Abstract
Purpose: In the present study we aimed to quantify marrubiin, as the major active compound, in the aerial parts of Marrubium vulgare from Iran using a HPTLC-densitometry technique.
Methods: Quantitative determination of marrubiin in M. vulgare methanol extract was performed by HPTLC analysis via a fully automated TLC scanner. Later on, the in vitro antioxidant activity of the M. vulgare methanol extract was determined using 1,1-diphenyl-2-picryl-hydrazil (DPPH) free radical scavenging assay. Furthermore, total phenolics and flavonoids contents of the methanol extract were quantified, spectrophotometrically.
Results: The amount of marrubiin was calculated as 156 mg/g of M. vulgare extract. The antioxidant assay revealed a strong radical scavenging activity for the M. vulgare methanol extract with RC50 value of 8.24μg/mL. Total phenolics and flavonoids contents for M. vulgare were determined as 60.4 mg gallic acid equivalent and 12.05 mg quercetin equivalent per each gram of the extract, correspondingly.
Conclusion: The presented fingerprint of marrubiin in M. vulgare extract developed by HPTLC densitometry afforded a detailed chemical profile, which might be useful in the identification as well as quality evaluation of herbal medications based on M. vulgare. Besides, the considerable antioxidant activity of M. vulgare was associated with the presence of marrubiin along with phenolics and flavonoids exerting a synergistic effect.
Keywords: Marrubium vulgare, Marrubiin, Folin-Ciocalteau, Free radicals, TLC scanner, Densitometry
Introduction
Marrubium vulgare (Lamiaceae), known as horehound in English and Khanak in Persian, is a popular medicinal herb which has been used traditionally as a remedy for a wide range of diseases such as hypertension, infections, pain and etc.1,2 M. vulgare shows strong antioxidant activity due to the presence of flavonoids, terpenes, and phenols.3 The main active ingredient of M. vulgare was found to be marrubiin which has been identified in 1984 as the first diterpene isolated from leaves of this plant. Marrubiin is a furan labdane diterpenoid which is produced and accumulated only in the aerial parts of the plant.1,4 It is important to mention that marrubiin is generated as an artifact from pre-marrubiin during the extraction procedure when heat is involved in the extraction or concentration procedure.5 Marrubiin has been associated with the bitter principle of the horehound and lots of other medicinal plants of the family Lamiaceae. The broadly known diterpenoid lactone, marrubiin, has been marked with assorted types of biological activities such as analgesic, vasorelaxant, cardioprotective, gastroprotective, antidiabetic, antioxidant, antispasmodic, anti-hypertensive, anti-edematogenic and immunomodulating properties.1,6-15 In a study conducted by Mnonopi et al, marrubiin which was isolated from Leonotis leonurus L. found to be a cardioprotective compound by inducing anticoagulant, antiplatelet and anti-inflammatory properties in obese rat models.6 Our previously published paper on cardioprotective effect of the total methanolic extract of M. vulgare, containing marrubiin as the major ingredient of the extract, in an animal model with myocardial infarction was in consistence with the reported activity related to the compound marrubiin.16 Furthermore, in a recent study gastroprotective activity of the methanolic extract of M. vulgare had been revealed due to the presence of marrubiin as evidenced by inhibitory effect of both marrubiin and the methanol extract of M. vulgare on the indomethacin-induced ulcers.17 Generally, considering loads of published data over the past century on the chemical and biological aspects of marrubiin in the genus Marrubium (Lamiaceae), we could consider this compound as a biomarker within the plants of this genus.
