Abstract
Background
Dyslipidemia and insulin resistance (IR), occurring in most infertile women with polycystic ovarian syndrome (PCOS), increase the risk of cardiovascular disease (CVD) and type 2 diabetes. This study aimed to assess the relationships between lipoprotein ratios and IR in PCOS women.
Materials and Methods
Thirty six infertile women with PCOS selected based on Androgen Excess Society (AES) criteria and 29 healthy women matched for age were recruited to this case-control study. After physical measurements, fasting serum glucose (Glu), insulin and lipid profile levels [triglycerides (TGs), total cholesterol (TC), low-density lipoproteincholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C)] were measured, while lipoprotein ratios (TC/HDL-C, LDL-C/HDL-C, TG/HDL-C) were calculated. IR was also calculated using homeostasis model assessment (HOMA)-IR. The optimal cutoffs of lipoprotein ratios in relation to HOMA-IR were calculated based on the Receiver Operating Characteristics (ROC) curve analysis using the area under curve (AUC).
Results
Waist circumference (WC), insulin levels, HOMA-IR, TG levels, and all lipoprotein ratios were significantly higher, while HDL-C was lower in PCOS group as compared to healthy controls. All lipoprotein ratios, TG levels, and WC are significantly correlated with insulin levels and HOMA-IR. Among lipoprotein ratios, the highest AUC of the ROC belonged to TG/HDL-C ratio with sensitivity of 63.6% and specificity of 84.4% (TG/HDL-C>3.19) as a marker of IR in infertile PCOS women.
Conclusion
Lipoprotein ratios, particularly TG/HDL-C, are directly correlated with insulin levels and can be used as a marker of IR (HOMA-IR) in infertile PCOS patients.
Keywords: Lipoprotein, Infertility, PCOS, Insulin Resistance
Introduction
Polycystic ovary syndrome (PCOS) is the most common gynecological endocrinopathy disorder and the frequent cause of oligo-ovulatory infertility (1). Abnormalities with ovulation are the cause of infertility in about one third of couples attending infertility clinics that count for 90% of these cases (2). PCOS is generally characterized by chronic anovulation, hyperandrogenism and ovarian polycystic changes that are detected by an ultrasound scan in a clinic (3). The estimated prevalence of PCOS based on the criteria used for diagnosis and recruitment process of the study population has been reported between 2.2 and 26% in different countries (4). Although the etiology of PCOS is still unknown, it has been demonstrated that PCOS is a metabolic disorders rather than a reproductive endocrine disease (3). Insulin is a key component in the pathophysiology of PCOS (1). On average, PCOS patients have higher triglyceride (TG), lower high density lipoprotein-cholesterol (HDL-C) and higher low density lipoprotein-cholesterol (LDL-C) levels than their non-PCOS matched group (5). Insulin resistance (IR), hyperinsulinaemia and dyslipidemia are diagnosed among 50 to 70% of patients with PCOS (6). There is a drastic improvement in PCOS complication when is accompanied by modulation of IR (1). Therefore, PCOS is associated with increased risk of metabolic abnormalities, indicating that the patients are at the risk of developing type 2 diabetes and cardiovascular disease (CVD) (3).
Despite modern treatment options for infertilities and considering economic aspects, it is reasonable to give specific attention to cost effective and easily applied methods for predicting metabolic abnormalities at population level (7). Routine methods for measuring IR are hyperinsulinemiceuglycemic clamp technique (a gold standard to assess insulin sensitivity) (8), homeostasis model assessment (HOMA)-IR, Bennett index, Li Guangwei index, quantitative insulin sensitivity check index (QUICKI), and fasting serum glucose (Glu)/insulin ratio (G/I). Due to being complex, expensive and time-consuming, the latter methods are of limited use in clinical and epidemiological studies (9). Thus for daily clinical practice, it is necessary to use other methods for measuring IR, which are lower in costs and applicable to the general population.
In order to provide a new idea to evaluate IR in infertilities associated with PCOS, the possibility of establishing the values of total cholesterol (TC)/ HDL-C, TG/HDL-C, and LDL-C/HDL-C ratios, waist circumference (WC) as surrogates, as well as LDL-C, TC, and TG levels to estimate insulin levels and IR was investigated. By using Receiver Operating Characteristic (ROC) curves in our subjects, the accuracy of the mentioned parameters was recived.
