Abstract
Background
Adiponectin and its receptors (AdipoR1 and AdipoR2), known as adiponectin system, have some proven roles in the fat and glucose metabolisms. Several studies have shown that adiponectin can be considered as a candidate in linking metabolism to testicular function. In this regard, we evaluated the correlation between sperm mRNA abundance of adiponectin and its receptors, with sperm motility indices in the present study.
Materials and Methods
In this completely randomized design study, semen samples from 6 adult rams were fractionated on a two layer discontinuous percoll gradient into high and low motile sperm cells, then quantitative parameters of sperm motility were determined by computer-assisted sperm analyzer (CASA). The mRNA abundance levels of Adiponectin, AdipoR1 and AdipoR2 were measured quantitatively using real-time reverse transcriptase polymerase chain reaction (qRT-PCR) in the high and low motile groups.
Results
Firstly, we showed that adiponectin and its receptors (AdipoR1 and AdipoR2) were transcriptionally expressed in the ram sperm cells. Using Pfaff based method qRT- PCR, these levels of transcription were significantly higher in the high motile rather than low motile samples. This increase was 3.5, 3.6 and 2.5 fold change rate for Adiponectin, AdipoR1 and AdipoR2, respectively. Some of sperm motility indices [curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity (VAP), linearity (LIN), wobble (WOB) and straightness (STR)] were also significantly correlated with Adiponectin and AdipoR1 relative expression. The correlation of AdipoR2 was also significant with the mentioned parameters, although this correlation was not comparable with adiponectin and AdipoR1.
Conclusion
This study revealed the novel association of adiponectin system with sperm motility. The results of our study suggested that adiponectin is one of the possible factors which can be evaluated and studied in male infertility disorders.
Keywords: Adiponectin, AdipoR1, AdipoR2, Sperm Motility
Introduction
Adiponectin is a member of the adipose-secreted proteins, called adipocytokines. Adiponectin was initially described as a 30 kDa adipocyte complementrelated protein (1). It is a 244-amino acids protein and the most abundant adipose-derived hormone secreted by adipocytes in white adipose tissue with relevant roles in lipid metabolism and glucose homeostasis (2). Adiponectin also plays role on stimulation of fatty acid oxidation in the liver and skeletal muscle, suppression of hepatic gluconeogenesis, stimulation of glucose uptake in skeletal muscle and increasing insulin secretion (3). Following production, the actions of adiponectin are supported by two distinct but structurally related adiponectin receptors (AdipoR), AdipoR1 and AdipoR2 (4). The metabolic importance of these receptors is now firmly established. So that, AdipoR1(/) and AdipoR2(/) mouse models exhibited various disorders due to aberration in the fat and glucose metabolisms (5).
In addition to the well-known metabolic effects, it has been shown that adiponectin could affect the reproductive system, in part, through central actions on the hypothalamus-pituitary axis (6). Hypothalamic neurons secrete gonadotropin-releasing hormone (GnRH) with a pulsatile pattern, stimulating the release of pituitary gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These gonadotropins regulate testicular steroidogenesis and spermatogenesis (7). AdipoR1 and AdipoR2 are generally expressed in human hypothalamus and pituitary (8), so adiponectin can presumably be involved in the modulation of the endocrine reproductive axis in humans. Adiponectin and its receptors are also expressed by different cell types of the male gonad, suggesting a possible regulation of testicular function by adiponectin, through endocrine and/or paracrine actions. In chicken, presence of the adiponectin system (adiponectin, AdipoR1 and AdipoR2) was demonstrated in peritubular and seminiferous tubule cells (9). In line with this, testicular expression of adiponectin receptors was found to be higher at mRNA or protein levels in adult compared to prepubertal chickens and rats (9,10), suggesting that sexual maturation is likely associated with an increased sensitivity to the changes in plasma adiponectin levels. A significant, positive relationship was also reported between plasma adiponectin and high-density lipoprotein cholesterol in men (11) which may contribute to testosterone production. In this regard, Laughlin et al. (12) showed that, in the men and women, serum adiponectin is positively related to testosterone and high-density lipoprotein.
In general, the previous studies demonstrated a close relationship between adiponectin system and reproductive function. So, we hypothesized that the sperm Adiponectin, AdipoR1 and AdipoR2 mRNA abundances might correlate with sperm motility indices.
