who underwent hemodialysis (DIAL group), and 16 age‐matched control, nondialysis patients (CTRL group) formed the second group (Table 1). Autopsies and surgical nephrectomies were performed at Tor Vergata University Hospital (PTV) in Rome between January 2012 and July 2014. The CTRL group was obtained from the tissue bank (autopsy and surgical specimens) of the Department of Pathology of the PTV selecting the first renal artery samples, according to the following criteria: absence of dialysis, matched for age and sex, length of the renal artery of at least 2.5 cm, at least 5 cases with chronic kidney diseases.
Table 1.
Patients’ Characteristics
| Parameters | Autopsy Cases | Surgery Samples | ||
|---|---|---|---|---|
| DIAL Group (5 Cases) | CTRL Group (8 Cases) | DIAL Group (4 Cases) | CTRL Group (8 Cases) | |
| Age, y | 72.4±10.7 | 65.8±16.4 | 61.2±5.8 | 53.8±13.0 |
| Sex, F/M | 2/3 | 2/5 | 3/1 | 3/5 |
| History of hypertension, Yes/No | 3/2 | 5/3 | 3/1 | 5/3 |
| Grade of renal arterioles ≥4 | 4 | 0 | 3 | 3 |
| CKD, Yes/No | 5/0 | 2/5 | 4/0 | 3/5 |
| Coronary artery disease, Yes/No | 3/2 | 5/3 | 2/2 | 0/8 |
| Arrhythmias, Yes/No | 2/3 | 1/7 | 0/4 | 1/7 |
| Type 2 diabetes, Yes/No | 1/4 | 3/5 | 1/3 | 2/6 |
| Dyslipidemia, Yes/No | 0/5 | 2/6 | 2/2 | 0/8 |
| Cause of death | ||||
| Acute myocardial infarction | 0 | 4 | ||
| Congestive heart failure | 3 | 0 | ||
| Bronchopneumonia | 2 | 2 | ||
| Pulmonary embolism | 0 | 2 | ||
| Histological changes of kidneys | ||||
| Glomerulosclerosis with interstitial fibrosis | 5 | 0 | 2 | 0 |
| Polycystic kidney disease | 0 | 0 | 2 | 2 |
| Chronic pyelonephritis | 0 | 8 | 0 | 0 |
| Tumors | 0 | 0 | 0 | 6 |
CKD indicates chronic kidney diseases; CTRL, control; DIAL, dialysis.
The renal arteries were collected from 13 subjects at autopsy (5 in the DIAL group and 8 in the CTRL group) and 12 surgical nephrectomies that were performed for therapeutic purposes (4 in the DIAL group and 8 in the CTRL group). Because both renal arteries were examined in autopsy cases, a total of 14 renal arteries from the DIAL patients and 24 from the CTRL group were analyzed.
This study was approved by and conducted in accordance with the guidelines of the Human Research Committee of our Institution and all surgical patients provided informed consent.
Histological Protocol
All autopsies were performed within 12 to 24 hours of death. Arteries, after sampling, to avoid folding and kinking were set without making any traction on a tablet and immediately fixed in buffered formalin. For renal arteries collected during the course of autopsies, at least 6 tissue specimens were sampled at distances of 5 mm from each other. Each artery, with the surrounding peri‐adventitial tissue, was equally divided into proximal and distal segments. The arteries harvested from the surgical nephrectomy specimens had a length of ≈2.5 cm; therefore, 4 tissue specimens were collected at distances of 5 mm from each other. Each segment was serially cut into 5‐mm‐thick sections. Two sections were stained with hematoxylin and eosin and Weigert stain. The other 3 sections were used for the immunohistochemistry analyses.
Moreover, in each patient, a sample of kidney tissue, including both cortex and medulla, was collected to evaluate the histological changes associated with hemodialysis and hypertension. A sample of the wall from the left ventricle of the heart was also biopsied in the autopsy cases to evaluate histological lesions and the sympathetic innervation of the pericardium.
Determination of Hypertensive Status by Histological Evaluation of the Renal Arterioles
To obtain an objective evaluation of the patients’ hypertensive status, 20 arterioles with a diameter ranging between 150 and 500 μm were evaluated from each kidney section according to the score system of Burke et al.27 A median score equal to 4 (concentric intimal thickening greater than the thickness of the media without concentric elastic duplication) or 5 (concentric intimal thickening greater than the thickness of the media with concentric elastic duplication in 3 or more vessels examined) was considered to be an objective marker of hypertensive nephropathy, independent of clinical history and therapy.