Materials and Methods
Subjects
In this case-control study, subjects were selected among women aged 19 to 35 years who visited a private reproductive medical center, Tabriz, Iran, during the period of February till April 2013, for infertility due to PCOS. Selection was done by the standardized protocol for the initial evaluation. A total of 35 patients were identified as PCOS cases according to the Androgen Excess Society (AES, 2006) criteria (1), while 29 age-matched healthy women (without any infertility and PCOS disorders) were recruited in the study as the control group. Inclusion criteria for case group were as follows: married, clinical and/or biochemical hyperandrogenism, and ovarian dysfunction (oligoanovulation and/or polycystic ovaries detected by ultrasound scans). Exclusion criteria were as follows: congenital adrenal hyperplasia, androgensecreting tumors, taking androgenic/anabolic medications, Cushing syndrome, severe IR syndrome, thyroid dysfunction, hyperprolactinemia, diabetes, hypertension, CVD, taking vitamins and supplements during the 3 months prior to the study, evidence of recent or recurrent infection, and smoking or drinking alcohol.
Physical measurements
Body weight was measured without shoes with minimal amount of clothing using a digital scale (SECA, Germany) to the nearest 0.1 kg. Height was measured using a non-stretchable stadiometer (SECA, Germany) to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight in kg divided to height in squared meters. WC was measured at the midpoint between the lowest rib and the top of the lateral border of iliac crest during minimal respiration. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using Spot Vital Signs Device (Welch Allyn, USA). Participants were asked to lie down and relax for approximately 8 to 10 minutes, after which three blood pressure measurements were recorded at fiveminute intervals.
Blood analysis
After 12-hour overnight fast, blood samples were collected. Serum and plasma samples were separated using a centrifuge (Beckman Coulter Inc., USA) at 1500 rpm for 15 minutes. Fasting insulin levels were measured using enzyme-linked immunosorbent assay (ELISA) kits (Monobind Inc., USA). Fasting plasma Glu was measured using enzymatic procedures by an automatic analyzer (Abbott, USA). IR was estimated by HOMA using the following formula: HOMA- IR=fasting insulin (μU/ml)×fasting Glu (mg/dl)/405 (10). The concentrations of TC and TG were measured using enzymatic procedure with commercial kits (Pars Azmon, IRI), while HDL-C was measured by a direct method using polyethylene-glycol-pretreated enzymes by an automatic analyzer (Abbott, USA). LDL-C was calculated using Friedewald’s formula (11). Lipoprotein ratios (TC/ HDL-C, TG/HDL-C and LDL-C/HDL) were then calculated.
Ethical considerations
This study was approved by the Medical Ethics Committee of Ahvaz Jundishapur University and all participants gave an informed consent before commencing the study. The code of Ethics Committee is ETH-702, and registered code of study is NRC-9110.
Statistical analysis
Results were expressed as mean ± SD. Levene’s test for equality of variances was used. The differences between concerning continuous and categorical variables were analyzed using unpaired t test (or Mann-Whitney U test for non-normally distributed data) and λ2 test, respectively. Correlations were determined by Spearman correlation coefficient method. ROC curves were used to estimate the sensitivity and specificity of serum lipoprotein ratios to diagnose IR. P values less than 0.05 were considered statistically significant. All statistical analyses were performed using Statistical Package for Social Sciences 20.0 (SPSS, SPCC Inc., USA) software.
Results
The control group was matched with the patient group for age. Although the values of BMI, BP, TC, LDL-C, TG and fasting serum Glu were found to be higher in the infertile PCOS group than in the control group, indicating that these differences were not statistically significant. A higher insulin level and HOMA-IR value were observed in patients group compared to the control group (P<0.001 and P=0.024, respectively). TG levels (P=0.009) as well as the values of TC/ HDL (P=0.002), TG/HDL (P=0.047), LDL/HDL (P=0.002) and WC (P<0.001) were significantly higher, while HDL-C levels (P=0.003) were lower in the cases compared to those of their healthy counterparts. The results are shown in Table 1.
