Abstract
Background
In acute respiratory distress syndrome (ARDS), gas exchange and respiratory system mechanics (compliance) are severely impaired. Besides ventilatory parameters, the degree of respiratory abnormality can be influenced by the circulatory state. This study investigated the influence of acute hypovolemia on the respiratory system.
Methods
We performed a secondary analysis of a previous study including 8 pigs with ARDS-like syndrome induced by lung lavage and surfactant depletion method (ARDS group) and 10 mechanically ventilated pigs with no intervention (CTRL group). Animals of both groups were subjected to hemorrhage and retransfusion successively. We reanalyzed the effect of acute blood volume variations on intrapulmonary shunt (shunt), arterial oxygenation (PaO2:FiO2), global oxygen delivery (DO2) and respiratory system compliance (Crs).
Results
In the ARDS group, after hemorrhage, shunt decreased (−28 +/− 3.5 % (p < 0.001)), respiratory system compliance (Crs) increased (+5.1 +/− 1.0 ml/cm H2O (p < 0.001)) moreover, there was a concurrent increase in PaO2:FiO2 (+113 +/− 19.1 mmHg; p < 0.001) but this did not prevent a reduction in DO2 (−317 +/− 49.8 ml/min; p < 0.001). Following retransfusion, shunt and Crs return towards pre-hemorrhage values. Similar changes, but of smaller magnitude were observed in the CTRL group, except that no significant changes in oxygenation occurred.
Conclusions
The present analysis suggests that an acute decrease in blood volume results in a decrease in shunt with a parallel improvement in arterial oxygenation and an increase in Crs during ARDS-like syndrome. Our results strengthen the importance to integrate the circulatory condition in the analysis of the state of the respiratory system. However, the translation of this physiological model in a clinical perspective is not straightforward because our model of acute and severe hemorrhage is not strictly equivalent to a progressive hypovolemia, as could be obtained in ICU by diuretic. Furthermore, the present model does not consider the impact of blood loss induced decrease of DO2 on other vital organs function.
Trial registration
‘Not applicable’.
Keywords: Transpulmonary blood flow, Lung compliance, Admission
Background
Several studies explored the influence of ventilation parameters during acute respiratory distress syndrome (ARDS) [1, 2]. However, the influence of hemodynamic parameters on respiratory characteristics is frequently neglected. Indeed, animal studies reported that a decrease in cardiac output (CO), induced by a reduction in blood volume [3–5] or by pharmacological intervention [6, 7], may be associated with a decrease in intrapulmonary shunt, in normal lung [3, 5] as well as during ARDS [3, 4, 6, 7]. In addition, other studies reported an increase in intrapulmonary shunt associated with an increase in CO [5, 8]. Human studies reported similar results, where a parallel change in CO and shunt has been observed in normal subjects during exercise [9] or during CO reduction following the use of positive end-expiratory pressure (PEEP) in patients with ARDS [10], even if the effect of PEEP on shunt is not only related to his effect on CO [11]. Concerning the respiratory system compliance (Crs), previous studies reported conflicting results as some observed an increase [12, 13], a decrease [5] or no significant changes [14, 15] in Crs related to hemorrhage. On the other hand, an increase in pulmonary blood volume has been shown to result in a decrease in lung compliance [16].
In a previous study exploring the influence of tidal volume (VT) and respiratory rate (RR) on pulse pressure variations in pigs with ARDS [17], we observed an unexpected improvement in oxygenation and an increase in Crs during acute hemorrhage. In order to complete previous studies on this issue, we analyzed retrospectively our data with the hypothesis that a decrease in CO due to hypovolemia, may decrease intrapulmonary shunt, improve Crs and may result in an improvement in arterial oxygenation during ARDS.
Methods
The present study was a second analysis from a previous study [17]. The initial experimentation was performed on 24 domestic pigs. Domestic pigs were selected for the experiment because of their strong similarity in term of physiologic values, hemodynamic and respiratory behavior with humans. In this first analysis only 16 animals have been included as 8 pigs were excluded. Indeed, five animals allowed to improve the model (among them 2 animals died during the experiment), and three animals were excluded because of technical problems (dysfunction of the measurement systems). For the present analysis, 18 animals (mean weight of 31.4 ± 3.1 kg) from the 24 considered in the first study have been included. Four pigs were excluded due to lack of data needed for the present analysis and two other animals died during the experiment.
Ethics statement
The study was approved by the Ethics Committee for Animal Research at the University Medical Centre and by the Cantonal Veterinary Office of Geneva, Switzerland (No 31.1.1043/3127/1). Animals received respectful care in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Preparation
Animals were premedicated with intramuscular azaperon 6 mg kg−1, midazolam 0.5 mg.kg−1, and atropine 0.5 mg. Anesthesia was induced by isoflurane and maintained by fentanyl 20 μg.kg−1.h−1, isoflurane 1.5–2.0 % and pancuronium 0.4 mg.kg−1.h−1. Animals were intubated and mechanically ventilated (Servo Ventilator 900, Siemens-Elema, Sweden). FiO2 was set at 0.4 for the control (CTRL) group and 1.0 for the ARDS group. Tidal volume (VT) was set at 10 ml.kg−1 without positive end expiratory pressure (PEEP) and respiratory rate (RR) was 15/min with a fixed inspiratory to expiratory time ratio (1:2). A Swan-Ganz catheter (CCOmboV, 7.5 F, Edwards Lifesciences™, Irvine, CA) was placed in the pulmonary artery. Arterial pressure was recorded in the aorta trough a carotid arterial catheter. A right internal jugular vein catheter was placed for central venous pressure (CVP) measurement, drug infusion and blood retransfusion. Heart rate and rhythm were recorded with a standard 3-lead electrocardiogram.
Measurements
Respiratory system compliance
Airway pressure was monitored (UltimaTM, Datex/Instrumentarium, Helsinki, Finland). VT was calculated by digital integration of a flow signal measured by a pneumotachograph (Gould Godart, model 17212). Quasi-static Crs was calculated after an inspiratory pause of 3 s as VT/(plateau airway pressure – PEEP). Flow tracings were visually checked to ensure the absence of signs of intrinsic PEEP [18].