Immunohistochemistry
Nerves were identified in tissue sections using the methods and antibodies reported by Sakakura et al.28 To obtain accurate nerve contour for nerve size measurements, immunostaining for neurofilament protein (Ventana Medical Systems, Inc, Tucson, AZ) and S100 (Ventana Medical Systems, Inc) was performed in all cases. Anti‐tyrosine hydroxylase antibody (EMD Millipore, Billerica, MA) has been used to identify efferent nerves, and a rabbit anti‐calcitonin gene‐related peptide antibody (Sigma‐Aldrich, St. Louis, MO) to identify afferent nerves.
Morphometric Analysis of Renal Nerves
The morphometric analysis of nerves in the peri‐adventitial tissue was performed in sections that were stained for neurofilament protein and S100 and evaluated using the Hamamatsu NanoZoomer Digital Pathology Virtual Slide Viewer software (Hamamatsu City, Shizouka, Japan). The following parameters were assessed: (1) number of nerves per unit area, (2) size of the nerve endings, and (3) distance of the nerves from the vascular lumen.
The measurements were related both to the entire area of the peri‐adventitial tissue and to 2 concentric rings that were located within 0.5 mm (called “internal area”) and between 0.5 to 1 mm (defined as “intermediate area”) from the beginning of the adventitia (Figure 1). All the remaining peri‐adventitial tissue, beyond 1 mm from the beginning of the adventitia, was defined as “external area.”
Figure 1.
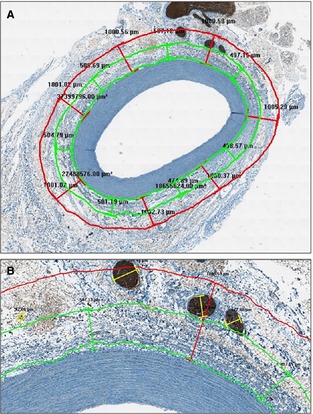
Morphometric analysis of renal nerves. Measurements were related both to the entire area of the peri‐adventitial tissue and to 2 concentric rings that were located within 0.5 mm (called the “internal area”) and between 0.5 and 1 mm (defined as the “intermediate area”) from the beginning of adventitia (A, ×2). B, A higher magnification of (A) (×10).
Statistical Analysis
Data were analyzed using the SPSS 16.0 (Statistical Package for the Social Sciences, SPSI Inc. Chicago, IL) software. The Shapiro–Wilk test was used to statistically assess the normal distribution of the data. Continuous variables with normal distribution were expressed as the mean±SE, while those with skewed distribution as median and range.
Data corresponding to the left and right renal arteries and those regarding internal area versus intermediate and external area, respectively, of adventitia were analyzed using the Wilcoxon ranks test for matched comparison. Because in our autopsy cases no significant differences were observed between the right and the left renal artery, for the further statistical analyses 1 artery per individual (by averaging the values of the left and right artery) was considered.
Differences between subjects undergoing dialysis and the control group were analyzed using the Mann–Whitney and Wilcoxon test for comparison of independent samples. The same test was used to correlate density and size of nerves with gender.
Categorical data were analyzed using the χ2 test or the Fisher exact test. The Spearman correlation coefficient was calculated to assess the correlations between nerve counts identified by neurofilament protein and S100 immunostains and the correlation between nerve density and age. All P values were determined by 2‐sided analysis, and P<0.05 were considered statistically significant.
Results
Histological Findings
In 10 of the 14 renal arteries from DIAL patients (71.4% of cases), the degree of stenosis was <30%, whereas it was between 60% and 75% in the remaining 4 cases (28.6%). In 9 cases (64.3%), the renal arteries showed a fibromuscular dysplasia that was associated with calcification of the tunica media in 2 cases. In the remaining 5 cases (35.7%), a fibrocalcified plaque was observed (Figure 2). Nineteen of the 24 renal arteries (79.2%) from patients in the CTRL group had a percentage of stenosis <30% and showed a fibromuscular dysplasia. The remaining 5 arteries (28.8%) had a fibroatheromatous plaque with between 60% and 75% of stenosis (Table 2). The thickness of the renal arterial wall ranged from 760 to 1920 μm.
Figure 2.