HOMA-IR value in the patients showed a positive correlation with TG levels (r=0.56, P<0.01) as well as the values of TC/HDL-C (r=0.34, P<0.05), TG/HDL-C (r=0.49, P<0.01), LDL-C/HDL-C (r=0.33, P<0.05), and WC (r=0.37, P<0.05). However, HOMA-IR value showed no significant correlation with TC, LDL and HDL concentrations. Serum insulin levels are positively correlated with TG level (r=0.46, P<0.01), TC level (r=0.33, P<0.05), and TG/HDL value (r=0.39, P<0.05). We found no significant correlation between serum insulin levels and LDL-C, HDL-C, TC/HDL, LDL/ HDL and WC values in our patients. The results are shown in Table 2.
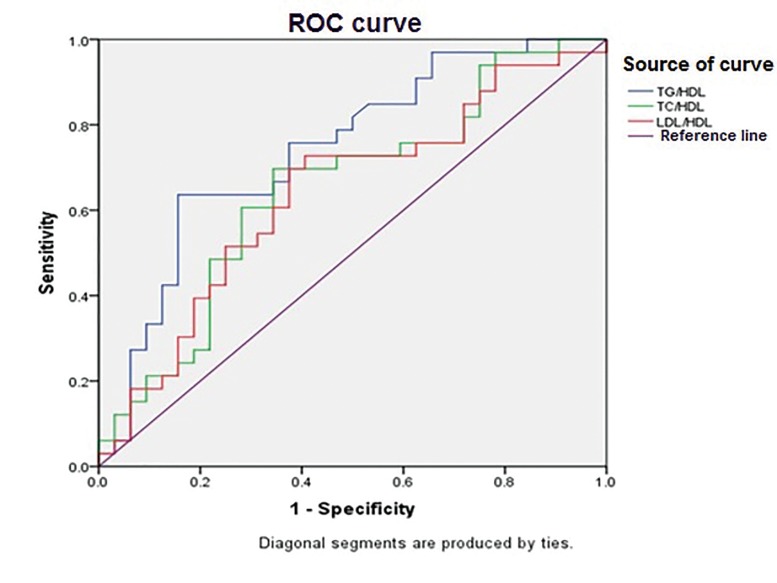
According to the ROC curve analysis, all lipid ratios (TG/HDL-C, TC/HDL-C, and LDL/HDLC) showed an area under curve (AUC) greater than 0.5. Thus, as an effective diagnostic marker for IR in PCOS patients, the AUC of TG/HDL-C was the highest with sensitivity of 63.6% and specificity of 84.4% (TG/HDL-C>3.19). The results are shown in Table 3 and Figure 1.
Table 1.
Baseline and clinical characteristics of two groups (age range 19-35 years)
| Variables | Infertile PCOS (n=36) | Healthy control (n=29) | P valueaa |
|---|---|---|---|
| Age | 26.36 ± 4.2 | 27.96 ± 2.47 | 0.107 |
| BMI (kg/m2) | 26.72 ± 4.39 | 25.55 ± 4.3 | 0.286 |
| BMI (%)c BMI≥25 | 72.2 | 48.3 | 0.049 |
| WC (cm) | 94.77 ± 10.36 | 85.06 ± 8.48 | <0.001 |
| SBP (mmHg) | 118.66 ± 8.98 | 116.89 ± 6.03 | 0.209 |
| DBP (mmHg) | 78.19 ± 6.98 | 76.37 ± 5.15 | 0.274 |
| Fasting serum Glu (mg/dL) | 94.47 ± 11.88 | 89.86 ± 8.25 | 0.081 |
| Insulin (μU/mL)b | 21.41 ± 14.14 | 16.24 ± 11.55 | 0.029 |
| HOMA-IRb | 5.16 ± 3.72 | 3.41 ± 2.53 | 0.024 |
| TC (mg/dL) | 214.83 ± 43.97 | 202.68 ± 46.44 | 0.285 |
| TG (mg/dL) | 139.28 ± 66.98 | 98.17 ± 50.72 | 0.009 |
| HDL-C (mg/dL) | 42.88 ± 10.2 | 52.06 ± 13.71 | 0.003 |
| LDL-C (mg/dL) | 143.69 ± 36.25 | 129.51 ± 35.70 | 0.119 |
| TC/HDL-C ratio | 5.16 ± 1.22 | 4.11 ± 1.36 | 0.002 |
| TG/HDL-C ratio | 3.62 ± 2.17 | 2.44 ± 2.52 | 0.047 |
| LDL-C/HDL-C ratio | 3.44 ± 0.98 | 2.62 ± 1.02 | 0.002 |
PCOS; Polycystic ovarian syndrome, BMI; Body mass index, WC; Waist circumference, SBP; Systolic blood pressure, DBP; Diastolic blood pressure, Glu; Glucose, HOMA-IR; Homeostasis model assessment of insulin resistance, TC; Total cholesterol, TG; Triglyceride, HDL-C; High density lipoprotein-cholesterol, LDL-C; Low density lipoprotein-cholesterol, a; Statistical analyses performed by unpaired t test for comparison, b; Statistical analyses performed by Mann-Whitney U test and c; Statistical analyses performed by Chi-squared test. Data are the mean ± SD.