Materials and Methods
Semen samples and spermatozoa preparations
In this completely randomized design, testicles of 6 adult rams were collected from an official abattoir and transferred to the laboratory at room temperature (20-25°C). All procedures to sacrifice the animals were carried out at abattoir in accordance with Iranian government rules. Semen collection was carried out within the first 2 hours after the slaughter of the ram. Epididymis-testicle complexes were dissected into two parts: testicle, epididymis. Sperm was obtained by slicing the tissue of the cauda epididymis with a scalpel; the fluid was collected by sampler and the volume was estimated. To prohibit contamination, epididymis samples were carefully dissected free of blood clots and extraneous tissues. Care was taken to no cut blood vessels.
Semen samples were washed with Hepes-buffered tissue culture medium (Hepes TCM, Gibco, Life technologies, USA)+10% bovine serum albumin (BSA, Gibco, Life technologies, USA) and sperm suspensions were centrifuged at 500 g for 2 minutes. The supernatant was then discarded. This procedure repeated two times. The sperm of 6 rams was subsequently separated into low and high motility categories, as described below.
Sperm separation procedures
Sperm suspension was layered on a two-layer discontinuous Percoll gradient, consisting of 1 ml of 45% (v/v) and 2 ml of 90% (v/v) Percoll (Uppsala, Sweden) in a 15 ml conical plastic tube (Falcon No. 2095, Fisher Scientific, Pittsburg, USA). The tube was centrifuged at 700 g for 20 minutes. After centrifugation, the separated fractions in the tube were carefully transferred into a new set of tubes, and the volume of each fraction was determined.
Spermatozoa evaluation
The assessment of motility parameters was carried out, using computer-assisted sperm analysis (CASA, HooshmandFanavar, Iran). Samples were diluted (10- 20×106 cells/ml) in the same Hepes TCM medium with 320 mOsm/kg, and kept warm in the 37°C incubator during examination. Subsequently, 5 μl sample drop was placed into a Makler counting cell chamber (20 μm depth) and evaluated. Evaluation was carried out on both groups of the separated sperm by Percoll gradient.
The CASA settings were selected as follow: 6 visionfields per sample, 20 frames per second with the time analysis of less than 15 seconds per frame, 0-180 μm/ second analysis power for sperm velocity, and magnifying power of ×4 for microscope objective lens.
In CASA analysis results, sperm motility was divided into classes A, B, C and D as rapid motility, slow motility, non-progressive motility and immotility, respectively. Besides, the followed sperm motion parameter indices were studied: curvilinear velocity (VCL), the time-average of velocity along the actual trajectory for a spermatozoon in micrometers per second, straight line velocity (VSL) representing the average velocity of sperm from the first to last position of a sperm head in a track by micrometers per second. A straight-line path from the first to last position of a sperm head was plotted, and velocity along this trajectory was termed VSL (micrometers per second). The average path of sperm cell motion was also computed, and averagetime of velocity along the average path was calculated and named as average path velocity (VAP, micrometers per second). For each centroid location of sperm, there was a deviation from the average path, called as the amplitude of lateral head displacement (ALH, micrometers). Beat cross frequency (BCF) was the frequency of sperm cell’s head cross, through the sperm cell’s average pathway in Hertz. Similarly, there were points where the curvilinear path intersects the average path, and the number of such intersections was termed as BCF (number per second). The linearity (LIN) represents the linearity of a curvilinear path in percentage. The wobble (WOB) was the measure of the actual path oscillation with regard to the average path, and the mean angular displacement (MAD) was the average time for the instantaneous turning angle absolute values of the sperm head, along with the curvilinear trajectory in degree (13).
RNA extraction and cDNA synthesis of sperm cells
Total RNA isolation was carried out on sperm cells, according to the acid guanidiniumthiocyanatephenol-chloroform single-step extraction protocol, as described earlier (14). Treatment of total RNA with RNase-free DNase (Sinaclon Bioscience, Iran) was performed to avoid amplification of contaminating genomic DNA. The quality and integrity of the purified RNA was controlled by measurement of the A260/A280 nm ratio as well as agarose gel electrophoresis. Only RNA samples showing integrity by electrophoresis and exhibiting an A260/A280 ratio of >1.9 were used for synthesis of cDNA.
Total RNA was reverse transcribed into cDNA using moloney murine leukemia virus reverse transcriptase (M-MLV RT, Sinaclon Bioscience, Iran). The reverse transcribed mixture was incubated at 75ºC for 15 minutes to denature the RNA, and then stored at -20ºC.