Blood gas tensions
Arterial blood gas (ABG) tensions, hemoglobin (Hb), oxygen saturation (arterial blood oxygen saturation (SaO2) and mixed venous blood oxygen saturation (SvO2) were analyzed by an automated oximeter (ABL-505 Analyzer, Radiometer, Copenhagen, Denmark).
Intrapulmonary shunt
In the ARDS group, shunt was calculated using the standard formula: Qs/Qt = (CcO2 ‐ CaO2)/(CcO2 ‐ CvO2) with the FiO2 set at 1.0.
In the present equation, Qs represents the shunt flow; Qt represents the systemic blood flow, the CcO2 represents the pulmonary capillary oxygen content; CaO2, the arterial oxygen content and CvO2, the mixed venous oxygen content. Oxygen content in arterial venous and capillary blood where calculated as: (1.34 × oxygen blood saturation × hemoglobin [Hb]) – (PO2 × 0.003). In the CTRL group, as FiO2 was set at 0.4 therefore, the calculation of shunt represent venous admixture.
Global oxygen delivery
DO2 was calculated as the product of CaO2 and CO, where CaO2 was calculated as 1.34 × Hb × SaO2 + (0.003 × PaO2).
Hemodynamic and respiratory measurements were digitized using an analog/digital interface converter (Biopac, Santa Barbara, CA, USA) and stored for off-line analysis.
Experimental protocol
Animals were separated into two groups. The CTRL group (n = 10) received anesthesia and controlled mechanical ventilation but no intervention. The ARDS group (n = 8) was submitted to a procedure of surfactant depletion to induce an ARDS-like syndrome [19]. This procedure consisted of lung lavage with NaCl 0.9 % (1000 ml) at 37 °C repeated until criteria for ARDS were fulfilled (PaO2/FiO2 < 200 mmHg). This required a mean ± SD of 3.1 ± 0.3 lung lavage at 12.6 ± 3.5 min intervals.
During hemorrhage total blood volume was reduced by 40 % of (estimated at 70 ml.kg−1 body weight) over 5–10 min. Removed blood was stored at body temperature in bags containing citrate-phosphate-dextrose (Baxter AG, Volketswil, Switzerland) and was totally retransfused during the retransfusion period. The mean time between the start of bleeding until completed retransfusion was 49 ± 11 min.
Measurement were assessed at pre-hemorrhage, hemorrhage, and retransfusion period in the CTRL group and at baseline (pre-ARDS), pre-hemorrhage (post-ARDS), hemorrhage and retransfusion in the ARDS group. As the original study was designed to investigate the influence of VT and RR on pulse pressure variations, three variants of ventilation were used successively during each period: V1 = VT 10 ml.kg−1 and RR 15 min−1; V2 = VT 6 ml.kg−1 and RR 25 min−1; and V3 = VT 6 ml.kg−1 and RR 15 min−1 (5–7 min each).
Statistical analysis
Data are presented as mean ± SD or as mean changes between periods ± SE. Baseline measures (pre-ARDS) in the ARDS group were not included in the statistical analyses. To determine the influence of hemorrhage on measured variables, we used a mixed effects analysis of variance (ANOVA) with fixed effect for the 3 periods of the experiment and random effect for pig (8 or 10). The same model was used to analyze the changes of the major determinants of DO2 (CaO2, Hb and SaO2,) in the ARDS group only. This statistical model estimates all comparisons between time periods simultaneously, reducing the risk of type I error. Furthermore, it takes into account repeated measurements of each pig across periods and across variants of ventilation. We determined the significance of the effect of hemorrhage and retransfusion on these parameters. As the goal of the analysis was to determine the effect of acute hemorrhage on shunt and Crs and because in the CTRL group venous admixture rather than true shunt was estimated, we did not compare the two groups of animals. Results are presented as the mean difference between two periods (hemorrhage vs pre-hemorrhage; retransfusion vs hemorrhage; retransfusion vs prehemorrhage) with respective p values. Because SvO2 may influence PaO2:FiO2 through venous admixture [20] we also analyzed PaO2:FiO2 adjusted for SvO2. After visual inspection of the data in order to determine the effect of successive ventilation mode on shunt in the ARDS group, we performed a mixed effects analysis of variance of the shunt across variant of ventilation. Comparison of blood loss between the CTRL group and the ARDS group were performed using unpaired t-test. All analyses were performed with R software (Version 2.15.1). A value of p less than 0.05 was considered significant and all statistical tests were two-tailed.
Results
Results are presented in Figs. 1 and 2, Tables 1 and 2. Mean difference and results of statistical analysis between periods are shown in Table 3.
Fig. 1.
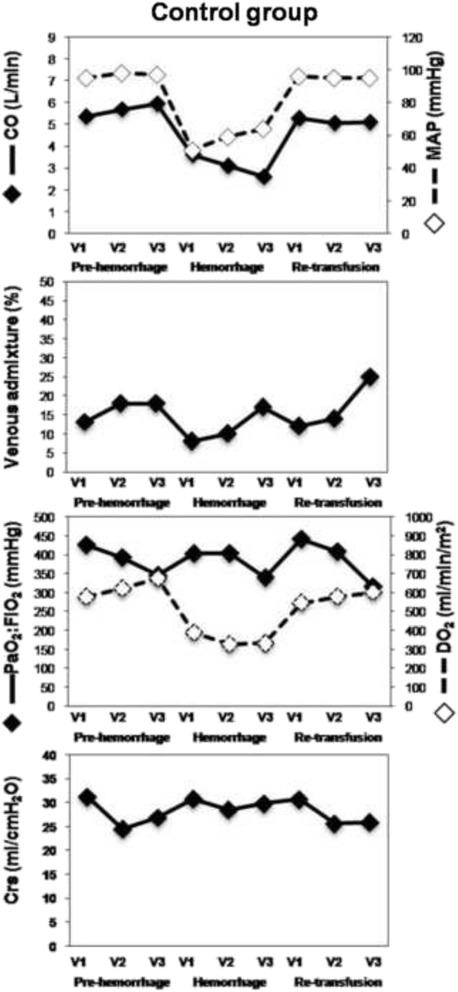
This figure shows the evolution of measured parameters during the experiment in the control group. All values are presented for each mode of ventilation (V1, V2, V3) in the successive phase of the experiment (Baseline, Hemorrhage, Re-transfusion). Crs, respiratory system compliance; CO, cardiac output; DO2, oxygen delivery; MAP, mean arterial pressure V1, tidal volume: 10 ml.kg−1 and respiratory rate: 15 min−1; V2 = tidal volume: 6 ml.kg−1 and respiratory rate: 25 min−1; V3, tidal volume 6 ml.kg−1 and respiratory rate: 15 min−1
Fig. 2.