Table 2.
Spearmanʼs correlations of lipid profile, lipoprotein ratios and WC values with serum insulin level and IR in infertile women with PCOS
| Variables | Serum insulin levels | IR |
|---|---|---|
| TGs | 0.46b | 0.56b |
| TC | 0.33a | 0.316 |
| LDL-C | 0.29 | 0.28 |
| HDL-C | 0.14 | 0.08 |
| TC/HDL | 0.3 | 0.34a |
| TG/HDL | 0.39a | 0.49b |
| LDL/HDL | 0.28 | 0.33a |
| WC | 0.32 | 0.37a |
IR; Insulin resistance, PCOS; Polycystic ovary syndrome, WC; Waist circumference, TC; Total cholesterol, TG; Triglyceride, HDL-C; High density lipoprotein-cholesterol, LDL-C; Low density lipoprotein-cholesterol, a; P<0.05 and b; P<0.01.
Table 3.
Serum lipoprotein ratios, AUC, cut-off points and sensitivity and specificity calculated from ROC curves for the detection of PCOS with IR
| Serum lipoprotein ratios | AUC± SE | 95% CI | Cut-off point | Infertile PCOS patients | P value | |
|---|---|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | |||||
| TG/HDL | 0.743 ± 0.062 | 0.622-0.864 | 3.19 | 63.6 | 84.4 | 0.001 |
| TC/HDL | 0.651 ± 0.069 | 0.515-0.786 | 4.37 | 69.7 | 65.6 | 0.037 |
| LDL/HDL | 0.638 ± 0.070 | 0.502-0.775 | 2.84 | 69.7 | 63.5 | 0.055 |
CI; Confidence interval, IR; Insulin resistance, AUC; Area under curve area, ROC; Receiver operating characteristic, PCOS; Polycystic ovarian syndrome, TC; Total cholesterol, TG; Triglyceride, HDL; High density lipoprotein and LDL; Low density lipoprotein.
Fig 1.
ROC curves of serum lipoprotein ratios for detection of insulin resistance in PCOS cases. Diagonal segments are produced by ties. PCOS; Polycystic overian syndrome and ROC; Receiver operating characteristic.
Discussion
Among the factors responsible for a reduction in fecundity and successful pregnancy, the hormonal changes associated with various factors are considered as an important cause for interrupting normal ovulatory menstrual cycles (12). Among these factors, visceral adiposity is a common finding in PCOS patient, even when the subjects are not classified as overweight (25<BMI<29.9) (13). According to our findings, we observed a significant different in WC between groups. Although the difference in BMI index between cases and controls was not significant, but in case group, percentage of overweight BMI was higher than that of controls. Also there was a significantly positive correlation between WC and IR. Our Findings are in agreement with those of some previous studies (13-15). However, the latter results differ from those of the study conducted by Iuhas et al. (16). In their study, visceral fat area showed no significant difference in PCOS and healthy subjects, which might be due to the difference in method of measuring visceral fat and larger sample size. Pathophysiology of PCOS is unknown. It is regarded as an endocrinal disorder due to IR, which presents in about 70% of PCOS patients (17, 18). In PCOS patients, IR is mostly associated with dyslipidemia. Methods used for measuring IR are mostly sophisticated and expensive that are not applicable for epidemiological studies. Hence, more reasonable methods for IR measurements have been investigated in several studies, of which lipoprotein ratios were proposed for the identification of IR as an alternative method. Our investigation was carried out in order to provide evidences for the application of lipoprotein ratios as an indicator of IR in infertile PCOS women. In this investigation, PCOS was diagnosed by AES criteria, while for the first time, subjects were selected among infertile PCOS women.