Real-time quantitative reverse transcriptasepolymerase chain reaction analysis
The levels of all three transcripts (Adiponectin, AdipoR1 and AdipoR2) were determined by real-time reverse transcriptase polymerase chain reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was selected as a housekeeping gene to normalize the difference of input load of cDNA between the samples. Specific primers for cDNA of Adiponectin, AdipoR1, AdipoR2 and GAPDH were designed using primer BLAST (http://www.ncbi.nlm.nih.gov/primerblast). Nucleotide sequences of the selected primer pairs and the length of amplified product are presented in the Table 1.
Table 1.
Characteristics of the used primers
| Gene | NIH GenBank accession no. | Product length (bp) | Primer sequence 5´-3´ |
|---|---|---|---|
| GAPDH | NM_001190390.1 | 116 | F: GTTCCACGGCACAGTCAAGG |
| R: ACTCAGCACCAGCATCACCC | |||
| Adiponectin | KJ159213.1 | 132 | F: CGGCACCACTGGCAAATTCC |
| R: TGGTCGTGGGTGAAGAGCAG | |||
| AdipoR1 | KJ159212.1 | 131 | F: CAGGGGTGCAGGAGGAACTT |
| R: GTGGGCTGAAGCTTGGTTGG | |||
| AdipoR2 | KF921623.1 | 155 | F: GCATCGCAGCCATCATCGTC |
| R: GATGGTGGCAGCCTTCAGGA | |||
Real-time quantitative reverse transcriptasePCR (qRT-PCR) analysis was performed on Rotor-Gene Q 6000 System (Qiagen, USA) using SYBR premix EX Tag ІІ (Takara, China). One microliter of cDNA was added to the master-mix (0.5 µM of each specific primer, and 10 µl of SYBR premix EX Tag ІІReady Mix) in a total volume of 20 µl. Aliquot of each reaction mixture was analyzed by electrophoresis in 1.5% agarose gel and stained with 0.5 μg/ ml ethidium bromide. The relative quantification of three gene transcripts was determined in low and high motile sperm groups. Reaction condition was performed as 95°C for 5 minutes, 45 cycles of 95°C for 40 seconds, 63°C for 30 seconds and 72°C for 30 seconds. The PCR amplification was performed in triplicate for each sample.
Gene expression data were normalized to GAPDH (as internal reference gene). Data were analyzed using LinRegPCR software version 2012.0 (Amsterdam, Netherland), to give the threshold cycle (Ct) number . Mean efficiency values (E) for each gene were also determined from the amplification profiles of individual samples with this software (15).
The mRNA level of each target gene relative to GAPDH was estimated for each sample in two experimental groups by following formula: E GAPDH (Ct high motile)/E Adiponectin(Ct high motile). Then, the comparison was statistically done between groups. To determine fold change for each gene, the relative gene expression of high motile group relative to low motile group were calculated as following (16,17).
To ensure product homogeneity, the melting curve analysis was performed after the real-time PCR procedure. The fluorescence signals were recorded continuously during temperature ramp (65-95°C).
Statistical analysis
Differences between experimental group means were analyzed through paired t test with SPSS, version 16.0 (SPSS Inc., USA). The Pearson correlation procedure was used to evaluate correlation between the level of mRNA abundance and all quantitative sperm motion parameters for the indicated genes. All results are shown as mean ± SEM and differences were considered significant at P< 0.05.
Results
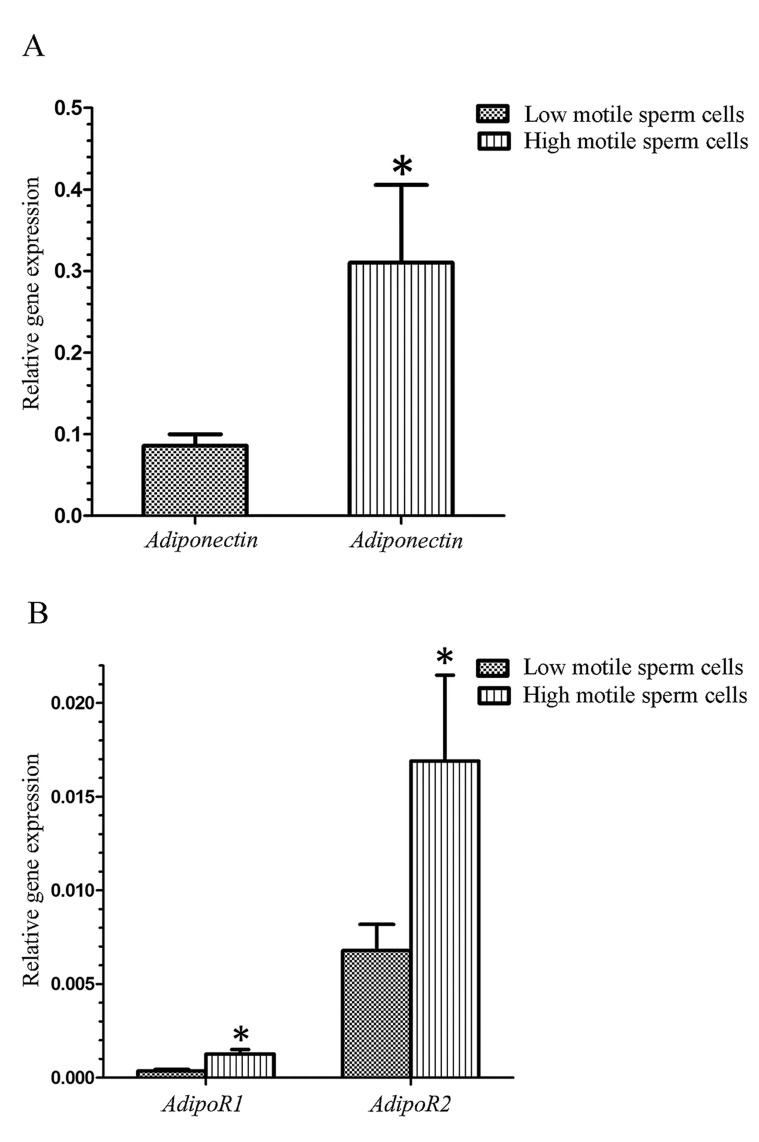
