Abstract
Purpose: A simple stability-indicating RP-HPLC assay method was developed and validated for quantitative determination of Chloramphenicol, Dexamethasone Sodium Phosphate and Tetrahydrozoline Hydrochloride in ophthalmic solution in the presence of 2-amino-1-(4-nitrophenyl)propane-1,3-diol, a degradation product of Chloramphenicol, and Dexamethasone, a degradation product of Dexamethasone Sodium Phosphate.
Methods: Effective chromatographic separation was achieved using C18 column (250 mm, 4.6 mm i.d., 5 μm) with isocratic mobile phase consisting of acetonitrile - phosphate buffer (pH 4.0; 0.05 M) (30:70, v/v) at a flow rate of 1 mL/minute. The column temperature was maintained at 40°C and the detection wavelength was 230 nm.
Results: The proposed HPLC procedure was statistically validated according to the ICH guideline, and was proved to be stability-indicating by resolution of the APIs from their forced degradation products.
Conclusion: The developed method is suitable for the routine analysis as well as stability studies.
Keywords: Chloramphenicol, Dexamethasone Sodium Phosphate, Tetrahydrozoline Hydrochloride, HPLC, Ophthalmic Solution, Stability-indicating
Introduction
Chloramphenicol (CAP), Figure 1,A, is a bacteriostatic antibiotic.1 Dexamethasone Sodium Phosphate (DSP), Figure 1,B, is an inorganic ester of dexamethasone, that suppresses the inflammatory response to a variety of agents.2 Tetrahydrozoline Hydrochloride (THC), Figure 1,C, is an imidazoline-derivative sympathomimetic amine, which temporary relief of conjunctival congestion, itching, and minor irritation.3 An ophthalmic solution contains CAP 0.5%, DSP 0.1%, and THC 0.025% is available in the market. It is indicated for keratitis, conjunctivitis acute and chronic infectious, inflammation of the uvea anterior, scleritis, and sympathetic ophthalmia.4
Figure 1.
Chemical structures of (A) CAP (B) DSP (C) THC (D) AMPD (E) DEX
2-amino-1-(4-nitrophenyl)propane-1,3-diol (AMPD), Figure 1,D, is the hydrolysis product of Chloramphenicol.5 British Pharmacopoeia states that it should be less than 8%, with respect to Chloramphenicol, in the ophthalmic solution.6
Dexamethasone (DEX), Figure 1,E, is the hydrolytic derivative of Dexamethasone Sodium Phosphate.7 The allowable maximum limit for Dexamethasone in the solution for Injection is 0.5%, with respect to Dexamethasone Sodium Phosphate.6
The literature survey revealed that few methods determined simultaneously Chloramphenicol and Dexamethasone Sodium Phosphate8,9 in the presence of Dexamethasone.10 Therefore, the aim of this work was to develop and validate a new simple stability-indicating HPLC method for simultaneous determination of Chloramphenicol, its hydrolysis derivative (AMPD), Dexamethasone sodium phosphate, its hydrolysis derivative (Dexamethasone) and Tetrahydrozoline in the ophthalmic solution.
Materials and Methods
Chemicals and solutions
CAP was purchased from CHEMO, Spain; DSP and DEX were purchased from SYMBIOTICA, Malaysia; THC was purchased from S.I.M.S, Italy; AMPD was purchased from British Pharmacopoeia Commission Laboratory; and excipients were kindly supplied by DIAMOND PHARMA, Syria.
Acetonitrile used was of HPLC grade. All the other reagents used were of analytical grade. Purified water was used for making the solutions.
Chromatographic conditions
Separations were performed with a HPLC (LA Chrom ELITE, VWR Hitachi, Germany, equipped with L-2130 pump, L-2200 auto sampler, L-2300 column oven, and UV photo diode array detector L-2455). The out-put signal was monitored and processed using EZ Chrom ELITE software.