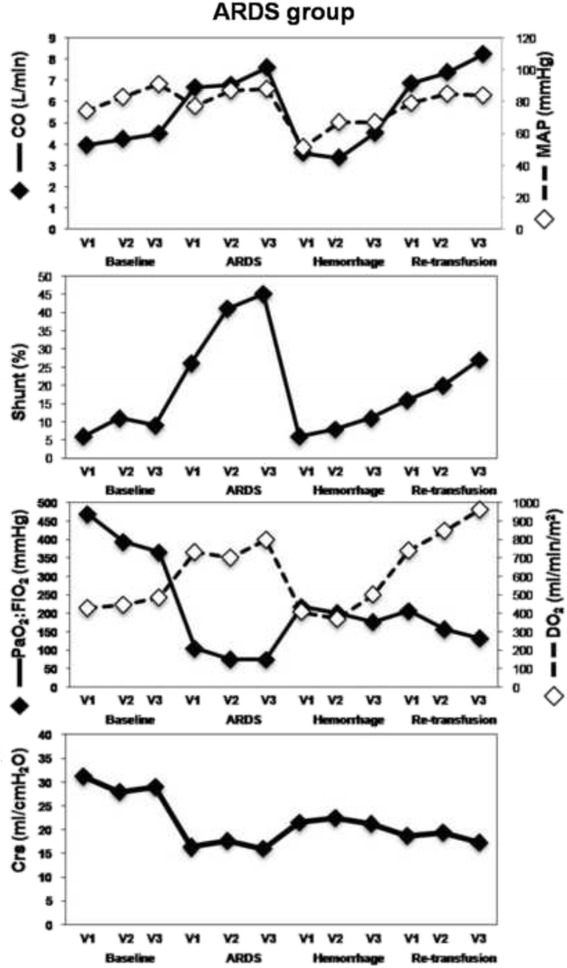
This figure shows the evolution of measured parameters during the experiment in the ARDS group. All values are presented for each mode of ventilation (V1, V2, V3) in the successive phase of the experiment (Baseline, ARDS, Hemorrhage, Re-transfusion). Crs, respiratory system compliance; CO, cardiac output; DO2, oxygen delivery; MAP, mean arterial pressure; shunt, intrapulmonary shunt; V1, tidal volume: 10 ml.kg−1 and respiratory rate: 15 min−1; V2 = tidal volume: 6 ml.kg−1 and respiratory rate: 25 min−1; V3, tidal volume 6 ml.kg−1 and respiratory rate: 15 min−1
Table 1.
Hemodynamic, respiratory and oxygenation parameters during each mode of ventilation at each time point in the control group
| Pre-hemorrhage Mean ± SD | Hemorrhage Mean ± SD | Retransfusion Mean ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ventilation mode: | ||||||||||||
| Tidal volume:ml.k g-1 | VT:10 | VT:6 | VT:6 | Average across variants of ventilation | VT:10 | VT:6 | VT:6 | Average across variants of ventilation | VT:10 | VT:6 | VT:6 | Average across variants of ventilation |
| Respiratory rate: breaths/min | RR: 15 | RR: 25 | RR: 15 | RR: 15 | RR: 25 | RR: 15 | RR: 15 | RR: 25 | RR: 15 | |||
| Cardiac output (l.min-1) | 5.4 ± 1.4 | 5.7 ± 1.5 | 6.0 ± 1.9 | 5.7 ± 1.6 | 3.6 ± 0.8 | 3.1 ± 1.1 | 2.6 ± 1.6 | 3.1 ± 1 | 5.3 ± 1.7 | 5.1 ± 2.5 | 5.1 ± 2.6 | 5.1 ± 2.2 |
| Heart Rate (bpm) | 101 ± 32 | 100 ± 26 | 103 ± 24 | 101 ± 27 | 130 ± 25 | 154 ± 50 | 163 ± 55 | 150 ± 46 | 111 ± 30 | 114 ± 25 | 120 ± 27 | 115 ± 26 |
| Mean arterial pressure (mmHg) | 95 ± 13 | 98 ± 15 | 97 ± 14 | 96.8 ± 13.7 | 51 ± 15 | 59 ± 16 | 64 ± 13 | 58.1 ± 15.4 | 96 ± 10 | 95 ± 9 | 95 ± 10 | 95.2 ± 9.3 |
| Central venous pressure (mmHg) | 8 ± 1 | 7 ± 1 | 7 ± 2 | 8 ± 1 | 5 ± 2 | 5 ± 3 | 6 ± 3 | 5 ± 3 | 9 ± 2 | 7 ± 3 | 8 ± 1 | 8 ± 2 |
| Veinous admixture (%) | 13 ± 4 | 18 ± 8 | 18 ± 9 | 16 ± 7 | 8 ± 4 | 10 ± 4 | 17 ± 14 | 11 ± 9 | 12 ± 4 | 14 ± 5 | 25 ± 12 | 16 ± 9 |
| PaO2:FiO2 (mmHg) | 426 ± 72 | 393 ± 92 | 345 ± 91 | 388 ± 89 | 403 ± 72 | 404 ± 87 | 340 ± 90 | 385 ± 84 | 441 ± 59 | 408 ± 54 | 316 ± 82 | 395 ± 79 |
| SVO2 (%) | 78 ± 8 | 78 ± 5 | 76 ± 8 | 77 ± 7.0 | 60 ± 12 | 65 ± 10 | 64 ± 15 | 63 ± 12 | 82 ± 5 | 78 ± 8 | 77 ± 6 | 79 ± 7 |
| Global oxygen delivery (ml.min-1.m-2) | 575 ± 160 | 620 ± 201 | 674 ± 305 | 623 ± 225 | 388 ± 99 | 326 ± 147 | 333 ± 159 | 347 ± 132 | 544 ± 228 | 577 ± 205 | 601 ± 258 | 570 ± 216 |
| Respiratory system compliance (ml.cm H2O−1) | 31.2 ± 3.6 | 24.5 ± 5.1 | 26.9 ± 4.6 | 27.5 ± 5.1 | 30.8 ± 5.4 | 28.5 ± 2.9 | 29.8 ± 7.4 | 29.7 ± 5.2 | 30.7 ± 4.1 | 25.6 ± 4.2 | 25.9 ± 4.3 | 27.3 ± 4.6 |
Table 2.