The results of CASA evaluation for sperm motility and sperm motility pattern are given in the Tables 2 and 3. After separation on Percoll gradient, the remaining sperm phase, in 45% Percoll, had significantly lower motile sperm cells (Table 2). The high motile sperm groups were also significantly better in other sperm motility parameters, such as VCL, VSL, VAP, LIN, WOB and STR (Table 3). This result showed that separation procedure was performed well. After separation, we analyzed the mRNA abundance of three genes between high and low motile sperm groups. As presented in the Figure 1, the mean level of Adiponectin, AdipoR1 and AdipoR2 gene abundances was significantly higher in high motile group than low motile. In the next step and for more evaluation, the correlation analysis was performed between the level of mRNA abundance and all sperm motion parameters for all three genes in motile and immotile sperm groups. In this analysis, all samples from high and low motile groups were considered together and the general correlation between motility indices and the level of mRNA abundance was calculated. The results of this analysis showed that mRNA abbundance for Adiponectin gene had a significant positive correlation with the class A of sperm motility, percent of progressive motile sperms, percent of motile sperms, VCL, VSL, VAP, LIN and WOB (Table 4). The amount of mRNA for AdipoR1 gene also showed a significant positive correlation with class A of sperm motility, percent of progressive motile sperms, LIN, WOB and STR (Table 4). For AdipoR2 gene, significant correlation was only observed with WOB.
Table 2.
Concentration, motility and progression of Percoll separated sperm samples (evaluated by CASA). Results are given as mean ± SE
| Groups | Sperm density (Mill/ml) | Motile sperm (%) | Progression (%) | |||
|---|---|---|---|---|---|---|
| Fast progressive (class A) | Slow progressive (class B) | Non progressive (class C) | Non motile (class D) | |||
| High motile (n=6) | 12.07 ± 2.56ns | 76.40 ± 2.27** | 58.53 ± 3.52**** | 12.01 ± 4.66ns | 5.85 ± 0.51ns | 23.60 ± 2.2** |
| Low motile (n=6) | 13.46 ± 1.73 | 58.49 ± 4.47 | 29.36 ± 2.41 | 16.34 ± 6.67 | 9.01 ± 3.85 | 44.00 ± 3.84 |
CASA; Computer-assisted sperm analyzer, ns; Not significant, **; P<0.01 and ****; P<0.0001.
Table 3.
Sperm motility pattern parameters of percoll separated sperm samples (evaluated by CASA). Results are given as mean ± SE
| Groups | Sperm motility pattern | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| VCL(μm/s) | VSL(μm/s) | VAP(μm/s) | MAD(%) | ALH(%) | BCF (%) | LIN (%) | WOB (%) | STR (%) | |
| High motile (n=6) | 80.93 ± 9.66* | 57.13 ± 8.47** | 65.58 ± 8.66* | 20.20 ± 3.28 ns | 3.03 ± 0.18 ns | 2.49 ± 0.77 ns | 56.53 ± 2.56** | 71.24 ± 1.59*** | 71.52 ± 1.95** |
| Low motile (n=6) | 49.75 ± 7.78 | 23.65 ± 3.06 | 32.39 ± 5.66 | 13.86 ± 3.18 | 2.67 ± 0.26 | 1.73 ± 0.75 | 36.31 ± 3.48 | 54.64 ± 2.66 | 57.19 ± 2.97 |
CASA; Computer-assisted sperm analyzer, VCL; Curvilinear velocity, VSL; Straight-line velocity, VAP; Average path velocity, MAD; Mean angular displacement, ALH; Amplitude of lateral head displacement, BCF; Beat cross frequency, LIN; Linearity, WOB; Wobble, STR; Straightness, ns; Not significant, *; P<0.05, **; P<0.01 and ***; P<0.001.
