ABSTRACT
Understanding the mechanisms by which cancer stem cells (CSCs) survive chemotherapy is essential for the development of new therapies. Recently, we demonstrated that ovarian CSCs survive cisplatin treatment through enhanced expression of DNA polymerase η (Pol η). Identification of micro RNA-93 (miR-93) as the regulator of Pol η provides a novel target to improve the outcome of platinum-based therapy.
Keywords: Cancer stem cell, cisplatin resistance, DNA repair, translesion synthesis
In recent years, cancer stem cells (CSCs) have gained much attention as key “tumor-initiating” cells. The survival of CSCs following cancer treatment is thought to be responsible for the subsequent tumor recurrence and metastasis. Understanding the mechanisms by which CSCs survive conventional chemotherapy, particularly with DNA damaging agents such as platinum (Pt)-based chemotherapeutic agents, is essential for developing new therapeutic strategies to prevent tumor relapse. We have recently demonstrated that ovarian CSCs survive cisplatin treatment through DNA-directed polymerase eta (POLH, best known as Pol η)-mediated translesion DNA synthesis (TLS). Specifically, enhanced expression of Pol η in ovarian CSCs facilitates bypass of the unrepaired cisplatin-induced DNA lesions and rescues DNA replication arrested by the DNA damage, leading to the survival of cisplatin-treated CSCs. Furthermore, we also revealed that enhanced expression of Pol η can be attributed to the low expression of micro RNA-93 (miR-93) in ovarian CSCs.1 Our results identified Pol η-mediated TLS as a key player in cisplatin resistance of ovarian CSCs, and have implications for targeting miR-93 to improve the efficacy of Pt-based chemotherapy (Fig. 1).
Figure 1.
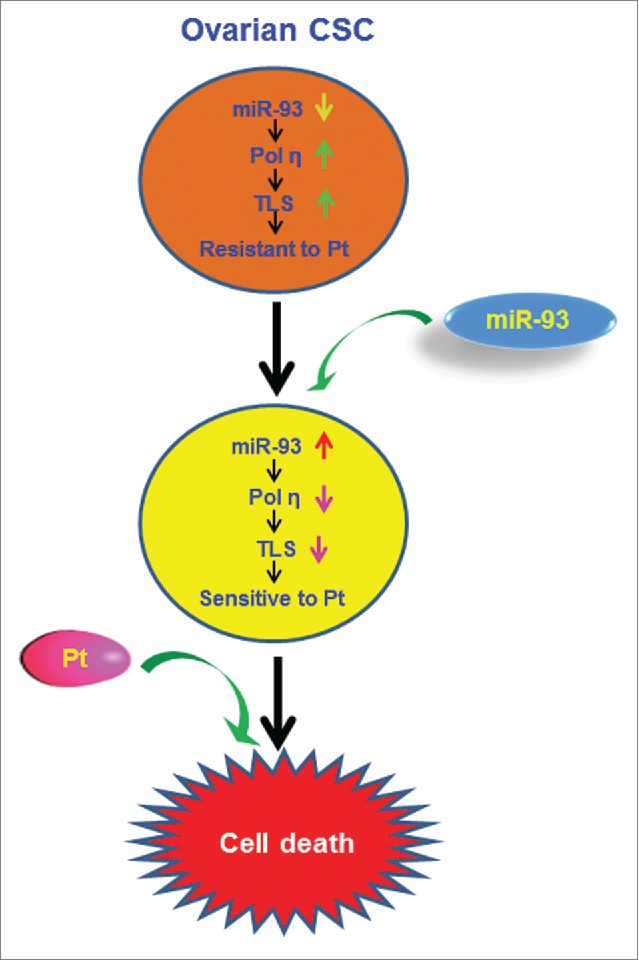
Sensitizing cancer stem cells to platinum-based therapy through miR-93. The depleted miR-93 in ovarian cancer stem cells (CSCs) is unable to suppress DNA polymerase η (Pol η) expression, which promotes translesion DNA synthesis (TLS)-mediated resistance to platinum (Pt). Overexpression of miR-93 in ovarian CSCs is expected to reduce the expression of Pol η, inhibit TLS, and sensitize CSCs to Pt-based chemotherapy.
One of the major mechanisms of cisplatin resistance is a decreased effective concentration of drug in the cell, resulting in reduced lethal DNA damage. This can result from either decreased influx or increased efflux of the drug. Increasing evidence indicates that the chemoresistance of CSCs is partially due to increased expression of ATP-binding cassette (ABC) transporters, which are also considered CSC markers.2 Therefore, it is logical to reason that the high capacity of CSCs to efflux drugs reduces the concentration of cisplatin in the cells, leading to fewer DNA intra-strand crosslinks. In addition, an enhanced DNA damage response has been described in various CSCs.3 Therefore, exploitation of an enhanced DNA repair mechanism by CSCs may also contribute to the inefficiency of DNA damaging agents. However, we were unable to show decreased cisplatin-induced DNA crosslinks in ovarian CSCs compared with corresponding bulk cancer cells when they were treated with equivalent doses of cisplatin, and did not observe an increased DNA repair efficiency to remove cisplatin-induced intra-strand crosslinks in the ovarian CSCs. Our data clearly indicate that inefficient formation of DNA lesions and enhanced DNA damage responses are unlikely to contribute to the cisplatin resistance of ovarian CSCs.