Hemodynamic, respiratory and oxygenation parameters during each mode of ventilation at each time point in the ARDS group (n = 8)
| Ventilation mode | Pre-hemorrhage mean ± SD | Hemorrhage mean ± SD | Retransfusion mean ± SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ventilation mode: | ||||||||||||
| VT:ml.kg-1 | VT:10 | VT:6 | VT:6 | Average across variants of ventilation | VT:10 | VT:6 | VT:6 | Average across variants of ventilation | VT:10 | VT:6 | VT:6 | Average across variants of ventilation |
| RR: breaths/min | RR: 15 | RR: 25 | RR: 15 | RR: 15 | RR: 25 | RR: 15 | RR: 15 | RR: 25 | RR: 15 | |||
| Cardiac output (l.min-1) | 6.7 ± 1.3 | 6.8 ± 1.5 | 7.6 ± 1.4 | 7.0 ± 1.4 | 3.6 ± 0.9 | 3.4 ± 0.8 | 4.5 ± 1.0 | 3.8 ± 1.0 | 6.9 ± 3.0 | 7.4 ± 2.6 | 8.2 ± 2.1 | 7.5 ± 2.5 |
| Heart rate (bpm) | 145 ± 18 | 157 ± 30 | 171 ± 36 | 158 ± 30 | 210 ± 24 | 215 ± 17 | 209 ± 29 | 212 ± 22 | 172 ± 32 | 170 ± 27 | 181 ± 24 | 174 ± 27 |
| Mean arterial pressure (mmHg) | 77 ± 19 | 87 ± 14 | 88 ± 13 | 84.0 ± 16.0 | 51 ± 13 | 67 ± 16 | 67 ± 12 | 62 ± 15 | 79 ± 10 | 85 ± 10 | 84 ± 12 | 83 ± 11 |
| Central venous pressure (mmHg) | 8 ± 2 | 7 ± 2 | 7 ± 2 | 8 ± 2 | 6 ± 2 | 5 ± 1 | 5 ± 2 | 5 ± 1 | 9 ± 1 | 8 ± 1 | 8 ± 1 | 9 ± 1 |
| Shunt (%) | 26 ± 15 | 41 ± 12 | 45 ± 15 | 37 ± 16 | 6 ± 7 | 8 ± 6 | 11 ± 9 | 9 ± 7 | 16 ± 17 | 20 ± 16 | 27 ± 19 | 20 ± 18 |
| PaO2:FiO2 (mmHg) | 105 ± 38 | 76 ± 20 | 75 ± 19 | 85 ± 30 | 218 ± 105 | 201 ± 95 | 177 ± 80 | 199 ± 91 | 207 ± 125 | 157 ± 77 | 133 ± 59 | 166 ± 93 |
| SVO2 (%) | 69 ± 11 | 70 ± 9 | 69 ± 11 | 69 ± 10 | 59 ± 14 | 67 ± 15 | 67 ± 17 | 64 ± 15 | 79 ± 7 | 79 ± 8 | 81 ± 8 | 80 ± 7 |
| Global oxygen delivery (ml.min-1.m-2) | 731 ± 146 | 701 ± 219 | 798 ± 265 | 744 ± 210 | 408 ± 113 | 369 ± 114 | 501 ± 160 | 427 ± 137 | 740 ± 392 | 846 ± 404 | 961 ± 323 | 849 ± 370 |
| Arterial content in oxygen (ml.dl-1) | 11.0 ± 0.9 | 10.2 ± 1.5 | 10.4 ± 1.7 | 10.5 ± 1.4 | 10.9 ± 1.3 | 10.9 ± 1.5 | 11.0 ± 1.5 | 11.0 ± 1.4 | 11.5 ± 1.0 | 11.2 ± 1.6 | 11.6 ± 1.1 | 11.4 ± 1.2 |
| Hemoglobin (g.dl-1) | 8.4 ± 0.4 | 8.4 ± 0.6 | 8.6 ± 0.8 | 8.5 ± 0.6 | 7.6 ± 0.6 | 7.7 ± 0.8 | 7.9 ± 0.7 | 7.8 ± 0.7 | 8.1 ± 1.0 | 8.1 ± 1.3 | 8.6 ± 0.8 | 8.3 ± 1.0 |
| SaO2 (%) | 92 ± 7 | 86 ± 8 | 85 ± 8 | 87 ± 8 | 97 ± 4 | 96 ± 4 | 95 ± 6 | 96 ± 5 | 97 ± 4 | 95 ± 4 | 94 ± 6 | 95 ± 5 |
| Respiratory sytem compliance (ml.cm H2O−1) | 16.4 ± 3.0 | 17.6 ± 3.9 | 16.0 ± 2.6 | 16.8 ± 3.2 | 21.5 ± 2.9 | 22.5 ± 4.6 | 21.2 ± 4.3 | 21.9 ± 4 | 18.7 ± 2.7 | 19.4 ± 5.2 | 17.3 ± 2.9 | 18.5 ± 3.9 |
Table 3.