Table 4.
Correlations between the amount of relative gene abundance for Adiponectin, AdipoR1 and AdipoR2 with quantitative sperm motion parameters
| Groups | Sperm motility pattern | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS(%) | Class A(%) | SPM(%) | VCL(μm/s) | VSL(μm/s) | VAP(μm/s) | MAD(%) | ALH(%) | BCF (%) | LIN (%) | WOB (%) | STR (%) | |
| Adiponectin relative abundance | 0.51* | 0.66* | 0.56* | 0.54* | 0.60** | 0.61** | -0.10ns | 0.03ns | 0.18ns | 0.53* | 0.64** | 0.06 ns |
| AdipoR1 relative abundance | 0.19ns | 0.44* | 0.44* | 0.47ns | 0.19ns | 0.23ns | 0.67ns | -0.014ns | 0.56ns | 0.46* | 0.55** | 0.47* |
| AdipoR2 relative abundance | 0.21ns | 0.36ns | 0.26ns | 0.10ns | 0.15ns | 0.16ns | -0.03ns | 0.11ns | 0.28ns | 0.32ns | 0.45* | 0.26ns |
MS; Motile sperm; Class A; Sperm with fast progressive motility, SPM; Sperm with progressive motility (class A+class B), VCL; Curvilinear velocity, VSL; Straight-line velocity, VAP; Average path velocity, MAD; Mean angular displacement, ALH; Amplitude of lateral head displacement, BCF; Beat cross frequency, LIN; Linearity, WOB; Wobble, STR; Straightness, ns; Not significant, *; P<0.05 and **; P<0.01.
Fig.1.
Relative abundance of A. Adiponectin, B. AdipoR1 and AdipoR2 mRNA in low and high motile sperm groups.
*; Significant difference between two groups.
Discussion
In the present study, associations between sperm mRNA abundance of Adiponectin and its receptors, AdipoR1 and AdipoR2, with quantitative parameters of sperm motility were evaluated in rams. Adiponectin is a plasma protein with about 0.01% of total serum proteins concentration (18). The primary amino acid sequences of Adiponectin are highly conserved across the species (19). For example, bovine adiponectin shows 92% homology with human Adiponectin and 82% homology with murine Adiponectin (20). Adiponectin is synthesized as a single monomer which undergoes multimerization to provide three multimer forms with different molecular weights (MWs): i. Low molecular weight (LMW) Adiponectin composed of three monomers that are combined to form a trimmer, ii. Middle molecular weight (MMW) Adiponectin, as a hexamer formed by two trimmers, and iii. High molecular weight (HMW) multimer of Adiponectin, comprised of 12-18 monomers.
Ejaculated sperm retains a complex and specific population of RNAs. It was recently proposed that these RNA molecules may have important roles in the sperm development, chromatin repackaging and even zygote development (21). Studies on sperm RNA are available for humans (22), stallion (23), cattle (24) and boars (25). Analysis of the mRNA profiles in the normal and abnormal sperms is a growing field, which can become a diagnostic and prognostic tool to evaluate male fertility and can eventually lead to identify specific genetic pathways which are necessary for production of the fertile sperm. For example, studies have been conducted to compare the genetic profiles of sperm samples from normal fertile men and teratozoospermic patients (26,27).
In the present study, the mRNA abundances of all three components of adiponectin system were significantly higher in the high motile sperm groups. The mRNA abundances also positively correlated with some of the most important parameters of sperm motility pattern, especially for Adiponectin and AdipoR1. Adiponectin and the relevant receptors play major roles in sperm morphology and function, contributing to increase fertility. A recent study by Kasimanickam et al. (28) showed that Adiponectin, AdipoR1, and AdipoR2 were immunolocalized in the acrosomal, postacrosomal, equatorial, and tail regions of bull sperm. In this study, serum Adiponectin concentration and sperm mRNA expressions for Adiponectin and its receptors showed a significant positive correlation with sire conception rate. In ram, transcripts for adiponectin system components, have been detected in the testis, all parts of epididymis, vesicular and bulbourethral glands (29). Expression of Adiponectin receptors was also reported in porcine epididymis (30).
