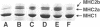Abstract
OBJECTIVE: In congestive heart failure (CHF) the skeletal muscle of the lower limbs develops a myopathy with atrophy and shift from the slow type to the fast type fibres. The aim was to test the hypothesis that this myopathy is specific and not simply related to detraining, by comparing patients with different degrees of CHF with patients with severe muscle atrophy due to disuse. DESIGN: Case-control study involving 50-150 micrograms needle biopsies of the gastrocnemius muscle. By an electrophoretic micromethod, the three isoforms of myosin heavy chains (MHC) were separated. PATIENTS: Five patients restricted to bed for more than one year because of stroke with disuse atrophy and normal ventricular function, and 19 with CHF were studied. There were seven age matched controls. MAIN OUTCOME MEASURES: The percentage of MHC1 (slow isoform), MHC2a (fast oxidative), and MHC2b (fast glycolytic) was determined by densitometric scan and correlated with indices of severity of cardiac failure. RESULTS: Ejection fraction was 42.5 (SD 15.2)% in CHF, 59.5 (1.0)% in disuse atrophy and 60.3 (1.4)% in controls (P < 0.001 v both). The degree of muscle atrophy as calculated by the body mass index/gastrocnemius cross sectional area, showed a profound degree of atrophy in patients with muscle disuse [0.94 (0.39)]. This was worse than in the controls [4.27 (0.16), P < 0.0005] and the CHF patients [2.60 (1.10), P < 0.005]. Atrophy in CHF patients was also greater than in controls (P < 0.005). MHC1 was lower in CHF than in disuse atrophy [51.83 (15.04) v 84.5 (17.04), P < 0.01] while MHC2b was higher [23.5 (7.4) v 7.25 (7.92), P < 0.001]. There was a similar trend for MHC2a [24.83 (15.01) v 8.25 (9.12), P < 0.05]. Within the CHF group there was a positive correlation between NYHA class and MHC2a (r = 0.47, P < 0.05) and MHC2b (r = 0.55, P < 0.01) and a negative correlation between NYHA class and MHC1 (r = -0.74, P < 0.001). Similarly, significant correlations were found for ejection fraction, diuretic consumption score, exercise test tolerance, and degree of muscle atrophy. CONCLUSIONS: The CHF myopathy appears to be specific and not related to detraining. The magnitude of MCH redistribution correlates with the severity of the disease. The electrophoretic micromethod used is very sensitive and reproducible. Biopsies are so well tolerated that can be repeated frequently, allowing thorough follow up.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler-Browne G. S., Bugaisky L. B., Cuénoud S., Schwartz K., Whalen R. G. Denervation of newborn rat muscle does not block the appearance of adult fast myosin heavy chain. Nature. 1982 Oct 28;299(5886):830–833. doi: 10.1038/299830a0. [DOI] [PubMed] [Google Scholar]
- Caforio A. L., Rossi B., Risaliti R., Siciliano G., Marchetti A., Angelini C., Crea F., Mariani M., Muratorio A. Type 1 fiber abnormalities in skeletal muscle of patients with hypertrophic and dilated cardiomyopathy: evidence of subclinical myogenic myopathy. J Am Coll Cardiol. 1989 Nov 15;14(6):1464–1473. doi: 10.1016/0735-1097(89)90383-5. [DOI] [PubMed] [Google Scholar]
- Carraro U., Catani C. A sensitive SDS-PAGE method separating myosin heavy chain isoforms of rat skeletal muscles reveals the heterogeneous nature of the embryonic myosin. Biochem Biophys Res Commun. 1983 Nov 15;116(3):793–802. doi: 10.1016/s0006-291x(83)80212-5. [DOI] [PubMed] [Google Scholar]
- Carraro U., Morale D., Mussini I., Lucke S., Cantini M., Betto R., Catani C., Dalla Libera L., Danieli Betto D., Noventa D. Chronic denervation of rat hemidiaphragm: maintenance of fiber heterogeneity with associated increasing uniformity of myosin isoforms. J Cell Biol. 1985 Jan;100(1):161–174. doi: 10.1083/jcb.100.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats A. J., Adamopoulos S., Meyer T. E., Conway J., Sleight P. Effects of physical training in chronic heart failure. Lancet. 1990 Jan 13;335(8681):63–66. doi: 10.1016/0140-6736(90)90536-e. [DOI] [PubMed] [Google Scholar]
- Drexler H., Riede U., Münzel T., König H., Funke E., Just H. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992 May;85(5):1751–1759. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- Drexler H. Skeletal muscle failure in heart failure. Circulation. 1992 Apr;85(4):1621–1623. doi: 10.1161/01.cir.85.4.1621. [DOI] [PubMed] [Google Scholar]
- Green H. J., Thomson J. A., Daub B. D., Ranney D. A. Biochemical and histochemical alterations in skeletal muscle in man during a period of reduced activity. Can J Physiol Pharmacol. 1980 Nov;58(11):1311–1316. doi: 10.1139/y80-199. [DOI] [PubMed] [Google Scholar]
- Harding S. E., Jones S. M., O'Gara P., del Monte F., Vescovo G., Poole-Wilson P. A. Isolated ventricular myocytes from failing and non-failing human heart; the relation of age and clinical status of patients to isoproterenol response. J Mol Cell Cardiol. 1992 May;24(5):549–564. doi: 10.1016/0022-2828(92)91843-t. [DOI] [PubMed] [Google Scholar]