Impact of hemorrhage and retransfusion on hemodynamic, respiratory and oxygenation parameters
| Phase | Hemorrhage vs. pre-hemorrhage | Retransfusion vs. hemorrhage | Retransfusion vs. pre-hemorrhage | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Group | Mean difference ± SE | p | Mean difference ± SE | p | Mean difference ± SE | p |
| Hemodynamic | |||||||
| Cardiac output, l.min−1 | CTRL | −2.6 ± 0.3 | <0.001 | 2.4 ± 0.3 | <0.001 | −0.2 ± 0.3 | 0.48 |
| ARDS | −3.2 ± 0.4 | <0.001 | 3.7 ± 0.4 | <0.001 | 0.5 ± 0.4 | 0.21 | |
| Mean arterial pressure, mmHg | CTRL | −36.7 ± 3.1 | <0.001 | 36.6 ± 3.2 | <0.001 | −0.1 ± 3.1 | 0.98 |
| ARDS | −22 .4 ± 4.0 | <0.001 | 21.4 ± 4.1 | <0.001 | −1.0 ± 4.1 | 0.80 | |
| Central venous pressure, mmHg | CTRL | −2.3 ± 0.3 | <0.001 | 2.9 ± 0.4 | <0.001 | 0.6 ± 0.3 | 0.09 |
| ARDS | −2.2 ± 0.3 | <0.001 | 3.2 ± 0.3 | <0.001 | 1 ± 0.3 | 0.003 | |
| Respiratory system and oxygenation | |||||||
| Venous admixture, % | CTRL | −6.1 ± 1.9 | 0.002 | 4.9 ± 1.9 | 0.01 | −1.2 ± 1.8 | 0.50 |
| Shunt, % | ARDS | −28.5 ± 3.5 | <0.001 | 12.3 ± 3.5 | <0.001 | −16.1 ± 3.5 | <0.001 |
| PaO2:FiO2, mmHg | CTRL | 1.7 ± 17.0 | 0.92 | 13.2 ± 17.2 | 0.45 | 14.5 ± 16.5 | 0.37 |
| ARDS | 113.4 ± 19.1 | <0.001 | −33.3 ± 19.1 | 0.09 | 80.2 ± 19.1 | <0.001 | |
| PaO2:FiO2 adjusted for mixed venous blood saturation, mmHg | CTRL | 42.6 ± 23.8 | 0.08 | −33.1 ± 25.3 | 0.20 | 9.4 ± 16.2 | 0.56 |
| ARDS | 125.7 ± 19.2 | <0.001 | −71.3 ± 22.5 | 0.002 | 54.4 ± 20.6 | 0.01 | |
| Global oxygen delivery, ml.min-1.m−2 | CTRL | −290.8 ± 37.0 | <0.001 | 229.6 ± 38.4 | <0.001 | −61.1 ± 35.9 | 0.09 |
| ARDS | −316.9 ± 49.8 | <0.001 | 422.1 ± 49.8 | <0.001 | 105.3 ± 49.8 | 0.04 | |
| Arterial content in oxygen, ml.dl−1 | ARDS | 0.4 ± 0.6 | 0.51 | 0.5 ± 0.6 | 0.48 | 0.9 ± 0.6 | 0.18 |
| Hemoglobin, g.dl−1 | ARDS | −0.7 ± 0.4 | 0.08 | 0.5 ± 0.4 | 0.18 | 0.2 ± 0.4 | 0.65 |
| Arterial blood saturation, % | ARDS | 9.0 ± 2.6 | 0.004 | −1.0 ± 2.6 | 0.76 | 8.0 ± 2.6 | 0.008 |
| Respiratory system compliance, ml.cm H2O−1 | CTRL | 2.5 ± 0.9 | 0.006 | −2.6 ± 0.9 | 0.01 | −0.1 ± 0.9 | 0.94 |
| ARDS | 5.1 ± 1.0 | <0.001 | −3.3 ± 1.0 | 0.001 | 1.8 ± 1.0 | 0.07 | |
Hemodynamics
The blood volume removed from the animals was 936 ± 100 ml (30 ml/kg) and 909 ± 94 ml (29 ml/kg) in the CTRL and ARDS group respectively (p > 0.05). CO (Figs. 1 and 2), mean arterial pressure and CVP decreased during the hemorrhage and recovered to baseline values during retransfusion (Table 3). The only exception was CVP in the ARDS group, which was slightly but significantly higher after retransfusion than pre-hemorrhage.
Gas exchange, oxygen delivery and compliance
In the CTRL group (Fig. 1), venous admixture decreased during hemorrhage and recovered to pre-hemorrhage values during retransfusion (Table 1). In the ARDS group (Fig. 2), shunt decreased during hemorrhage and recovered during re-transfusion, but did not fully return to pre-hemorrhage values (Table 2). In the CTRL group (Fig. 1), even if shunt changed, PaO2:FiO2, was similar across pre-hemorrhage, hemorrhage and retransfusion phases (Table 1). On the other hand, in the ARDS group (Fig. 2), PaO2:FiO2 varies inversely from shunt: increased during hemorrhage and did not recover completely during retransfusion (Table 2). The adjustment of PaO2:FiO2 for SvO2 did not change these results.
For both groups, DO2 varied in accordance with CO. That is, DO2 decreased during hemorrhage and then recovered during retransfusion (CTRL group: Fig. 1; Table 1; ARDS group: Fig. 2; Table 2). The main determinants of DO2 were examined in the ARDS group. CaO2 did not change across the phases (Table 2), Hb remained stable across the phases and SaO2 increased during hemorrhage but did not recover during re-transfusion (Table 2).
For both groups, Crs increased during hemorrhage and recovered to pre-hemorrhage values during retransfusion (CTRL group: Fig. 1; Table 1; ARDS group: Fig. 2; Table 2).
Impact of changes in ventilation settings on shunt in the ARDS group
At baseline (pre-ARDS), shunt did not change with ventilation setting (V1 to V2 change = 5.12 % (p = 0.19), V2 to V3 change = −1.83 (p = 0.61)). After ARDS induction but before hemorrhage (pre-hemorrhage period), shunt was only affected by the change from V1 to V2 (change = 15.16, p = 0.01) but not from V2 to V3 (change = 3.10, p = 0.57). After hemorrhage and retransfusion, there were no statistically significant differences in shunt values when ventilation was switched from V1 to V2 or from V2 to V3.
Discussion
In the present animal study, the authors investigated the effect of a decrease in CO through acute severe hypovolemia (with neither a pharmacologic intervention nor a mechanical procedure) on intra-pulmonary shunt in healthy and ARDS animals. Our analysis suggests that severe hypovolemia induced by acute hemorrhage is associated with a decrease in shunt and an increase in Crs in a pig model of ARDS. These changes were associated with improvements in PaO2:FiO2 only at high values of shunt (ARDS-like syndrome). The improvement in PaO2:FiO2 did not compensate for the decrease in CO and, as a consequence, DO2 decreased. To our knowledge, this is the first study showing the effects of acute hemorrhage on Crs in ARDS pig model.