In view of these facts, High Performance Thin Layer Chromatography (HPTLC) has been widely used as a rapid, precise and cost-effective method for determination of biological compounds from medicinal plants; accordingly, in this study we adopted HPTLC as a promising technique for determination of marrubiin quantitatively within the M. vulgare extract.18 It is of note to mention that HPTLC encompasses the use of chromatographic layers of utmost separation efficiency, utilization of instrumentation for all steps in the approach, defined sample applications, validated reproducible chromatogram developments and software controlled analysis. Nonetheless, adaptation on optimized new approaches would not be inevitable in displacement of existing methods but a supplement to already existing techniques. The standardized method might be useful in identification as well as quality evaluation of herbal medications based on a specific phytochemical. Following our previous work, in the present study a preliminary phytochemical screening of the methanol extract of M. vulgare from Iran was performed. Additionally, an HPTLC-densitometry assay was conducted for the purpose of quantitative determination of marrubiin content in the extract.
Materials and Methods
Materials
All the chemicals, including solvents, were of analytical grade from Merck Company Germany. 1,1-Diphenyl-2-picryl-hydrazyl (DPPH), quercetin, gallic acid, Folin-Ciocalteu reagent, and aluminum chloride from Sigma-Aldrich chemical company Madrid-Spain, potassium acetate, and silica gel 60 F254 HPTLC (20cm × 20cm) plates (Merck, Darmstadt, Germany) were used in this study.
Plant Material, Extraction and Preparation
The aerial parts of M. vulgare were collected in 2012 during flowering stage on June from Kiasar (Mazandaran Province, Iran). They were authenticated by Dr. M. Mazandarani (Department of Biology, Azad Islamic University, Gorgan, Iran). Voucher specimens (No. 712 Tbz-Fph) have been deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran. Air dried aerial parts (200g) were grounded and extracted with methanol (2L×4) by maceration at room temperature (25-30 °C). The obtained extract was concentrated to dryness under vacuum at 40 °C using a rotary evaporator. A greenish residue weighing 17.8 % (w/w) was obtained and kept in air tight bottle in a refrigerator until use. To identify the chemical constituents, the resultant methanol extract was subjected to preliminary phytochemical analysis.
Determination of Total Phenolic Content
Total phenolic content was determined by Folin-Ciocalteau method as described by Ghasemi et al and Ebrahimzadeh et al.19,20 Briefly, 0.5 ml of the extract was mixed with Folin-Ciocalteau reagent (5 mL, 1:10 diluted with distilled water) for 5 min and aqueous Na2CO3 (4 mL, 1 M) was then added. The mixture was allowed to stand for 15 min where the phenolics were determined by colorimetry at 765 nm (Shimadzu 2100, Japan). The standard curve was prepared by 50, 100, 150, 200, and 250 mg/mL solutions of gallic acid in methanol: water (50:50, v/v). Total phenol values are expressed in terms of gallic acid equivalent (mg/g of the extract) by reference to calibration curve: y=0.0067x+0.0132, (R2=0.987).
Determination of Total Flavonoids Content
In order to determine the total flavonoids content of the M. vulgare extract, the colorimetric aluminum chloride method was employed according to the method described by Ghasemi et al and Ebrahimzadeh et al.19,20 Briefly, 0.5 mL solution of methanolic extract were mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate and 2.8 mL of distilled water, and were left at room temperature for 30 min. The absorbance of the reaction mixture was measured spectrophotometrically at 415 nm. Total flavonoids content were calculated as quercetin from a calibration curve prepared using 31.25-250 μg/mL solutions of quercetin in methanol as standard (y=0.008x-0.068, R2=0.999).
Determination of In Vitro Antioxidant Activity
The free radical scavenging capacity of the extract was measured from the bleaching of the purple colored methanol solution of 1,1-diphenyl-2-picryl-hydrazil. The stock concentration 1 mg/ml of the methanol extract of the M. vulgare was prepared followed by dilution in order to obtain concentrations of 5×10-1, 2.5×10-1, 1.25×10-1, 6.25×10-2, 3.13×10-2 and 1.56×10-2 mg/mL. The obtained concentrations in equal volumes of 2 mL were added to 2 mL of a 0.004% of DPPH solution. After a 30 min of incubation period at 25 ºC, the absorbance at 517 nm was determined against a blank. Tests were carried out in triplicate where the average absorption was noted for each concentration. Furthermore, as the positive control the same procedure was repeated with quercetin. The inhibition percentages of DPPH free radicals of by the samples were calculated following the equation:
R (%) = 100 × [(A blank – A sample) / A blank]
Hereon, “A blank” represents the absorbance value of the control reaction and “A sample” is the absorbance value for each sample. Besides, RC50 value, concentration of the extract reducing 50% of the DPPH free radicals, was calculated from the graph of inhibition percentages against concentrations of M. vulgare extract in mg/mL.