Our results showed a novel evidence for the presence of Adiponectin and its two receptors in at least sperm cells from cauda-epididymides. In this context, Rahmanifar and Tabandeh (29) also determined that Adiponectin, AdipoR1 and AdipoR2 were expressed in all parts of epididymides (caput, corpus, and cauda), while AdipoR2 mRNA expression was higher than AdipoR1. These results, in addition to the finding reported by Kasimanickam et al. (28) indicating that gene expression of Adiponectin and its receptors during preand post-capacitation in spermatozoa, provide evidences of possible production of fertile sperm by local actions of Adiponectin at the testis level.
In this regard, it should be noted that a spermspecific ATP-binding cassette (ABC) transporter regulates intracellular lipid metabolism in rodents (31). Kitajima et al. (32) showed that Adiponectin and its receptors (AdipoR1 and AdipoR2) increased cholesterol efflux and reconstituted high-density lipoprotein-induced efflux, at least partially through an ABCA1 pathway. In that study, AdipoR1and AdipoR2-transfected cells showed greater cholesterol efflux when treated with Adiponectin. In contrast, down-regulation of adiponectin receptors decreased reconstituted high-density lipoprotein-induced cholesterol efflux. Adiponectin related signaling pathways in the sperm cell are not well studied until now. But Adiponectin and its receptors might participate in cholesterol efflux via a sperm-specific ABC transporter and thereby affect sperm hyperactivation and capacitation (33).
The positive correlation of obesity with male infertility could be the evidence of clinical importance of Adiponectin in the fertility. In rodents, the obesity leads to sub-fertility caused by reduced sperm motility (34). Obese men have also reduced sperm concentration and total sperm count (35). In an interesting study performed by Thomas et al. (36), normal-weight men showed higher concentrations of Adiponectin in the seminal plasma and blood serum. In addition, Adiponectin concentration in seminal plasma positively correlated with sperm concentration and normal morphology of spermatozoa. Hammoud et al. (37) also found that asthenozoospermia and oligozoospermia were increased due to high body-mass index and worsened from overweight to obese men. One possible reason can be the correlation between plasma Adiponectin and testosterone concentrations. In this regard, Ribot et al. (38) confirmed that the diet-induced obesity in rats leads to decrease in the effective production of Adiponectin. Studies showed that Adiponectin played roles in gonadal steroidogenesis. As a paralog of Adiponectin, CTRP3 (a member of the C1q/TNF-related protein superfamily) was expressed at high level in the adipose tissue. In adult mouse testis, CTRP3 was expressed in Leydig cells and contributes to increase testosterone production by up-regulating Cyp11A1 and Star protein expressions (39). Interestingly, Adiponectin has been shown to regulate the expression of steroidogenic genes (Star, Cyp11Aa1 and Cyp19A1) in human, rat, chicken and swine ovary (40,41), suggesting that Adiponectin might affect steroidogenesis in Leydig cells through regulation of steroidogenic gene expressions as well. This finding was also confirmed by Erdemir et al. (42). In this study, the male fat rats, had significantly lower levels of testosterone compared to the controls. In terms of pathologic evaluation, Johnsen Score (a 1-10 degree score for microscopic evaluation of spermatogenesis quality in testicular tissue) was significantly lower in the fat male rats.
Conclusion
The findings indicated that the products of Adiponectin gene may be involved in the physiology of sperm cell movement. Although the exact role of Adiponectin in the male reproductive system remains hypothetical, demonstrated expression of this gene in epididymal spermatozoa in this study and all parts of epididymidis suggests a possible role of Adiponectin in maturational spermatozoa changes, as they transit the duct.
Moreover, considering the demonstrated correlation between obesity and fertility impairment, the results of such studies will help to find the molecular mechanisms involved in the pathogenesis of this disorder.
Acknowledgments
This work was financially supported by the Applied Research Centre, Vice Chancellor for Research of Shahrekord University. All authors declare that they have no conflicts of interest.