In the present study, we observed that shunt and blood oxygenation were influenced by acute hemorrhage (shunt decrease) and retransfusion (shunt increase). Moreover, as during ARDS oxygenation is closely related to shunt [21, 22], PaO2:FiO2 varied similarly to shunt. This relation between global perfusion and shunt has already been reported during a decrease and an increase in perfusion [4, 6–10]. However, the present study extends these results to demonstrate that this relation is associated with concomitant changes in respiratory system compliance and that the effects on PaO2:FiO2 are not observed for relatively moderate values of shunt.
The decrease in shunt during hemorrhage could be explained by several physiological mechanisms. Firstly, it may be related to a preferential decrease in the perfusion of low- and/or non-ventilated regions. Indeed, to have a significant impact on oxygenation, the decrease in lung perfusion must be larger in the shunted part of the lung than in other normal lung regions. In ARDS, shunt essentially resides in the injured areas of the lung, in which perfusion is already limited by microvascular obstruction [23], adaptative hypoxic vasoconstriction [24], and gravitational compression of vessels [25, 26]. Secondly, in regions with lung injury, the impaired oxygenation and apparent “shunt” could also be explained by a decrease in pulmonary transit time in which the blood cannot be fully oxygenated because of increased red cell velocity [27, 28]. Thus, when the animals are hemorrhaged and pulmonary flow reduced, the reduced flow velocity may be coupled to a more efficient blood oxygenation. Finally, changes in Crs may also have influenced PaO2:FiO2 as suggested by the significant correlation between the changes in Crs and the changes in PaO2:FiO2 observed in our analysis and reported in Fig. 3. As the increase in Crs decreased alveolar pressures it may result in an improvement in the perfusion of West zone II [29] that may have reduced shunt.
Fig. 3.
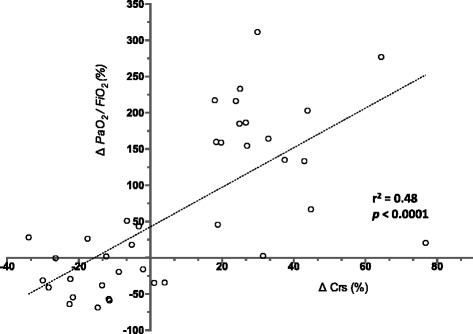
This figure report the relation between the changes (%) in PaO2:FiO2 and the changes (%) in Crs after hemorrhage and retransfusion. The correlation coefficient (r2) describes how two variables vary together
The other means by which shunt could be decreased is to recruit poorly or non-ventilated lung. However, in our analysis, the decrease in shunt and the concurrent improvement in PaO2:FiO2 remained significant whatever the VT value. This may be related to the fact that, during ARDS, pure shunt is predominant [6, 10] and small changes in VT value will not have a significant impact. Indeed, in the ARDS group, shunt significantly increased when VT was decreased from 10 ml.kg−1 (V1) to 6 ml.kg−1 (V2) during normovolemia but not during hemorrhage, confirming that the circulatory state was the main determinant of the shunt during Hypovolemia (Fig. 1; Table 2). As well, although the calculated shunt in the CTRL group was overestimated (since the calculation of shunt at FiO2 lower than 100 % represents venous admixture and not a true shunt [30]), the impact of bleeding on CTRL group PaO2:FiO2 was low, demonstrating that the circulatory state remains a determinant of the shunt only when lungs are affected by atelectasis.
However, even if gas exchange (PaO2:FiO2) and SaO2 improved during hemorrhage, it does not result in better tissue oxygenation. Indeed, the improvement in SaO2 was not large enough to influence CaO2, due in part to a slight decrease in hemoglobin. As a consequence, the decrease in CO reduced DO2. However, as the initial study was not designed to measure tissue oxygenation abnormalities, the impact of this decrease of DO2 on other vital organs function have not be investigated in the present analysis. Nevertheless, our observation is relevant to clinical settings, as it indicates that an improvement in PaO2:FiO2 and SaO2 can falsely mask a worsening in systemic oxygen delivery.
A key result in this study is the observed changes in Crs following blood volume shift (hemorrhage and retransfusion) (Table 3). An influence of blood volume on Crs has already been reported during blood volume decrease [12, 13] or increase [16], but previous studies reported conflicting results as they reported either a decrease [5, 31] or a stable [14, 15] Crs following hemorrhage. None of these studies investigated this effect during ARDS. One explanation for the inconsistency in previous results could be related to the variable and interrelated impact of blood volume on the total lung volume (including air, blood and tissue) and the participation of the microvascular network to the stiffness of the lung tissue. Indeed, considering the relation between pulmonary vascular pressure/filling and lung mechanical characteristics, various studies suggests that the pressurized pulmonary capillary network act as a tether for the lung tissue which may influence respiratory mechanics stability [32, 33].
This study acknowledges some limitations. First of all, the present report and analysis are based on previous experimentations [17] in which the main goal was far away from considering the impact of CO on intrapulmonary shunt. However, this secondary analysis of previously collected data is an ethical way to study further concepts without performing new experimentations and minimizing, by the way, the use of animals. Moreover, even if the design of the initial study was not specifically adapted to the aim of the present analysis, our results are in accordance with physiological laws and support previous works on this subject. Second, animals were ventilated without PEEP. Adding PEEP could have limited the development of atelectatic regions resulting in a model without significant shunt and decreased Crs, which could not allow investigating the specific effect of hemorrhage on these parameters. Consequently, we could not totally extrapolate the effects of hemorrhage on shunt and Crs in situations of ARDS ventilated with PEEP. However in patients with ARDS, even if high PEEP is used, the remaining hypoxemia is predominantly related to persistent shunt that could be influenced by the changes in CO. Third, our model of ARDS induced by a surfactant depletion method is a classical model of ARDS-like syndrome in animals resulting in reproducible and stable changes in physiological parameters similar to human ARDS [34]. However, this model is mainly characterized by atelectasis and less inflammatory lesions. Third, due to the design of the initial study [17], animals were ventilated with three successive ventilation modes. These changes in ventilatory settings could have influenced measured parameters. However, we used a mixed model ANOVA taking into account the effect of ventilation mode. Moreover, when we analyzed the impact of changes in ventilatory settings on shunt in the ARDS group, the mode of ventilation affected shunt only in the pre-hemorrhage period and between V1:VT: 10 ml.kg−1, RR: 15 min−1 and V2: VT: 6 ml.kg-1, RR: 25 min−1 but not in other period nor other change in ventilation mode. Therefore, the ventilation mode used in this study does not seem to influence significantly the measured parameters. Fourth, the translation of this physiological model in a clinical perspective is not straightforward: our model of acute and severe hemorrhage is not strictly equivalent to a progressive hypovolemia, as could be obtained by diuretic for example. However, our results consolidate the hypothesis that decrease in blood volume may influence respiratory system characteristics, especially when these characteristics are already altered. Moreover it emphasizes that the influence of a decrease in blood volume on SaO2 or PaO2:FiO2 may be at the expense of global oxygen delivery. Our model may be more closely encountered for example during the institution or the weaning of extracorporeal membrane oxygenation (ECMO) where large and acute changes in circulating blood volume may influence respiratory system characteristics. Finally, the compensatory release of catecholamine associated with the acute severe hemorrhage model may have influenced our observation. Indeed, catecholamine increase pulmonary vascular resistance [35] which may have amplified the decrease in pulmonary shunt observed during hemorrhage.