The column used was Thermo Hypersil C18 column (250 mm, 4.6 mm i.d., 5 μm). The isocratic mobile phase comprised of mixture of acetonitrile - potassium dihydrogen phosphate buffer (pH 4.0; 0.05 M) (30:70, v/v). The mobile phase was filtered through 0.45 μm membrane filter, degassed in ultrasonic bath and pumped from the respective solvent reservoir at a flow rate of 1 mL/minute. The column temperature was maintained at 40°C and the detection wavelength was 230 nm. The injection volume was 20 µL. The column was equilibrated for about 60 minutes prior to injection.
Preparation of standard solution
CAP (5000 μg/mL), DSP (1000 μg/mL), THC (250 μg/mL) and AMPD (400 μg/mL) stock solutions were prepared in mobile phase.
DEX solution (250 μg/mL) was prepared in acetonitrile. Then, dilution was made with mobile phase to obtain DEX stock solution with concentration of (5 μg/mL).
2 mL of each of the stock solutions were transferred into a 25 mL volumetric flask and diluted with mobile phase. The concentrations obtained were 400, 80, 20, 32, 0.4 μg/mL for CAP, DSP, THC, AMPD and DEX, respectively.
The standard solution was filtered using a 0.45 μm filter.
Method validation
The proposed HPLC method was validated according to ICH guideline.11
Forced degradation studies
Stock solutions
CAP (4000 μg/mL), DSP (800 μg/mL) and THC (200 μg/mL) stock solutions were prepared in mobile phase.
Degradation studies
5 mL of each of the stock solutions were transferred into a 50 mL volumetric flask, in each study.
For acidic hydrolysis, 2 mL of 2 M HCl was added, and the volumetric flask was kept at 70°C for about 3 hours in water bath. Then the solution was allowed to attend ambient temperature, neutralized by 2 mL of 2 M NaOH, and the volume was made up with mobile phase.
For alkaline hydrolysis, 1 mL of 0.1 M NaOH was added, and the volumetric flask was kept at 70°C for about 60 minutes in water bath. Then the solution was allowed to attend ambient temperature, neutralized by 1 mL of 0.1 M HCl, and the volume was made up with mobile phase.
For oxidative degradation, 3 mL of 3% H2O2 was added, and the volumetric flask was kept at 70°C for about 3 hours in water bath. Then the solution was allowed to attend ambient temperature and the volume was made up with the mobile phase.
For thermal degradation, the volumetric flask was kept at 70°C for 3 hours in water bath. Then the solution was allowed to attend ambient temperature and the volume was made up with mobile phase.
For photolytic degradation, the volumetric flask was subjected to both of the cool white fluorescent and near ultraviolet lamp with a maximum energy emission at 365 nm for 30 minutes. Then the solution was allowed to attend ambient temperature and the volume was made up with mobile phase.
All solutions were filtered with a 0.45 μm filter and injected in stabilized chromatographic conditions.
Results and Discussion
Method validation
The results of system suitability test from five replicate injections of standard solution were within the acceptable limits as per FDA guideline.12
The chromatograms of standard solution and excipients solution showed the absence of interfering peaks at the retention times of analytes in the excipients chromatogram, which demonstrates specificity of the method.
Good linearity was obtained in the studied ranges, as the correlation coefficients of the peak area responses versus concentrations calibration curves were more than 0.999.
This method was found to be precise as the RSD% of assay values at three concentrations (50%, 100% and 200%) for repeatability and intermediate precision (performed by three analysts) were less than 2%.
Recovery % of the analytes at each of added concentration (50%, 100% and 200%) was within the range of 98% to 102%, indicating that the method is accurate.
The summary of validation parameters of the proposed method is tabulated in Table 1.