- Jakubiec-Puka A., Catani C., Carraro U. Myosin heavy-chain composition in striated muscle after tenotomy. Biochem J. 1992 Feb 15;282(Pt 1):237–242. doi: 10.1042/bj2820237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D. C., Anand I. S., Bennett J. G., Pepper J. R., Yacoub M. H., Rothery S. M., Severs N. J., Poole-Wilson P. A. Ultrastructural analysis of skeletal muscle. Microvascular dimensions and basement membrane thickness in chronic heart failure. Eur Heart J. 1994 Nov;15(11):1470–1476. doi: 10.1093/oxfordjournals.eurheartj.a060416. [DOI] [PubMed] [Google Scholar]
- Lipkin D. P., Jones D. A., Round J. M., Poole-Wilson P. A. Abnormalities of skeletal muscle in patients with chronic heart failure. Int J Cardiol. 1988 Feb;18(2):187–195. doi: 10.1016/0167-5273(88)90164-7. [DOI] [PubMed] [Google Scholar]
- Mancini D. M., Coyle E., Coggan A., Beltz J., Ferraro N., Montain S., Wilson J. R. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989 Nov;80(5):1338–1346. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- Massie B. M., Conway M., Yonge R., Frostick S., Sleight P., Ledingham J., Radda G., Rajagopalan B. 31P nuclear magnetic resonance evidence of abnormal skeletal muscle metabolism in patients with congestive heart failure. Am J Cardiol. 1987 Aug 1;60(4):309–315. doi: 10.1016/0002-9149(87)90233-5. [DOI] [PubMed] [Google Scholar]
- Massie B., Conway M., Yonge R., Frostick S., Ledingham J., Sleight P., Radda G., Rajagopalan B. Skeletal muscle metabolism in patients with congestive heart failure: relation to clinical severity and blood flow. Circulation. 1987 Nov;76(5):1009–1019. doi: 10.1161/01.cir.76.5.1009. [DOI] [PubMed] [Google Scholar]
- McMurray J., Abdullah I., Dargie H. J., Shapiro D. Increased concentrations of tumour necrosis factor in "cachectic" patients with severe chronic heart failure. Br Heart J. 1991 Nov;66(5):356–358. doi: 10.1136/hrt.66.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti J. R., Christoph I., Oka R., Weiner M. W., Wells L., Massie B. M. Impaired skeletal muscle function in patients with congestive heart failure. Relationship to systemic exercise performance. J Clin Invest. 1991 Dec;88(6):2077–2082. doi: 10.1172/JCI115537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minotti J. R., Pillay P., Chang L., Wells L., Massie B. M. Neurophysiological assessment of skeletal muscle fatigue in patients with congestive heart failure. Circulation. 1992 Sep;86(3):903–908. doi: 10.1161/01.cir.86.3.903. [DOI] [PubMed] [Google Scholar]
- Patel A. N., Razzak Z. A., Dastur D. K. Disuse atrophy of human skeletal muscles. Arch Neurol. 1969 Apr;20(4):413–421. doi: 10.1001/archneur.1969.00480100089013. [DOI] [PubMed] [Google Scholar]
- Rossini K., Rizzi C., Sandri M., Bruson A., Carraro U. High-resolution sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunochemical identification of the 2X and embryonic myosin heavy chains in complex mixtures of isomyosins. Electrophoresis. 1995 Jan;16(1):101–104. doi: 10.1002/elps.1150160118. [DOI] [PubMed] [Google Scholar]
- Sabbah H. N., Hansen-Smith F., Sharov V. G., Kono T., Lesch M., Gengo P. J., Steffen R. P., Levine T. B., Goldstein S. Decreased proportion of type I myofibers in skeletal muscle of dogs with chronic heart failure. Circulation. 1993 May;87(5):1729–1737. doi: 10.1161/01.cir.87.5.1729. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol (1985) 1994 Aug;77(2):493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Green H. J., Cobb F. R. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Relation to skeletal muscle aerobic enzyme activity. Circulation. 1991 Oct;84(4):1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Green H. J., Cobb F. R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990 Feb;81(2):518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- Sullivan M. J., Green H. J., Cobb F. R. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990 Feb;81(2):518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- Swan J. W., Walton C., Godsland I. F., Clark A. L., Coats A. J., Oliver M. F. Insulin resistance in chronic heart failure. Eur Heart J. 1994 Nov;15(11):1528–1532. doi: 10.1093/oxfordjournals.eurheartj.a060425. [DOI] [PubMed] [Google Scholar]
- Vescovo G., Harding S. E., Jones S. M., Dalla Libera L., Pessina A. C., Poole-Wilson P. A. Comparison between isomyosin pattern and contractility of right ventricular myocytes isolated from rats with right cardiac hypertrophy. Basic Res Cardiol. 1989 Sep-Oct;84(5):536–543. doi: 10.1007/BF01908205. [DOI] [PubMed] [Google Scholar]
- Vescovo G., Jones S. M., Harding S. E., Poole-Wilson P. A. Isoproterenol sensitivity of isolated cardiac myocytes from rats with monocrotaline-induced right-sided hypertrophy and heart failure. J Mol Cell Cardiol. 1989 Oct;21(10):1047–1061. doi: 10.1016/0022-2828(89)90803-1. [DOI] [PubMed] [Google Scholar]
- Wilson J. R., Mancini D. M., Dunkman W. B. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993 Feb;87(2):470–475. doi: 10.1161/01.cir.87.2.470. [DOI] [PubMed] [Google Scholar]