Conclusion
Our observations show that there is an impact of blood volume on respiratory system characteristics and oxygenation parameters during ARDS. Indeed, we observed that an acute hemorrhage results in a decrease in shunt with a parallel improvement in arterial oxygenation. Moreover, after hemorrhage, Crs improves. Our observations illustrate the link between physiologic mechanisms and clinical observations and may explain why in clinical practice a rapid modest improvement in lung compliance and/or a large improvement in oxygenation parameters in patients with ARDS may not necessarily reflect an improvement in patient condition but rather may be related to an acute decrease in transpulmonary blood flow. Our analysis thus strengthens the importance to integrate the circulatory condition in the analysis of the state of the respiratory system. However, the caveats of the study (use of a model not replicable in clinical activity; lack of data on the impact of oxygen delivery on clinical outcome) do not allow having a reliable take-home message for the clinical management of ARDS.
Ethics approval
The study was approved by the Ethics Committee for Animal Research at the University Medical Centre and by the Cantonal Veterinary Office of Geneva, Switzerland (No 31.1.1043/3127/1). Animals received respectful care in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Consent for publication
“Not applicable”.
Availability of data and material
The data supporting our results are presented within the article.
Availability of data and materials
Data are available upon request.
Acknowledgments
“Not applicable”.
Source of funding
This study was funded by an institutional grant from the Anesthesiology, Pharmacology and Intensive Care Department of the Geneva University Hospital (APSI 06–07). The authors declare no conflicts of interest for this study. No reprints will be ordered.
Abbreviations
- ABG
arterial blood gas
- ARDS
acute respiratory distress syndrome
- CaO2
arterial oxygen content
- CcO2
pulmonary capillary oxygen content
- CO
cardiac output
- Crs
respiratory system compliance
- CTRL group
no intervention
- CvO2
mixed venous oxygen content
- CVP
central venous pressure
- DO2
global oxygen delivery
- Hb
hemoglobin
- PaO2:FiO2
arterial oxygenation
- PEEP
positive end-expiratory pressure
- Qs
shunt flow
- Qs/Qt
Intrapulmonary shunt
- Qt
represents the systemic blood flow
- RR
respiratory rate
- SaO2
oxygen saturation (arterial blood oxygen saturation
- SvO2
mixed venous blood oxygen saturation
- VT
tidal volume
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NS conceive the study, analyze and interpret the results, write the paper; RG participate to the interpretation of the results and to the writing of the paper; DSC performed the statistical analysis and participate to the writing of the paper; CUW, performed the data acquisition and participate to the writing of the paper; KB conceived the original and the actual study, analyze and interpret the results and participate to the writing of the paper. All authors read and approved the final manuscript.
Contributor Information
Nils Siegenthaler, Email: nils.siegenthaler@hcuge.ch.
Raphael Giraud, Email: Raphael.giraud@hcuge.ch.
Delphine S. Courvoisier, Email: Delphine.Courvoisier@hcuge.ch
Claes U. Wiklund, Email: claes.wiklund@ki.se
Karim Bendjelid, Phone: +41 223827446, Email: karim.bendjelid@hcuge.ch.
References
- 1.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303:865–73. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8. [DOI] [PubMed]
- 3.Smith G, Cheney FW, Winter PM. The effect of change in cardiac output on intrapulmonary shunting. Br J Anaesth. 1974;46:337–42. doi: 10.1093/bja/46.5.337. [DOI] [PubMed] [Google Scholar]
- 4.Breen PH, Schumacker PT, Sandoval J, Mayers I, Oppenheimer L, Wood LD. Increased cardiac output increases shunt: role of pulmonary edema and perfusion. J Appl Physiol. 1985;59:1313–21. doi: 10.1152/jappl.1985.59.4.1313. [DOI] [PubMed] [Google Scholar]
- 5.Fulton RL, Fischer RP. Pulmonary changes due to hemorrhagic shock resuscitation with isotonic and hypertonic saline. Surgery. 1974;75:881–91. [PubMed] [Google Scholar]
- 6.Lynch JP, Mhyre JG, Dantzker DR. Influence of cardiac output on intrapulmonary shunt. J Appl Physiol. 1979;46:315–21. doi: 10.1152/jappl.1979.46.2.315. [DOI] [PubMed] [Google Scholar]
- 7.Light RB, Ali J, Breen P, Wood LD. The pulmonary vascular effects of dopamine, dobutamine, and isoproterenol in unilobar pulmonary edema in dogs. J Surg Res. 1988;44:26–35. doi: 10.1016/0022-4804(88)90119-9. [DOI] [PubMed] [Google Scholar]
- 8.Fredén F, Cigarini I, Mannting F, Hagberg A, Lemaire F, Hedenstierna G. Dependence of shunt on cardiac output in unilobar oleic acid edema. Distribution of ventilation and perfusion. Intensive Care Med. 1993;19:185–90. doi: 10.1007/BF01694768. [DOI] [PubMed] [Google Scholar]
- 9.Lovering AT, Romer LM, Haverkamp HC, Pegelow DF, Hokanson JS, Eldridge MW. Intrapulmonary shunting and pulmonary gas exchange during normoxic and hypoxic exercise in healthy humans. J Appl Physiol. 2008;104:1418–25. doi: 10.1152/japplphysiol.00208.2007. [DOI] [PubMed] [Google Scholar]
- 10.Dantzker DR, Lynch JP, Weg JG. Depression of cardiac output is a mechanism of shunt reduction in the therapy of acute respiratory failure. Chest. 1980;77:636–42. doi: 10.1378/chest.77.5.636. [DOI] [PubMed] [Google Scholar]