Table 1. Summary of validation parameters .
| Parameter | AMPD | THC | DSP | CAP | DEX | |
| System suitability | RSD% of area | 0.1 | 0.2 | 0.2 | 0.1 | 0.3 |
|
RSD% of retention time |
0 | 0.2 | 0.6 | 0.4 | 0.7 | |
| Tailing factor | 0.8 | 1.6 | 1.2 | 1.2 | 1.1 | |
| Resolution | - | 6.7 | 4.4 | 11.6 | 21.1 | |
| Theoretical plates | 9745 | 7521 | 9646 | 14337 | 16507 | |
| Linearity | Range (µg/mL) | 100 – 800 | 20 – 160 | 5 – 40 | 8 – 200 | 0.1 – 50 |
| Precision | Repeatability, RSD% | 0.9 | 0.4 | 0.5 | 0.5 | 1.1 |
| Intermediate precision, RSD% | 1.4 | 1.5 | 1.4 | 1.3 | 0.6 | |
| Accuracy | Mean recovery % | 99.6 | 100.4 | 99.9 | 100.9 | 100.1 |
| Sensitivity | Detection limit, (µg/mL) | 0.06 | 0.03 | 0.04 | 0.04 | 0.02 |
| Quantification limit, (µg/mL) | 0.21 | 0.11 | 0.13 | 0.13 | 0.07 | |
The robustness was evaluated by making small changes in some method parameters including mobile phase composition (± 1%), mobile phase pH (± 0.1), flow rate (± 0.1 mL/minute), column temperature (± 2°C), wavelength (± 2 nm), and injection volume (± 10 μL); System suitability parameters were within the acceptable limits in all varied chromatographic conditions, indicating that the method is robust.
However, in extended robustness study to evaluate the effect of larger variation in the chromatographic conditions, the resolution between THC and DSP peaks was found to be susceptible to the acetonitrile percentage increasing, as it became about 1.9 when the percentage was 32%. Thus, it's recommended to suitably control the mobile phase composition to get the best resolution.
Forced degradation studies
In the chromatograms resulted from all degradation studies, there was no interference between the tested drugs and the degradation products.
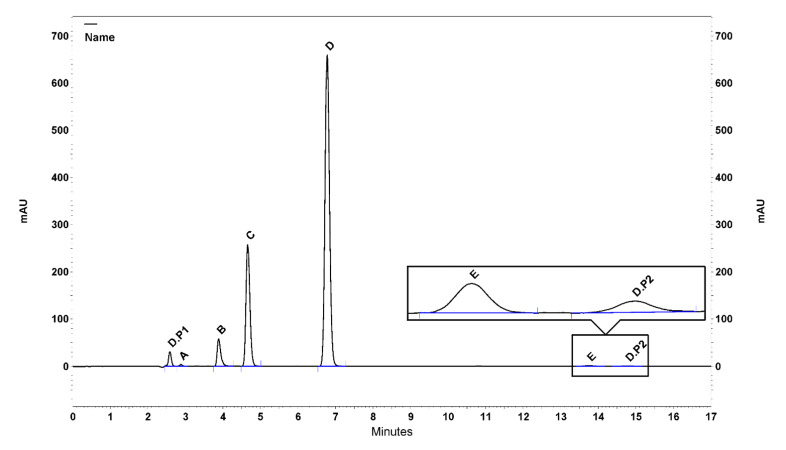
The chromatogram resulted from oxidative degradation study is represented in (Figure 2), as an example.
Figure 2.
Chromatogram resulted from oxidative degradation study, in which (A) AMPD, (B) THC, (C) DSP, (D) CAP, (E) DEX, (D.P1) and (D.P2) Degradation products
The peak purity spectrum of each tested drugs was recorded using PDA detector. Peak purity results were greater than 0.99, which indicates that the peaks are homogeneous in all stress conditions tested and thus establishing the specificity and confirming the stability-indicating power of the developed method.13,14
Table 2 presents the forced degradation studies results.
Table 2. Forced degradation studies results .