References
- 1.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Budak E, Fernández Sánchez M, Bellver J, Cerveró A, Simón C, Pellicer A. Interactions of the hormones leptin, ghrelin, adiponectin, resistin, and PYY3-36 with the reproductive system. Fertil Steril. 2006;85(6):1563–1581. doi: 10.1016/j.fertnstert.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 4.Martin LJ. Implications of adiponectin in linking metabolism to testicular function. Endocrine. 2014;46(1):16–28. doi: 10.1007/s12020-013-0102-0. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148(1):401–410. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 7.Maruska KP, Fernald RD. Social regulation of gene expression in the hypothalamic-pituitary-gonadal axis. Physiology. 2011;26(6):412–423. doi: 10.1152/physiol.00032.2011. [DOI] [PubMed] [Google Scholar]
- 8.Psilopanagioti A, Papadaki H, Kranioti EF, Alexandrides TK, Varakis JN. Expression of adiponectin and adiponectin receptors in human pituitary gland and brain. Neuroendocrinology. 2008;89(1):38–47. doi: 10.1159/000151396. [DOI] [PubMed] [Google Scholar]
- 9.Ocon-Grove OM, Krzysik-Walker SM, Maddineni SR, Hendricks GL, Ramachandran R. Adiponectin and its receptors are expressed in the chicken testis: influence of sexual maturation on testicular ADIPOR1 and ADIPOR2 mRNA abundance. Reproduction. 2008;136(5):627–638. doi: 10.1530/REP-07-0446. [DOI] [PubMed] [Google Scholar]
- 10.Caminos JE, Nogueiras R, Gaytan F, Pineda R, Gonzalez CR, Barreiro ML, et al. Novel expression and direct effects of adiponectin in the rat testis. Endocrinology. 2008;149(7):3390–3402. doi: 10.1210/en.2007-1582. [DOI] [PubMed] [Google Scholar]
- 11.Pfaehler A, Nanjappa MK, Coleman ES, Mansour M, Wanders D, Plaisance EP, et al. Regulation of adiponectin secretion by soy isoflavones has implication for endocrine function of the testis. Toxicol Lett. 2012;209(1):78–85. doi: 10.1016/j.toxlet.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin GA, Barrett-Connor E, May S. Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int J Obes(Lond) 2007;31(3):457–465. doi: 10.1038/sj.ijo.0803427. [DOI] [PubMed] [Google Scholar]
- 13.Amann RP, Waberski D. Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology. 2014;81(1):5–17. doi: 10.1016/j.theriogenology.2013.09.004. e1-3. [DOI] [PubMed] [Google Scholar]
- 14.Hassanpour H, Yazdani A, Khabir Soreshjani K, Asgharzadeh S. Evaluation of endothelial and inducible nitric oxide synthase genes expression in the heart of broiler chickens with experimental pulmonary hypertension. Br Poult Sci. 2009;50(6):725–732. doi: 10.1080/00071660903141005. [DOI] [PubMed] [Google Scholar]
- 15.Ruijter J, Ramakers C, Hoogaars W, Karlen Y, Bakker O, Van den Hoff M, et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6):e45–e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorak MT. Real-time PCR. 1st ed. New York: Taylor & Francis; 2007. pp. 66–68. [Google Scholar]
- 17.Pfaffl MW. Quantification strategies in real-time PCR. In: Bustin SA, editor. AZ of quantitative PCR. 1st ed. San Diego, CA: International University Line; 2004. pp. 89–113. [Google Scholar]
- 18.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008;409(3):623–633. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 20.Sato C, Yasukawa Z, Honda N, Matsuda T, Kitajima K. Identification and adipocyte differentiation-dependent expression of the unique disialic acid residue in an adipose tissue-specific glycoprotein, adipo Q. J Biol Chem. 2001;276(31):28849–28856. doi: 10.1074/jbc.M104148200. [DOI] [PubMed] [Google Scholar]
- 21.Miller D, Ostermeier GC, Krawetz SA. The controversy, potential and roles of spermatozoal RNA. Trends Mol Med. 2005;11(4):156–163. doi: 10.1016/j.molmed.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Goodrich R, Johnson G, Krawetz SA. The preparation of human spermatozoal RNA for clinical analysis. Arch Androl. 2007;53(3):161–167. doi: 10.1080/01485010701216526. [DOI] [PubMed] [Google Scholar]
- 23.Das PJ, Paria N, Gustafson-Seabury A, Vishnoi M, Chaki SP, Love CC, et al. Total RNA isolation from stallion sperm and testis biopsies. Theriogenology. 2010;74(6):1099–1106. doi: 10.1016/j.theriogenology.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 24.D'Amours O, Frenette G, Fortier M, Leclerc P, Sullivan R. Proteomic comparison of detergent-extracted sperm proteins from bulls with different fertility indexes. Reproduction. 2010;139(3):545–556. doi: 10.1530/REP-09-0375. [DOI] [PubMed] [Google Scholar]