- 11.Matamis D, Lemaire F, Harf A, Teisseire B, Brun-Buisson C. Redistribution of pulmonary blood flow induced by positive end-expiratory pressure and dopamine infusion in acute respiratory failure. Am Rev Respir Dis. 1984;129:39–44. doi: 10.1164/arrd.1984.129.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Cahill JM, Byrne JJ. Ventilatory mechanics in hypovolemic shock. J Appl Physiol. 1964;19:679–82. doi: 10.1152/jappl.1964.19.4.679. [DOI] [PubMed] [Google Scholar]
- 13.Proctor HJ, Moss GS, Homer LD, Litt BD. Changes in lung compliance in experimental hemorrhagic shock and resuscitation. Ann Surg. 1969;169:82–92. doi: 10.1097/00000658-196901000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sprung J, Mackenzie CF, Green MD, O’Dwyer J, Barnas GM. Chest wall and lung mechanics during acute hemorrhage in anesthetized dogs. J Cardiothorac Vasc Anesth. 1997;11:608–12. doi: 10.1016/S1053-0770(97)90014-8. [DOI] [PubMed] [Google Scholar]
- 15.Martins MA, Zin WA, Younes RN, Negri EM, Sakae RS, Lin CA, et al. Respiratory system mechanics in guinea pigs after acute hemorrhage: role of adrenergic stimulation. Crit Care Med. 1990;18:515–9. doi: 10.1097/00003246-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Hauge A, Bø G, Waaler BA. Interrelations between pulmonary liquid volumes and lung compliance. J Appl Physiol. 1975;38:608–14. doi: 10.1152/jappl.1975.38.4.608. [DOI] [PubMed] [Google Scholar]
- 17.Wiklund CU, Morel DR, Orbring-Wiklund H, Romand J-A, Piriou V, Teboul J-L, et al. Influence of tidal volume on pulse pressure variations in hypovolemic ventilated pigs with acute respiratory distress-like syndrome. Anesthesiology. 2010;113:630–8. doi: 10.1097/ALN.0b013e3181e908f6. [DOI] [PubMed] [Google Scholar]
- 18.Brochard L. Intrinsic (or auto-) positive end-expiratory pressure during spontaneous or assisted ventilation. Intensive Care Med. 2002;28:1552–4. doi: 10.1007/s00134-002-1515-z. [DOI] [PubMed] [Google Scholar]
- 19.Lachmann B, Robertson B, Vogel J. In vivo lung lavage as an experimental model of the respiratory distress syndrome. Acta Anaesthesiol Scand. 1980;24:231–6. doi: 10.1111/j.1399-6576.1980.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 20.West JB. State of the art: ventilation-perfusion relationships. Am Rev Respir Dis. 1977;116:919–43. doi: 10.1164/arrd.1977.116.5.919. [DOI] [PubMed] [Google Scholar]
- 21.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31(6):776–84. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 22.Dantzker DR, Brook CJ, Dehart P, Lynch JP, Weg JG. Ventilation-perfusion distributions in the adult respiratory distress syndrome. Am Rev Respir Dis. 1979;120:1039–52. doi: 10.1164/arrd.1979.120.5.1039. [DOI] [PubMed] [Google Scholar]
- 23.Tomashefski JF, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112:112–26. [PMC free article] [PubMed] [Google Scholar]
- 24.Brimioulle S, Julien V, Gust R, Kozlowski JK, Naeije R, Schuster DP. Importance of hypoxic vasoconstriction in maintaining oxygenation during acute lung injury. Crit Care Med. 2002;30:874–80. doi: 10.1097/00003246-200204000-00027. [DOI] [PubMed] [Google Scholar]
- 25.Pelosi P, D’Onofrio D, Chiumello D, Paolo S, Chiara G, Capelozzi VL, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;42:48s–56s. [DOI] [PubMed]
- 26.Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20:1017–28. doi: 10.1183/09031936.02.00401702. [DOI] [PubMed] [Google Scholar]
- 27.Granger WM, Miller DA, Ehrhart IC, Hofman WF. The effect of blood flow and diffusion impairment on pulmonary gas exchange: a computer model. Comput Biomed Res. 1987;20:497–506. doi: 10.1016/0010-4809(87)90037-1. [DOI] [PubMed] [Google Scholar]
- 28.Hopkins SR, Belzberg AS, Wiggs BR, McKenzie DC. Pulmonary transit time and diffusion limitation during heavy exercise in athletes. Respir Physiol. 1996;103:67–73. doi: 10.1016/0034-5687(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 29.West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J Appl Physiol. 1964;19:713–24. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- 30.Ab L. Nunn’s applied respiratory physiology sixth edition. Philadelphia: Elsevier/Butterworth Heinemann; 2005. [Google Scholar]
- 31.Kakvan M, Masuoka S, Simeone FA. Pulmonary function in irreversible hemorrhagic shock in dogs. Surg Forum. 1971;22:29–31. [PubMed] [Google Scholar]
- 32.Peták F, Habre W, Hantos Z, Sly PD, Morel DR. Effects of pulmonary vascular pressures and flow on airway and parenchymal mechanics in isolated rat lungs. J Appl Physiol 1985. 2002;92:169–78. doi: 10.1152/jappl.2002.92.1.169. [DOI] [PubMed] [Google Scholar]
- 33.Albu G, Habre W, Fontao F, Morel DR, Petak F. The contribution of the pulmonary microvascular pressure in the maintenance of an open lung during mechanical ventilation. Respir Physiol Neurobiol. 2007;157:262–9. doi: 10.1016/j.resp.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Muellenbach RM, Kredel M, Zollhoefer B, Bernd Z, Johannes A, Kuestermann J, et al. Acute respiratory distress induced by repeated saline lavage provides stable experimental conditions for 24 hours in pigs. Exp Lung Res. 2009;35:222–33. doi: 10.1080/01902140802534975. [DOI] [PubMed] [Google Scholar]
- 35.Barman SA. Effect of catecholamines on pulmonary circulation at elevated vascular tone. J Appl Physiol. 1995;78:1452–8. doi: 10.1152/jappl.1995.78.4.1452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.