This study showed the case group had higher TG levels and lower HDL-C levels compared to control group. While no significant difference was detected in TC and LDL-C levels between groups. Most studies have shown low levels of HDL-C in women with PCOS, but composition of HDL in PCOS is still unknown. There is still a need for further studies in order to determination of the HDL-C composition in these patients. One of the mechanisms that could explain the observed difference is the activity of hepatic lipase (HL) enzyme induced by IR and hyperandrogenemia, which removes lipid from HDL and plays as a key role of the lipid-depleted HDL particles in PCOS patients. Also insulin-resistant states along with low HDL levels are frequently associated with hypertriglyceridemia. However, another possible mechanism of dyslipidemia in PCOS could be a reduction in clearance of triglyceride-rich proteins (19).
Result of this study demonstrated a significant association in TC/HDL-C, TG/HDL-C and LDLC/ HDL-C ratios and TG with IR (HOMA-IR) in PCOS patients. In a study on women with PCOS, Xiang et al. (20) also suggested that serum lipoprotein ratios could be used as a marker of IR due to the significant positive correlation of the indices with IR. However, in their study, Rotterdam criteria were used for diagnosing PCOS, so there was a significant difference in terms of BMI between case and control groups, which could be a confounding factor. Hence the present study was designed more specifically by use of updated criteria (AES) on infertile women for diagnosing PCOS. Moreover in our study BMI was not significantly different between case and control groups, which could justify the confounding impact of BMI on results. Serum lipoprotein ratios were also reported to be significantly correlated to IR in type 2 diabetes patients (21). Furthermore TG/HDL could be considered as a simple reliable indicator to determine IR in healthy (22) and severely obese nondiabetic individuals (23). Our results on women with ovulatory disorder infertility also confirmed these findings. ROC curve analysis showed that TG/HDL-C, TC/HDL-C, and LDL-C/HDL-C with an AUC greater than 0.5 were effective and useful diagnostic markers for IR in infertile PCOS women. AUC of TG/HDL-C was the highest with sensitivity of 63.6% and specificity of 84.4% (TG/ HDL-C>3.19). Xiang et al. (20) have also shown that AUC of TC/HDL-C had the highest sensitivity and specificity (TC/HDL-C>3.6). This discrepancy could be partially due to lesser sample size in our study or possible racial differences.
Future studies with higher sample size and more specific markers are needed to show the correlation between lipid ratios and IR in an extended level.
Conclusion
Our investigation demonstrated that despite the routine methods used for measuring IR, TC/ HDL-C, TG/HDL-C, and LDL/HDL ratios could be regarded as simple, reliable and economic indicators of IR in PCOS infertile women. Moreover the combination of higher serum lipoprotein ratios and TG levels with abdominal obesity may predispose a group of patients to more marked risks for IR.
Acknowledgments
This work has been as a part of Aisa Ghaffarzadʼs M.Sc. thesis and was financially supported by Vice-Chancellor for Research, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran (grant number=NRC-9110). There is no conflict of interests between the authors.