- 25.Yang CC, Lin YS, Hsu CC, Wu SC, Lin EC, Cheng WT. Identification and sequencing of remnant messenger RNAs found in domestic swine (Sus scrofa) fresh ejaculated spermatozoa. Anim Reprod Sci. 2009;113(1-4):143–155. doi: 10.1016/j.anireprosci.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 26.Moldenhauer JS, Ostermeier GC, Johnson A, Diamond MP, Krawetz SA. Diagnosing male factor infertility using microarrays. J Androl. 2003;24(6):783–789. doi: 10.1002/j.1939-4640.2003.tb03122.x. [DOI] [PubMed] [Google Scholar]
- 27.Ostermeier GC, Dix DJ, Miller D, Khatri P, Krawetz SA. Spermatozoal RNA profiles of normal fertile men. Lancet. 2002;360(9335):772–777. doi: 10.1016/S0140-6736(02)09899-9. [DOI] [PubMed] [Google Scholar]
- 28.Kasimanickam VR, Kasimanickam RK, Kastelic JP, Stevenson JS. Associations of adiponectin and fertility estimates in Holstein bulls. Theriogenology. 2013;79(5):766–777. doi: 10.1016/j.theriogenology.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Rahmanifar F, Tabandeh MR. Adiponectin and its receptors gene expression in the reproductive tract of ram. Small Ruminant Res. 2012;105(1):263–267. [Google Scholar]
- 30.Dai MH, Xia T, Zhang G, Chen XD, Gan L, Feng SQ, et al. Cloning, expression and chromosome localization of porcine adiponectin and adiponectin receptors genes. Domest Anim Endocrinol. 2006;30(2):117–125. doi: 10.1016/j.domaniend.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Ban N, Sasaki M, Sakai H, Ueda K, Inagaki N. Cloning of ABCA17, a novel rodent sperm-specific ABC (ATPbinding cassette) transporter that regulates intracellular lipid metabolism. Biochem J. 2005;389(Pt 2):577–585. doi: 10.1042/BJ20050159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitajima K, Miura SI, Yamauchi T, Uehara Y, Kiya Y, Rye KA, et al. Possibility of increasing cholesterol efflux by adiponectin and its receptors through the ATP binding cassette transporter A1 in HEK293T cells. Biochem Biophys Res Commun. 2011;411(2):305–311. doi: 10.1016/j.bbrc.2011.06.131. [DOI] [PubMed] [Google Scholar]
- 33.Naseer Z, Ahmad E, Aksoy M. Cholesterol efflux from sperm: approaches and applications. Turk J Vet Anim Sci. 2014;38(6):653–659. [Google Scholar]
- 34.Fernandez CD, Bellentani FF, Fernandes GS, Perobelli JE, Favareto AP, Nascimento AF, et al. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32–32. doi: 10.1186/1477-7827-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mah PM, Wittert GA. Obesity and testicular function. Mol Cell Endocrinol. 2010;316(2):180–186. doi: 10.1016/j.mce.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Thomas S, Kratzsch D, Schaab M, Scholz M, Grunewald S, Thiery J, et al. Seminal plasma adipokine levels are correlated with functional characteristics of spermatozoa. Fertil Steril. 2013;99(5):1256–1263. doi: 10.1016/j.fertnstert.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 37.Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008;90(6):2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 38.Ribot J, Rodríguez AM, Rodríguez E, Palou A. Adiponectin and resistin response in the onset of obesity in male and female rats. Obesity. 2008;16(4):723–730. doi: 10.1038/oby.2008.113. [DOI] [PubMed] [Google Scholar]
- 39.Otani M, Kogo M, Furukawa S, Wakisaka S, Maeda T. The adiponectin paralog C1q/TNF-related protein 3 (CTRP3) stimulates testosterone production through the cAMP/ PKA signaling pathway. Cytokine. 2012;58(2):238–244. doi: 10.1016/j.cyto.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian theca and granulosa cell function. Mol Cell Endocrinol. 2008;284(12):38–45. doi: 10.1016/j.mce.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Richards JS, Liu Z, Kawai T, Tabata K, Watanabe H, Suresh D, et al. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil Steril. 2012;98(2):471–479. doi: 10.1016/j.fertnstert.2012.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erdemir F, Atilgan D, Markoc F, Boztepe O, Suha-Parlaktas B, Sahin S. The effect of diet induced obesity on testicular tissue and serum oxidative stress parameters. Actas Urol Esp. 2012;36(3):153–159. doi: 10.1016/j.acuro.2011.06.019. [DOI] [PubMed] [Google Scholar]