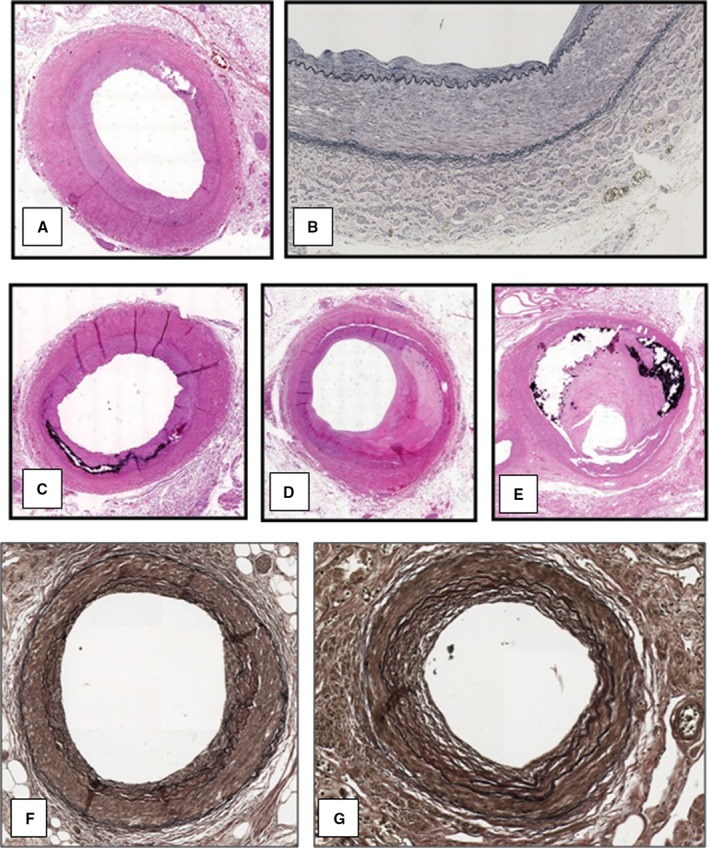
Histological findings of renal arteries and arterioles in patients of the DIAL group. Renal arteries with a fibromuscular dysplasia (A, H‐E—×2, and B, Weigert stain—×10), associated with a calcification of the tunica media (C, H‐E—×2) and with a fibrocalcified plaque (D and E, H‐E—×2). Renal arterioles with a score equal to 3 (F, Weigert stain—×10) and to 4 (G, Weigert stain—×10). DIAL indicates dialysis; H‐E, hematoxylin and eosin.
Table 2.
Histological Findings of Renal Arteries and Arterioles
| DIAL Group (n=9 Pts, 14 Renal Arteries and Kidneys) | CTRL Group (n=16 Pts, 24 Renal Arteries and Kidneys) | P Value | |
|---|---|---|---|
| Stenosis of renal arteries, N (%) | |||
| <30% | 10 (71.4) | 19 (79.2) | 0.59 |
| 30 to 59% | 0 | 0 | |
| 60 to 75% | 4 (28.6) | 5 (28.8) | |
| >75% | 0 | 0 | |
| Histological findings of renal arteries, N (%) | |||
| Fibromuscular dysplasia | 9 (64.3) | 19 (79.2) | 0.31 |
| Fibrocalcified plaque | 5 (35.7) | 5 (28.8) | |
| Arteriolar damage score a, N kidneys (%) | |||
| 2 | 0 | 9 (37.5) | 0.001 |
| 3 | 3 (21.4) | 12 (50.0) | |
| 4 | 10 (71.4) | 3 (12.5) | |
| 5 | 1 (7.2) | 0 | |
CTRL indicates control; DIAL, dialysis.
According to Burke et al (Ref. 27).
Seven of the 9 patients in the DIAL group (4 of the 5 autopsy samples, with similar lesions in both kidneys, and 3 of the 4 surgical specimens) showed signs of arteriolar damage due to hypertension (Table 2), although a clinical history of hypertension had been established for only 6 of them (Table 1). In particular, in 11 of the 14 kidneys examined in the DIAL group (78.6%), the arteriolar score was ≥4, whereas it was equal to 3 in 3 cases (21.4%) (Figure 2). Conversely, in the control group, 10 of the 16 patients (61.2%) had a history of hypertension but only 3 showed evidence of severe arteriolar damage (with a score=4) (Table 2).
Distribution of Peri‐Adventitial Nerves: General Findings
The overall nerve density in the peri‐adventitial tissue was 1.14±0.13/mm2. The nerve density was significantly greater in the internal area (3.27±0.23/mm2) than in the intermediate (1.15±0.12/mm2) and external areas (1.12±0.44/mm2) of the adventitia (P=0.001).
The mean diameter of the nerves measured 74.88±54.29 μm. The diameter of the nerves in the adventitial internal area was 48.2±16.6 μm, which was less than that of the nerves in the intermediate and external areas (105.08±64.02 μm, P=0.001). In the external area at a distance of at least 3.8 mm from the beginning of adventitia, few very large nerves, with a diameter >3 mm, were observed in both the autopsy and surgical samples.
No significant differences were found between the nerve measurements performed using neurofilament protein or S100 immunostains (P=0.98).
The density and size of nerves were not correlated with the sex and age of patients. In the 13 cases in which both renal arteries were evaluated at autopsy, no significant differences were found in the density of nerves from the left and right sides (2.63±1.27 versus 2.84±0.99/mm2, P=0.54).