Quantitative Analysis of Marrubiin
Preliminary HPTLC analysis of the M. vulgare extract was performed on silica gel 60 F254 HPTLC plate with benzene-acetone (17:3) as the mobile phase. Later on, detection of the spots after spraying with anisaldehyde-sulfuric acid reagent revealed the presence of marrubiin at RF=0.82 as the major compound in the extract such as expressed by Popa et al, in 1968. Following the preliminary qualitative analysis of HPTLC analysis of the M. vulgare extract, quantitative determination of marrubiin, as the major bioactive component in M. vulgare, was performed by photodensitometric method via a fully automated TLC scanner (CAMAG TLC scanner 3 coupled with Automatic TLC Sampler 4, Automatic Developing chamber 2 and a TLC Visualizer). In addition, winCATS (Planar chromatography manager) software was used for analyzing results of the plate scan.
As far as we know, automatic sample application is a key factor for productivity of the HPTLC laboratory evaluations, to this end; Automatic TLC Sampler 4 was used in spraying samples onto plates in the form of bands in the presence of nitrogen air. In this regard, samples were applied on the plate as 4 mm wide bands with constant application rate from 1 to 7µL s-1, an automatic TLC sampler under a flow of N2 gas, 20 mm from the bottom, 15 mm from the side, and spaces among the spots were 8 mm of the plate. In order to quantitative determine the content of marrubiin in the extract and obtain a standard curve, stock solution of marrubiin was prepared in methanol to achieve the concentration of 0.44 mg/ml. Samples of marrubiin on the HPTLC plate with volume of 1µl to 7µl were consequently spotted to afford 0.44, 0.88, 1.31, 1.75, 2.19, 2.63 and 3.06 µg marrubiin (spots number 1 to 7). Similarly, a stock solution of extract in methanol (10 mg/ml) was prepared and spotted with three volumes of 1, 2 and 3µl immediately after standard solutions' spots (spots number 8 to 10). Subsequently, the linear ascending development was carried out in a twin trough chamber, which was pre-saturated with 25 mL mobile phase with benzene-acetone (17:3) for 30 min, at room temperature (25 ± 2 °C) and 50 ± 5% relative humidity. The developing chamber of the HPTLC system automatically performed the development step minimizing environmental effects. Thus, the activity and preconditioning of the layer, chamber saturation, developing distance and final drying were completely pre-set and monitored during this step. When the Linomat is operated under software, plate dimensions, number and distance of tracks, designation, sample volumes and sequence are software controlled. All the operating data were automatically transferred to the densitometric processing evaluation step. Ultimately, the HPTLC plate was placed on a TLC Scanner and scanned under the UV 509 nm light (A 509-nm UV light was used to illuminate the plate).
Results
Phytochemical Screening of M. vulgare Extract
Preliminary phytochemical screening of M. vulgare extract indicated the presence of flavonoids and phenolic compounds in the plant extract. Regarding the absorbance values of the extract solutions, reacted with Folin-Ciocalteu and aluminum chloride reagents compared to the standard solutions as described in the methods section, the amount of total phenolic and flavonoids contents for M. vulgare were determined as 60.4 mg gallic acid equivalent/g extract and 12.05 mg quercetin equivalent/g of the extract, respectively.
In Vitro Antioxidant Activity of M. vulgare Extract
The free radical scavenging activity of M. vulgare extract was evaluated using the DPPH method in vitro. According to our results, the RC50 values for methanol extract of M. vulgare and quercetin were found to be 8.24 and 3μg/ml, correspondingly. Our results showed that the M. vulgare methanol extract has a considerable free radical scavenging activity comparable with the standard compound, quercetin.