References
- 1.Hernandez MI, Mericq V. Polycystic ovarian syndrome.Brook's clinical pediatric endocrinology. 6th ed. WileyBlackwell: A John Wiley & Sons, Ltd: Publication; 2009. pp. 559–570. [Google Scholar]
- 2.Balen AH, Rutherford AJ. Managing anovulatory infertility and polycystic ovary syndrome. BMJ. 2007;335(7621):663–666. doi: 10.1136/bmj.39335.462303.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsilchorozidou T, Overton C, Conway GS. The pathophysiology of polycystic ovary syndrome. Clin Endocrinol (Oxf) 2004;60(1):1–17. doi: 10.1046/j.1365-2265.2003.01842.x. [DOI] [PubMed] [Google Scholar]
- 4.Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol. 2011;9:39–39. doi: 10.1186/1477-7827-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wild RA. Dyslipidemia in PCOS. Steroids. 2012;77(4):295–299. doi: 10.1016/j.steroids.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Diamanti-Kandarakis E, Papavassiliou AG. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol Med. 2006;12(7):324–332. doi: 10.1016/j.molmed.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110(5):1050–1058. doi: 10.1097/01.AOG.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- 8.Traub ML. Assessing and treating insulin resistance in women with polycystic ovarian syndrome. World J Diabetes. 2011;2(3):33–40. doi: 10.4239/wjd.v2.i3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena P, Prakash A, Nigam A. Efficacy of 2-hour post glucose insulin levels in predicting insulin resistance in polycystic ovarian syndrome with infertility. J Hum Reprod Sci. 2011;4(1):20–22. doi: 10.4103/0974-1208.82355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 12.Bohler H Jr, Mokshagundam S, Winters SJ. Adipose tissue and reproduction in women. Fertil Steril. 2010;94(3):795–825. doi: 10.1016/j.fertnstert.2009.03.079. [DOI] [PubMed] [Google Scholar]
- 13.Douchi T, Ijuin H, Nakamura S, Oki T, Yamamoto S, Nagata Y. Body fat distribution in women with polycystic ovary syndrome. Obstet Gynecol. 1995;86(4 Pt 1):516–519. doi: 10.1016/0029-7844(95)00250-u. [DOI] [PubMed] [Google Scholar]
- 14.Kirchengast S, Huber J. Body composition characteristics and body fat distribution in lean women with polycystic ovary syndrome. Hum Reprod. 2001;16(6):1255–1260. doi: 10.1093/humrep/16.6.1255. [DOI] [PubMed] [Google Scholar]
- 15.Svendsen PF, Nilas L, Nørgaard K, Jensen JE, Madsbad S. Obesity, body composition and metabolic disturbances in polycystic ovary syndrome. Hum Reprod. 2008;23(9):2113–2121. doi: 10.1093/humrep/den211. [DOI] [PubMed] [Google Scholar]
- 16.Iuhas CI, Costin N, Niţă C, Mihu D. Body fat distribution in women with polycystic ovary syndrome. Rom J Diabetes Nutr Metab Dis. 2013;20(2):107–115. [Google Scholar]
- 17.Freeman R, Pollack R, Rosenbloom E. Assessing impaired glucose tolerance and insulin resistance in polycystic ovarian syndrome with a muffin test: an alternative to the glucose tolerance test. Endocr Pract. 2010;16(5):810–817. doi: 10.4158/EP09330.OR. [DOI] [PubMed] [Google Scholar]
- 18.Ovalle F, Azziz R. Insulin resistance, polycystic ovary syndrome, and type 2 diabetes mellitus. Fertil Steril. 2002;77(6):1095–1105. doi: 10.1016/s0015-0282(02)03111-4. [DOI] [PubMed] [Google Scholar]
- 19.Diamanti-Kandarakis E, Papavassiliou AG, Kandarakis SA, Chrousos GP. Pathophysiology and types of dyslipidemia in PCOS. Trends Endocrinol Metab. 2007;18(7):280–285. doi: 10.1016/j.tem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Xiang SK, Hua F, Tang Y, Jiang XH, Zhuang Q, Qian FJ. Relationship between serum lipoprotein ratios and insulin resistance in polycystic ovary syndrome. Int J Endocrinol. 2012;2012:173281–173281. doi: 10.1155/2012/173281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tangvarasittichai S, Poonsub P, Tangvarasittichai O. Association of serum lipoprotein ratios with insulin resistance in type 2 diabetes mellitus. Indian J Med Res. 2010;131:641–648. [PubMed] [Google Scholar]
- 22.González-Chávez A, Simental-Mendía LE, Elizondo-Argueta S. Elevated triglycerides/HDL-cholesterol ratio associated with insulin resistance. Cir Cir. 2011;79(2):126–131. [PubMed] [Google Scholar]
- 23.Brehm A, Pfeiler G, Pacini G, Vierhapper H, Roden M. Relationship between serum lipoprotein ratios and insulin resistance in obesity. Clin Chem. 2004;50(12):2316–2322. doi: 10.1373/clinchem.2004.037556. [DOI] [PubMed] [Google Scholar]