In the autopsy cases, no significant differences were observed between the nerve density in the proximal segment of the renal artery (up to 2 cm from the aortic origin) and that in the distal segment (between 2 cm from the aortic origin and the hilum of the kidney) measuring 2.72±1.08 and 2.82±1.10 mm2, respectively (P=0.22). Based on these results, it can be assumed that the specimens from the autopsy series, in which each renal artery was analyzed from the aorta to the renal hilum, were comparable to the surgical specimens, in which sampling of the renal arteries was limited to the distal segment. Since the aortorenal paravertebral ganglia are located near the ostia of the renal arteries and were probably not included in the surgical specimens, their numbers were very low and thus did not influence the statistical significance of the results.
Distribution of Peri‐Adventitial Nerves in Hemodialysis Patients
Patients in the DIAL group showed a significant increase in nerve density in the internal area of the peri‐adventitial tissue (within the first 0.5 mm from the beginning of adventitia) compared with the CTRL group (4.01±0.30 versus 2.87±0.28 mm2, P=0.01) (Figures 3A and 4A through 4C). On the contrary, the nerve density observed in these groups was not different in the intermediate and external areas. The nerve density was independent of underlying causes of ESRD in the 9 DIAL patients.
Figure 3.
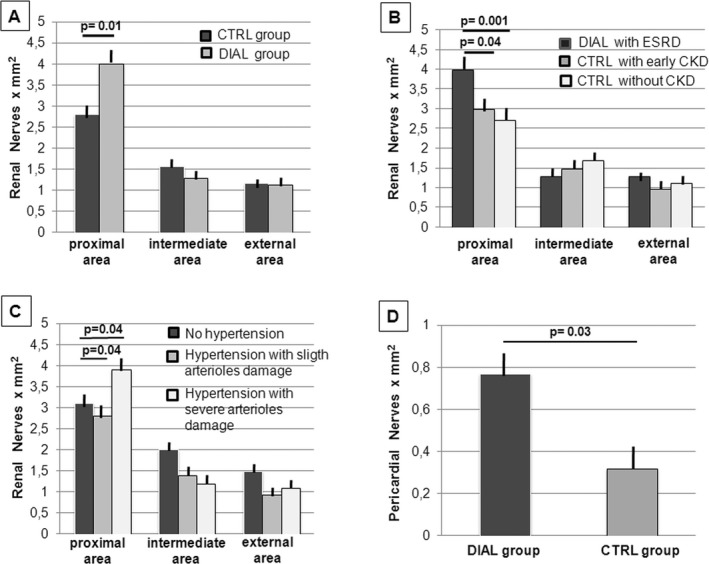
Distribution of peri‐adventitial and pericardial nerves. A, Patients in the DIAL group showed a significant increase in nerve density in the internal area of the peri‐adventitial tissue compared with the CTRL group (P=0.01). No significant differences were observed in the intermediate and external areas. B, Patients in the CTRL group with chronic kidney diseases in an earlier stage of the disease showed a smaller number of nerve endings in the internal area of the adventitia compared with dialysis patients with ESRD (P=0.03) and no significant differences compared with CTRL patients with normal renal function (P=0.93). No significant differences were observed in the intermediate and external area. C, Regardless of dialysis, hypertensive patients with signs of severe arteriolar damage showed a nerve density in the internal area of the adventitia that was significantly higher than that of hypertensive patients with minimal arteriolar damage (P=0.04). In this last group, the nerve density was similar to that of normotensive patients. D, Patients of the DIAL group showed a significant increase in nerve density in the pericardium compared with the CTRL group (P=0.03). CKD indicates chronic kidney disease; CTRL, control; DIAL, dialysis; ESRD, end stage renal disease.
Figure 4.