Marrubiin Quantification
Preliminary HPTLC analysis of the extract revealed marrubiin with Rf value of 0.82 as a major compound present in the M. vulgare extract. The quantitative determination of marrubiin on the fluorescent HPTLC plate on TLC scanner yielded a 3D graph on the basis of optical density (densitogram). In Figure 1, densitograms 1 to 7 are the increasing concentrations of pure compound marrubiin. The calibration curve was calculated automatically according to the densitograms 1 to 7 that in this curve the Y axis is marrubiin concentration and the X axis is the peak height of the corresponding concentration (Figure 2). The densitograms 8 to 10 are three repeated samples of the methanol extract from aerial parts of M. vulgare where the mean value of the heights of these three points were used to quantify the marrubiin content in the extract. Eventually, the amount of Marrubiin was calculated as 156 mg/g of M. vulgare extract by reference to the standard curve: (y=0.335x-27.87, R2= 0.999) (Figure 2).
Figure 1.
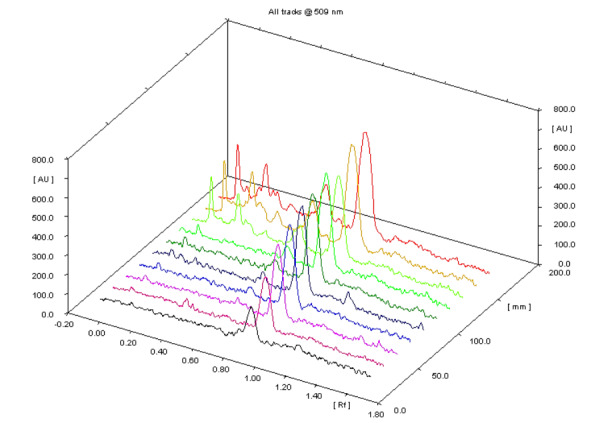
Photodensitogram of the fluorescent HPTLC plate of marrubiin (densitograms 1-7) and M. vulgare extract (densitograms from 8-10) depicted by TLC scanner 3 (CAMAG) in which the X, Y and Z axis represents RF of each detected spot, height of the peaks (Spot’s density), and location on the plate, respectively.
Figure 2.
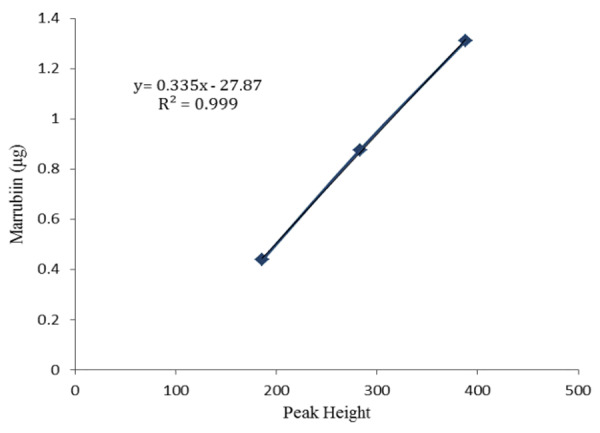
The calibration curve drawn automatically by TLC Scanner used to quantify marrubiin in the methanol extract of M. vulgare.
Discussion
Our preliminary phytochemical findings in this study revealed the presence of phenolic compounds, flavonoids and marrubiin as the major diterpenoid in the methanolic extract of M. vulgare. It was found that the considerable radical scavenging activity of the extract against the DPPH free radicals was in line with the high phenolic and flavonoid contents of M. vulgare extract. The naturally occurring flavonoids and phenolics are believed to possess the ideal chemical structure for scavenging free radicals.21,22
Owing to the fact that free radicals are molecules with an odd, unpaired electron that these unpaired electrons make them unstable and highly reactive,23 they could be extremely toxic to human cells attacking fatty acids, leading to lipid peroxidation of membranes, reacting with proteins, destruction and oxidation of amino acids, oxidation of sulfhydryl groups and polypeptide chain scission. Accordingly, these free radicals are the important factors in diseases related to oxidative stress conditions such as cardiovascular and neurodegenerative diseases.24 The use of natural antioxidants, especially phenolics and flavonoids, might be very promising in treatment of these kinds of diseases. Hence, in recent years more investigations have been focused on the plants especially those with remarkable antioxidant activities.22,25 Overall, here in this study it was suggested that the observed scavenging activity of the M. vulgare methanol extract could be assigned to the hydrogen-donating capacity of the phenolic and flavonoid components, in cooperation with the presence of marrubiin in the extract.