| Condition | THC | DSP | CAP | |||
| Assay | Peak purity | Assay | Peak purity | Assay | Peak purity | |
| Acidic | 98.2% | 1.000 | 96.9% | 1.000 | 92.2% | 1.000 |
| Alkaline | 97.2% | 0.998 | 96.1% | 1.000 | 97.4% | 1.000 |
| Oxidative | 99.3% | 1.000 | 96.9% | 1.000 | 97.7% | 1.000 |
| Thermal | 98.6% | 1.000 | 97.5% | 1.000 | 97.3% | 1.000 |
| Photolytic | 97.1% | 1.000 | 92.5% | 1.000 | 94.8% | 1.000 |
Conclusion
A simple HPLC method has been developed and validated for the analysis of Chloramphenicol, Dexamethasone Sodium Phosphate and Tetrahydrozoline Hydrochloride in the presence of Dexamethasone and AMPD in ophthalmic solutions. The results of the stress testing revealed that the method is stability-indicating; therefore, this method can be used to analyze samples during stability studies and the routine assay. In addition, the method can be applied for the determination of Dexamethasone and AMPD in ophthalmic solutions.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Sweetman SC, Pharm B, Pharm SFR. Martindale: The Complete Drug Reference. 36th ed. London: Pharmaceutical Press; 2009. [Google Scholar]
- 2. Dexamethasone Sodium Phosphate. Drugs.com. Available from: http://www.drugs.com/pro/dexamethasone-sodiumphosphate.html.
- 3. Tetrahydrozoline Hydrochloride. Drugs.com. Available from: http://www.drugs.com/monograph/tetrahydrozoline-hydrochloride.html.
- 4.AlAani H, Alnukkary Y, Alashkar I. Stability and kinetic studies for the estimation of shelf life of chloramphenicol, dexamethasone sodium phosphate, and tetrahydrozoline hydrochloride ophthalmic solution. Int J Pharm Sci Rev Res. 2015;30(1):327–30. [Google Scholar]
- 5.Boer Y, Pijnenburg A. Hplc determination of chloramphenicol degradation in eye drops. Pharm Weekbl Sci. 1983;5(3):95–101. doi: 10.1007/BF01960984. [DOI] [PubMed] [Google Scholar]
- 6.British Pharmacopoeia. London: Stationery Office; 2013. [Google Scholar]
- 7.Sulaimana S, Khamis M, Nir S, Lelario F, Scrano L, Bufo SA. et al. Stability and removal of dexamethasone sodium phosphate from wastewater using modified clays. Environ Technol. 2014;35(13-16):1945–55. doi: 10.1080/09593330.2014.888097. [DOI] [PubMed] [Google Scholar]
- 8.Shadoul WA, Gad Kariem EA, Adam ME, Ibrahim KEE. Simultaneous determination of dexamethasone sodium phosphate and chloramphenicol in ophthalmic solutions. Int J Chem Sci Tech. 2011;1(2):66–9. [Google Scholar]
- 9.Prakash K, Sireesha KR, Kumari AS. Stability indicating HPLC method for simultaneous determination of dexamethasone sodium phosphate and chloramphenicol in bulk and formulations. Int J Pharm Pharmaceut Sci. 2012;4 Supple 4:505–10. [Google Scholar]
- 10.Iqbal MS, Shad MA, Ashraf MW, Bilal M, Saeed M. Development and validation of an hplc method for the determination of dexamethasone, dexamethasone sodium phosphate and chloramphenicol in presence of each other in pharmaceutical preparations. Chromatographia. 2006;64(3/4):219–22. doi: 10.1365/s10337-006-0019-3. [DOI] [Google Scholar]
- 11. Validation of analytical procedures: text and methodology, Q2(R1). International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, ICH harmonised tripartite guideline. Available from: http://www.ich.org/fileadmin/Public-Web-Site/ICH-Products/Guidelines/Quality/Q2-R1/Step4/Q2-R1-Guideline.pdf.
- 12. Validation of chromatographic methods. Center for drug evaluation and research (CDER), Reviewer guidance. Available from: http://www.fda.gov/downloads/Drugs/Guidances/UCM134409.pdf.1994.
- 13.AlAani H, Alashkar I, Karabet F. Development and validation of stability-indicating RP-HPLC method for the determination of levocabastine HCl in bulk drug and in ophthalmic suspensions. Arab J Chem. 2013 doi: 10.1016/j.arabjc.2013.11.051. [DOI] [Google Scholar]
- 14.AlAani H, Alashkar I. Development and validation of stability-indicating RP-HPLC method for the analysis of levocetirizine dihydrochloride and fexofenadine hydrochloride in the presence of parabens in liquid dosage forms. Int J Pharm Sci Rev Res. 2013;23(2):64–71. [Google Scholar]