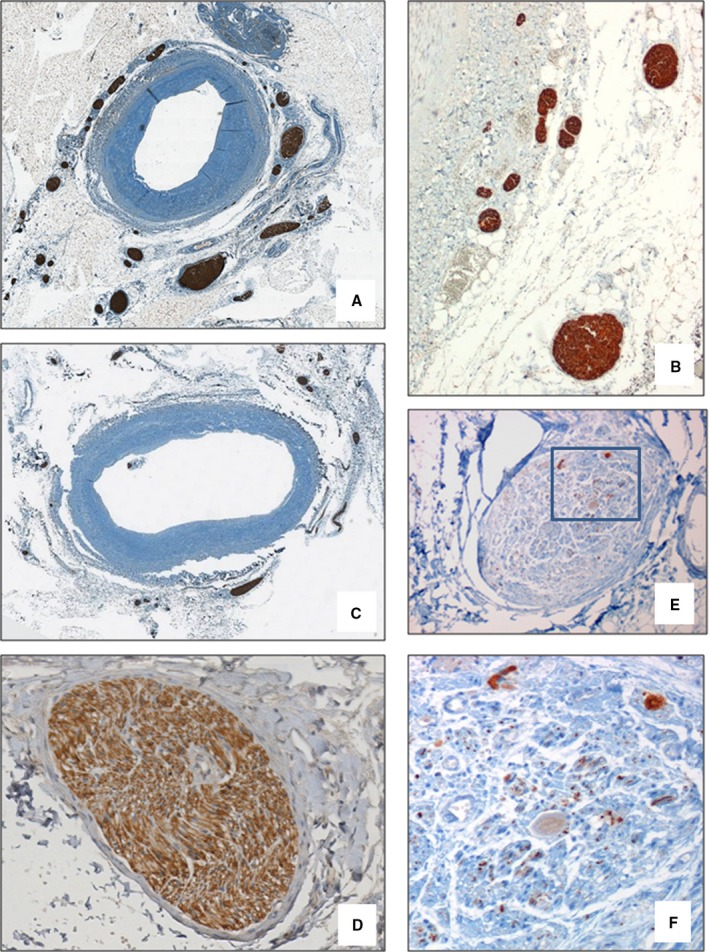
Morphological findings of peri‐adventitial nerves. In patients in the DIAL group, a significant increase in nerve density in the internal area of the peri‐adventitial tissue (within the first 0.5 mm from the beginning of adventitia) was observed (A, NF stain—×2 and B, ×4) compared with the CTRL group (C, NF stain—×2). The efferent nerve fibers (D, tyrosine hydroxylase stain—×20) were more numerous than afferent fibers (E, calcitonin gene–related peptide stain—×20; F, Detail of area delimited by the rectangle in D, ×40). CTRL indicates control; DIAL, dialysis; NF, neurofilament protein.
In the CTRL group, 5 patients (2 autopsy and 3 surgical cases) suffered from chronic kidney diseases at an early stage of the disease. This subgroup of patients showed a minor number of nerve endings in the internal area of the adventitia compared with the dialysis patients with ESRD (3.07±0.45 versus 4.01±0.30 mm2, P=0.03), with no significant differences compared with the CTRL patients with normal renal function (P=0.93) (Figure 3B).
In each group, the patients were divided into 2 subgroups: those with a clinical history of hypertension and those who were normotensive. No significant differences were detected within each group between the normotensive and hypertensive patients. However, as shown in Figure 3C, if we considered all cases, regardless of hemodialysis, the hypertensive patients with signs of severe arteriolar damage showed a nerve density in the internal area of the adventitia that was significantly higher than that of hypertensive patients with minimal arteriolar damage (3.90±0.36 versus 2.87±0.41 mm2, P=0.04). In this last group, the nerve density was similar to that of normotensive patients. If the analysis was limited to the DIAL group, patients with severe arteriolar damage also had a greater number of nerve endings in the most internal adventitia than did the patients without severe arteriolar damage (4.44±0.27 versus. 3.79±0.42 mm2), although it was not possible to verify the statistical significance of this finding given that only 2 of the 9 dialysis patients had no severe damage to the renal arterioles.
The efferent nerve fibers (immunopositive for tyrosine hydroxylase) were more numerous than afferent fibers (immunopositive for calcitonin gene–related peptide) (Figures 4D through 4F). In the DIAL group, as compared to CTRL group, a significative increase of both efferent and afferent sympathetic nerves density in the internal area of the adventitia of renal arteries was observed. In the DIAL group, efferent fibers showed an increase of ≈40% compared to the CTRL group (3.80±0.28 versus 2.89±0.26×mm2, P=0.01). A similar increase was observed for afferent fibers (0.16±0.01 versus 0.11±0.01, P=0.04), although the percentage of the afferent fibers remained constant in both groups (representing 3.5% of all fibers).
Distribution of Nerves in the Pericardium
Patients from the DIAL group showed a significant increase in nerves in the pericardium compared with the CTRL group (0.77±0.12 versus 0.32±0.11 mm2, P=0.03) (Figure 3D). No significant correlation was observed between nerve density and heart weight (R 2=0.22, P=0.20), probably due to the limited sample numbers.
Discussion
Our study provides evidence that in patients with ESRD on hemodialysis, there is a significant increase in nerve endings in the internal area of the adventitia compared with patients who had less chronic kidney disease or normal renal function. Moreover, regardless of hemodialysis, hypertensive patients with histological signs of arteriolar damage had a significantly higher nerve density in the internal area of the adventitia than did hypertensive patients without arteriolar damage.