Formerly in a report by our team, the methanol extract of M. vulgare showed significant improvements to the hemodynamic, electrocardiographical and histopathological changes in a myocardial infarction model induced by isoproterenol.16 However, our new findings in this paper provided sufficient evidence for the presence of high quantities of a diterpenoid marrubiin along with flavonoids and phenolics corroborating the cardioprotective effect of M. vulgare. Furthermore, we can come to the conclusion that these compounds might have a synergistic antioxidant effect through which they induce protection against oxidative stress. Taken together, M. vulgare from Iran have the potential to be considered as a suitable applicant to be probed as a source of bioactive components in search of new efficient versatile phytomedicines for treating various ailments especially those caused by oxidative stress.
On the other hand, a high performance thin layer chromatography method coupled with densitometric analysis was developed in this study for the identification and quantification of marrubiin in M. vulgare. This method was considered to be precise, consistent, rapid and low-cost which can be used for quantitative determination of marrubin in the extracts from Marrubium species. According to the findings, the presented chemical fingerprint of M. vulgare extract that was developed using HPTLC densitometry afforded a detailed chemical profile which might be useful in the identification as well as quality evaluation of herbal medications based on M. vulgare. As follows, the developed fingerprint could be of value in preparation of a standardized herbal product with consistent biological activity. Likewise, providing these kinds of chemical fingerprints may also be helpful in differentiation of the plant from adulterants according to the simplicity, flexibility and cost efficiency of the HPTLC technique in both qualitative and quantitative aspect with minimal time requirement.
Conclusion
Regarding the phytochemical findings of the present study it can be concluded that the sizeable radical scavenging activity of M. vulgare methanol extract could be attributed, to some extent, to its remarkable flavonoids and phenolics content. Moreover, it was revealed that marrubiin was the main compound in the aerial parts of M. vulgare grown in Iran. The quantification of marrubiin could be performed using HPTLC technique which is a precise, simple and relatively inexpensive method. According to our knowledge, there is no report on this method for analyzing marrubiin in the genus Marrubium so far. Thereby, this method could be used simply and rapidly for the routine analysis and quality control of marrubiin in the Marrubium species.
Acknowledgments
The authors would like to thank Biotechnology Research Center of Tabriz University of Medical Sciences for their help in conducting the HPTLC analysis. Financial support of this work by the Research Vice-Chancellor of Tabriz University of Medical Sciences is faithfully acknowledged. Authors are also thankful to Dr. M. Mazandarani for authenticating the plant.