Translesion synthesis is believed to contribute to the development of cisplatin resistance by rescuing cells from the collapse of the replication fork induced by DNA intra-strand crosslinks following cisplatin treatment.4 TLS allows the DNA replication machinery to bypass a site of unrepaired DNA damage using special polymerases. Among many polymerases tested in vitro, the Y-family DNA Pol η is the most efficient and accurate at bypassing cisplatin-induced intra-strand crosslinks.4 Our data have revealed elevated expression of Pol η in ovarian CSCs, indicating that Pol η might be a critical contributor to the chemoresistant property of CSCs.1 It is well known that downregulation of Pol η in cancer cells results in increased sensitivity to cisplatin.5 Our in vitro and mouse xenograft studies also demonstrated that downregulation of Pol η significantly enhanced the response of ovarian CSCs to cisplatin treatment,1 indicating that downregulation of Pol η can facilitate the eradication of ovarian CSCs by cisplatin. Given that CSCs are believed to be responsible for the initiation and recurrence of tumors, inhibition of the Pol η-mediated TLS pathway in CSCs would be a promising therapeutic strategy to promote the eradication of CSCs by platinum-based chemotherapy.
Novel therapeutic approaches are currently being developed based on selective inhibitors for polymerases involved in DNA repair and/or TLS. However, it is difficult to develop a selective inhibitor by rational drug design because of the common structural features of currently characterized DNA polymerases.6 To solve this problem, the crystal structure of human Pol η has been analyzed and a unique hydrophobic pocket by W297 has been identified that might serve as a potential target for the development of selective inhibitors of human Pol η.7 Furthermore, we demonstrated in our study that Pol η expression is regulated by miR-93.1 Our data also revealed a depletion of miR-93 in ovarian CSCs. Enforced expression of miR-93 in ovarian CSCs reduced Pol η expression and increased their sensitivity to cisplatin.1 Therefore, targeting miR-93 could be a better approach to increase the efficacy of cisplatin treatment in ovarian cancer.
The extensive involvement of miRNAs in various human diseases suggests that they could be used as new therapeutic targets. Their small size and conserved sequence make them potential candidates from a development standpoint. As therapeutic agents, miRNAs have the ability to target multiple genes. Moreover, the direct downstream targets of a single miRNA are often linked genes that function in a signaling cascade. This indicates that targeting a single miRNA is likely to have a dramatic effect as a result of the combinatorial effect of gene expression changes in several related downstream targets.8 One of the major challenges in the use of miRNA therapeutics is tissue-specific delivery.9 In recent years, many efforts have been made to improve the delivery of miRNA in order to treat different types of cancer. Several approaches, such as hydrodynamic injection, engineered viral vectors, and nanoparticles, have been developed for delivery of miRNA into cells to modulate gene expression.10 Entrapping miR-93 mimics in suitable carriers might be a novel molecular approach to increase the efficacy of cisplatin treatment in ovarian cancer.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH grant CA151248 (QEW). AKS is supported by Pelotonia Postdoctoral Fellowship
References
- 1.Srivastava AK, Han C, Zhao R, Cui T, Dai Y, Mao H, Zhao W, Zhang X, Yu J, Wang QE. Enhanced expression of DNA polymerase eta contributes to cisplatin resistance of ovarian cancer stem cells. Proc Natl Acad Sci U S A 2015; 112:4411-6; PMID:25831546; http://dx.doi.org/ 10.1073/pnas.1421365112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moitra K, Lou H, Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin Pharmacol Ther 2011; 89:491-502; PMID:21368752; http://dx.doi.org/ 10.1038/clpt.2011.14 [DOI] [PubMed] [Google Scholar]
- 3.Maugeri-Sacca M, Bartucci M, De MR. DNA damage repair pathways in cancer stem cells. Mol Cancer Ther 2012; 11:1627-36; PMID:22844074; http://dx.doi.org/ 10.1158/1535-7163.MCT-11-1040 [DOI] [PubMed] [Google Scholar]
- 4.Bassett E, King NM, Bryant MF, Hector S, Pendyala L, Chaney SG, Cordeiro-Stone M. The role of DNA polymerase eta in translesion synthesis past platinum-DNA adducts in human fibroblasts. Cancer Res 2004; 64:6469-75; PMID:15374956; http://dx.doi.org/ 10.1158/0008-5472.CAN-04-1328 [DOI] [PubMed] [Google Scholar]
- 5.Chen YW, Cleaver JE, Hanaoka F, Chang CF, Chou KM. A novel role of DNA polymerase eta in modulating cellular sensitivity to chemotherapeutic agents. Mol Cancer Res 2006; 4:257-65; PMID:16603639; http://dx.doi.org/ 10.1158/1541-7786.MCR-05-0118 [DOI] [PubMed] [Google Scholar]
- 6.Berdis AJ. DNA polymerases as therapeutic targets. Biochemistry 2008; 47:8253-60; PMID:18642851; http://dx.doi.org/ 10.1021/bi801179f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Biertumpfel C, Gregory MT, Hua YJ, Hanaoka F, Yang W. Structural basis of human DNA polymerase eta-mediated chemoresistance to cisplatin. Proc Natl Acad Sci U S A 2012; 109:7269-74; PMID:22529383; http://dx.doi.org/ 10.1073/pnas.1202681109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iorio MV and Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med 2012; 4:143-59; PMID:22351564; http://dx.doi.org/ 10.1002/emmm.201100209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aagaard L and Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 2007; 59:75-86; PMID:17449137; http://dx.doi.org/ 10.1016/j.addr.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang Z, Gemeinhart RA. Progress in microRNA delivery. J Control Release 2013; 172:962-74; PMID:24075926; http://dx.doi.org/ 10.1016/j.jconrel.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]