To our knowledge, our work is the first to use morphological methods to demonstrate an increase in the density of sympathetic fibers in patients with ESRD. Previously, increased activity of the sympathetic system in these patients had been demonstrated only by physiological methods.12
Activation of the sympathetic nervous system is considered to be an important mechanism of disease progression in patients with chronic renal failure.1, 2, 3, 4 Nevertheless, a significant increase in the density of nerves in the internal periadventitial area was observed only in patients with ESRD who underwent hemodialysis. These patients had twice the number of nerve endings in the internal area of the adventitia compared with patients who had less severe renal failure, with a significative increase in both efferent and afferent sympathetic nerves.
Although this was not the primary purpose of our work, the results showed that hypertensive patients with severe renal insufficiency and signs of arteriolar damage had a significantly higher density of nerves in the internal area of the adventitia than did hypertensive patients without arteriolar damage or normotensive patients. Based on our results, it is not possible to determine whether these changes are due to the duration and degree of renal disease. It is likely that lesions of the renal arterioles occur in patients with a long‐term hypertension not responding to treatment (“malignant” hypertension). Moreover, as shown in Table 1, because 7 of the 10 patients with arteriolar damage were subjected to hemodialysis, it was not possible to exclude the effect of this treatment as a potential explanation for this difference. These data are consistent with previous studies1 that used muscle sympathetic nerve activity (MSNA) to show an exponential increase in sympathetic activity at various stages of chronic renal failure and a remarkable level of hyperactivity in patients with ESRD who were on hemodialysis that was greater than that observed in essential hypertension. Converse et al1 showed that sympathetic activity was normalized in hemodialysis patients who underwent bilateral nephrectomy, which resulted in a significant reduction in blood pressure and supported the hypothesis that signals originating within the damaged kidneys were responsible for the increased activity of the sympathetic central nuclei.
In contrast to our findings, Sakakura et al26 recently reported that renal nerve anatomy in hypertensive patients was not different from that of normotensive patients. Some observations can be made to account for the different results. If the measurement area used in our study included all areas of the perirenal adipose tissue, no significant differences in the density of nerves were observed. A significant difference was observed only in the most internal area of the adventitia (within the first 500 μm). In addition, our results showed that hypertensive patients without arteriolar damage had the same number of nerve endings in the internal area of the adventitia as did normotensive patients. Differences were observed only in hypertensive patients with arteriolar damage. The work of Sakakura et al26 does not specifically address this subgroup of patients.
The increase in the density of the small nerves in the internal adventitia is compatible with the mechanism of nerve sprouting29 that has been well characterized and demonstrated in previous studies in the damaged ventricular wall of human and experimental infarcted hearts.30, 31 In the case of nerve injury, the Wallerian degeneration of the nerves is followed by neurilemma cell proliferation and axonal regeneration (nerve sprouting). Excessive sympathetic nerve sprouting has also been demonstrated in humans after heart transplantation, which increases the inotropic response.32 These findings support the idea that the release of some metabolites in chronic renal failure could promote the release of nerve growth factor by activated Schwann cells in the peri‐adventitial tissue.
Data obtained from autopsy cases have demonstrated that in hemodialysis patients there was also a significant increase in the density of sympathetic nerves in the pericardium compared with controls. Although it was observed in a small series, this result confirms and provides a morphological basis for previous clinical studies that found a significant association between sympathetic nervous activity and increased cardiovascular risk and poor clinical outcome in hemodialysis patients with chronic heart failure.5, 12, 33 Since there is strong evidence that sympathetic efferent neuronal activity is increased in congestive heart failure and can also trigger malignant arrhythmias leading to sudden cardiac death,34 it could be hypothesized that in the 3 autopsy cases of the DIAL group in which the cause of death was congestive heart failure (see Table 1), the latter may be correlated with the increase of the density of sympathetic nerves in the pericardium.
Moreover, our results could provide useful information regarding renal sympathetic denervation procedures in patients with chronic kidney disease on hemodialysis. The observation of an increase in sympathetic endings only in the internal area of the adventitia could influence the amount of energy required to achieve catheter‐based renal denervation. It is noteworthy that the maximum density of nerves was observed in the area closest to the vessel wall, as also demonstrated in previous work.25, 26
The majority of patients with ESRD also have hypertension, which is often difficult to control using conventional therapy. To this end, the use of catheter‐based renal denervation might be effective in reducing blood pressure and improving cardiovascular function, as demonstrated by recent trials.13, 14, 15, 16, 17, 18, 19 Nevertheless, few data on the safety and efficacy of renal denervation in patients with ESRD and uncontrolled hypertension have been reported.20, 21, 22, 23, 24 Renal denervation also appears to reduce left ventricular hypertrophy in patients with ESRD who are on hemodialysis.24
The most important limitation of our study was that although we investigated a large number of renal nerves, the number of cases in some subgroups was limited. In particular, we were unable to examine the renal arteries of a larger group of patients with ESRD and hemodialysis without arteriolar damage. Moreover, a clinical instrumental study was not conducted to confirm the hyperactivity of the sympathetic nervous system in our patients, although numerous studies in the literature have shown the existence of sympathetic hyperactivity in patients with ESRD.1, 2, 3, 4 Although we measured statistically significant differences in nerve densities, the sample size of our study on the nerve density of the pericardium was limited in that it was carried out only in the 13 autopsy cases (5 of the DIAL group and 8 CTRL). Further studies will be needed to confirm these results.
Our results could have a substantial clinical impact on the refinement of strategies of renal denervation in patients with ESRD. The results of our study demonstrated a significant increase in the density of the sympathetic fibers only in the most internal area of the adventitia in patients with ESRD who were on hemodialysis. The increase in the nerve ending density was correlated with hypertensive damage to the arterioles, but not the hypertensive clinical history. The findings from this morphological study lend support to the morphological basis of sympathetic hyperactivity in patients with ESRD and might provide useful information to improve the use of renal denervation in this group of patients.
Disclosures
None.
(J Am Heart Assoc. 2015;4:e002426 doi: 10.1161/JAHA.115.002426)
References
- 1. Converse RL Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad‐Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med. 1992;327:1912–1918. [DOI] [PubMed] [Google Scholar]
- 2. Augustyniak RA, Tuncel M, Zhang W, Toto RD, Victor RG. Sympathetic overactivity as a cause of hypertension in chronic renal failure. J Hypertens. 2002;20:3–9. [DOI] [PubMed] [Google Scholar]
- 3. Masuo K, Lambert GW, Esler MD, Rakugi H, Ogihara T, Schlaich MP. The role of sympathetic nervous activity in renal injury and end‐stage renal disease. Hypertens Res. 2010;33:521–528. [DOI] [PubMed] [Google Scholar]
- 4. Schlaich MP. Sympathetic activation in chronic kidney disease: out of the shadow. Hypertension. 2011;57:683–685. [DOI] [PubMed] [Google Scholar]
- 5. Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, Mallamaci F, Massy ZA, Rossignol P, Vanholder R, Wiecek A, Zoccali C, London GM. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843. [DOI] [PubMed] [Google Scholar]
- 6. Agarwal R. Systolic hypertension in hemodialysis patients. Semin Dial. 2003;16:208–213. [DOI] [PubMed] [Google Scholar]
- 7. Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end‐stage renal disease in men. N Engl J Med. 1996;334:13–18. [DOI] [PubMed] [Google Scholar]
- 8. Agarwal R, Flynn J, Pogue V, Rahman M, Reisin E, Weir MR. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol. 2014;25:1630–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grassi G, Seravalle G, Quarti‐Trevano F. The ‘neuroadrenergic hypothesis’ in hypertension: current evidence. Exp Physiol. 2010;95:581–586. [DOI] [PubMed] [Google Scholar]
- 10. DiBona GF. Sympathetic nervous system and hypertension. Hypertension. 2013;61:556–560. [DOI] [PubMed] [Google Scholar]
- 11. Grassi G, Quarti‐Trevano F, Seravalle G, Arenare F, Volpe M, Furiani S, Dell'Oro R, Mancia G. Early sympathetic activation in the initial clinical stages of chronic renal failure. Hypertension. 2011;57:846–851. [DOI] [PubMed] [Google Scholar]
- 12. Rubinger D, Backenroth R, Sapoznikov D. Sympathetic nervous system function and dysfunction in chronic hemodialysis patients. Semin Dial. 2013;26:333–343. [DOI] [PubMed] [Google Scholar]
- 13. Symplicity HTN‐1 Investigators. Catheter‐based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. [DOI] [PubMed] [Google Scholar]
- 14. Esler MD, Krum H, Schlaich M, Schmieder RE, Bohm M, Sobotka PA. Renal sympathetic denervation for treatment of drug‐resistant hypertension: one‐year results from the Symplicity HTN‐2 randomized, controlled trial. Circulation. 2012;126:2976–2982. [DOI] [PubMed] [Google Scholar]
- 15. Veelken R, Schmieder RE. Renal denervation–implications for chronic kidney disease. Nat Rev Nephrol. 2014;10:305–313. [DOI] [PubMed] [Google Scholar]
- 16. Thorp AA, Larsen RN, Schlaich MP. Renal sympathetic nerve ablation for the management of resistant hypertension: an update. Curr Opin Nephrol Hypertens. 2013;22:607–614. [DOI] [PubMed] [Google Scholar]
- 17. Schlaich MP, Schmieder RE, Bakris G, Blankestijn PJ, Bohm M, Campese VM, Francis DP, Grassi G, Hering D, Katholi R, Kjeldsen S, Krum H, Mahfoud F, Mancia G, Messerli FH, Narkiewicz K, Parati G, Rocha‐Singh KJ, Ruilope LM, Rump LC, Sica DA, Sobotka PA, Tsioufis C, Vonend O, Weber MA, Williams B, Zeller T, Esler MD. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;62:2031–2045. [DOI] [PubMed] [Google Scholar]
- 18. Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha‐Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment‐resistant hypertension: final 3‐year report of the Symplicity HTN‐1 study. Lancet. 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 19. Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier‐Vehier C, Courand PY, Lantelme P, Denolle T, Dourmap‐Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet. 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 20. Hering D, Mahfoud F, Walton AS, Krum H, Lambert GW, Lambert EA, Sobotka PA, Bohm M, Cremers B, Esler MD, Schlaich MP. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiuchi MG, Maia GL, de Queiroz Carreira MA, Kiuchi T, Chen S, Andrea BR, Graciano ML, Lugon JR. Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J. 2013;34:2114–2121. [DOI] [PubMed] [Google Scholar]
- 22. Schlaich MP, Bart B, Hering D, Walton A, Marusic P, Mahfoud F, Bohm M, Lambert EA, Krum H, Sobotka PA, Schmieder RE, Ika‐Sari C, Eikelis N, Straznicky N, Lambert GW, Esler MD. Feasibility of catheter‐based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end‐stage renal disease. Int J Cardiol. 2013;168:2214–2220. [DOI] [PubMed] [Google Scholar]
- 23. Di Daniele N, De Francesco M, Violo L, Spinelli A, Simonetti G. Renal sympathetic nerve ablation for the treatment of difficult‐to‐control or refractory hypertension in a haemodialysis patient. Nephrol Dial Transplant. 2012;27:1689–1690. [DOI] [PubMed] [Google Scholar]
- 24. Di Daniele N, Rovella V, Violo L, De Francesco M, Sperandio M, Spinelli A, Tesauro M, Romeo F, Simonetti G. Reduction of left ventricular hypertrophy detected by cardiac magnetic resonance in a patient after renal denervation. J Cardiovasc Med (Hagerstown). 2015;16:721. [DOI] [PubMed] [Google Scholar]
- 25. Atherton DS, Deep NL, Mendelsohn FO. Micro‐anatomy of the renal sympathetic nervous system: a human postmortem histologic study. Clin Anat. 2012;25:628–633. [DOI] [PubMed] [Google Scholar]
- 26. Sakakura K, Ladich E, Cheng Q, Otsuka F, Yahagi K, Fowler DR, Kolodgie FD, Virmani R, Joner M. Anatomic assessment of sympathetic peri‐arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635–643. [DOI] [PubMed] [Google Scholar]
- 27. Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. [DOI] [PubMed] [Google Scholar]
- 28. Sakakura K, Ladich E, Edelman ER, Markham P, Stanley JR, Keating J, Kolodgie FD, Virmani R, Joner M. Methodological standardization for the pre‐clinical evaluation of renal sympathetic denervation. JACC Cardiovasc Interv. 2014;7:1184–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409–416. [DOI] [PubMed] [Google Scholar]
- 30. Vracko R, Thorning D, Frederickson RG. Nerve fibers in human myocardial scars. Hum Pathol. 1991;22:138–146. [DOI] [PubMed] [Google Scholar]
- 31. Vracko R, Thorning D, Frederickson RG. Fate of nerve fibers in necrotic, healing, and healed rat myocardium. Lab Invest. 1990;63:490–501. [PubMed] [Google Scholar]
- 32. Bengel FM, Ueberfuhr P, Schiepel N, Nekolla SG, Reichart B, Schwaiger M. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med. 2001;345:731–738. [DOI] [PubMed] [Google Scholar]
- 33. Zoccali C, Mallamaci F, Parlongo S, Cutrupi S, Benedetto FA, Tripepi G, Bonanno G, Rapisarda F, Fatuzzo P, Seminara G, Cataliotti A, Stancanelli B, Malatino LS. Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end‐stage renal disease. Circulation. 2002;105:1354–1359. [DOI] [PubMed] [Google Scholar]
- 34. Fukuda K, Kanazawa H, Aizawa Y, Ardell JL, Shivkumar K. Cardiac innervation and sudden cardiac death. Circ Res. 2015;116:2005–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]