Ethical Issues
Not applicable.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Piccoli PN, Bottini R. Accumulation of the labdane diterpene marrubiin in glandular trichome cells along the ontogeny of marrubium vulgare plants. Plant Growth Regul. 2008;56:71–6. doi: 10.1007/s10725-008-9286-3. [DOI] [Google Scholar]
- 2.Pukalskas A, Venskutonis PR, Salido S, Waard PD, van Beek TA. Isolation, identification and activity of natural antioxidants from horehound (marrubium vulgare L.) cultivated in lithuania. Food Chem. 2012;130(3):695–701. doi: 10.1016/j.foodchem.2011.07.112. [DOI] [Google Scholar]
- 3.Vanderjagt TJ, Ghattas R, Vanderjagt DJ, Crossey M, Glew RH. Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico. Life Sci. 2002;70(9):1035–40. doi: 10.1016/S0024-3205(01)01481-3. [DOI] [PubMed] [Google Scholar]
- 4.Appleton RA, Fulke JWB, Henderson MS, McCrindle R. The stereochemistry of marrubiin. J Chem Soc C. 1967:1943–7. doi: 10.1039/J39670001943. [DOI] [Google Scholar]
- 5.Henderson MS, McCrindle R. Premarrubiin. A diterpenoid from Marrubium vulgare L. J Chem Soc C. 1969:2014–5. doi: 10.1039/J39690002014. [DOI] [Google Scholar]
- 6.Mnonopi N, Levendal RA, Davies-Coleman MT, Frost CL. The cardioprotective effects of marrubiin, a diterpenoid found in Leonotis leonurus extracts. J Ethnopharmacol. 2011;138(1):67–75. doi: 10.1016/j.jep.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 7.De Jesus RA, Cechinel-Filho V, Oliveira AE, Schlemper V. Analysis of the antinociceptive properties of marrubiin isolated from Marrubium vulgare. Phytomedicine. 2000;7(2):111–5. doi: 10.1016/S0944-7113(00)80082-3. [DOI] [PubMed] [Google Scholar]
- 8.de Souza MM, de Jesus RA, Cechinel-Filho V, Schlemper V. Analgesic profile of hydroalcoholic extract obtained from Marrubium vulgare. Phytomedicine. 1998;5(2):103–7. doi: 10.1016/S0944-7113(98)80005-6. [DOI] [PubMed] [Google Scholar]
- 9.El Bardai S, Morel N, Wibo M, Fabre N, Llabres G, Lyoussi B. et al. The vasorelaxant activity of marrubenol and marrubiin from Marrubium vulgare. Planta Med. 2003;69(1):75–7. doi: 10.1055/s-2003-37042. [DOI] [PubMed] [Google Scholar]
- 10.Meyre-Silva C, Yunes RA, Schlemper V, Campos-Buzzi F, Cechinel-Filho V. Analgesic potential of marrubiin derivatives, a bioactive diterpene present in Marrubium vulgare (Lamiaceae) Farmaco. 2005;60(4):321–6. doi: 10.1016/j.farmac.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Meyre-Silva C, Cechinel-Filho V. A review of the chemical and pharmacological aspects of the genus marrubium. Curr Pharm Des. 2010;16(31):3503–18. doi: 10.1016/j.jep.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Schlemper V, Ribas A, Nicolau M, Cechinel Filho V. Antispasmodic effects of hydroalcoholic extract of Marrubium vulgare on isolated tissues. Phytomedicine. 1996;3(2):211–6. doi: 10.1016/S0944-7113(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 13.Mnonopi N, Levendal RA, Mzilikazi N, Frost CL. Marrubiin, a constituent of Leonotis leonurus, alleviates diabetic symptoms. Phytomedicine. 2012;19(6):488–93. doi: 10.1016/j.phymed.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Karioti A, Skopeliti M, Tsitsilonis O, Heilmann J, Skaltsa H. Cytotoxicity and immunomodulating characteristics of labdane diterpenes from Marrubium cylleneum and Marrubium velutinum. Phytochemistry. 2007;68(11):1587–94. doi: 10.1016/j.phytochem.2007.03.027. [DOI] [PubMed] [Google Scholar]
- 15.Stulzer HK, Tagliari MP, Zampirolo JA, Cechinel-Filho V, Schlemper V. Antioedematogenic effect of marrubiin obtained from Marrubium vulgare. J Ethnopharmacol. 2006;108(3):379–84. doi: 10.1016/j.jep.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Yousefi K, Soraya H, Fathiazad F, Khorrami A, Hamedeyazdan S, Maleki-Dizaji N. et al. Cardioprotective effect of methanolic extract of Marrubium vulgare L. On isoproterenol-induced acute myocardial infarction in rats. Indian J Exp Biol. 2013;51(8):653–60. [PubMed] [Google Scholar]
- 17.de Oliveira AP, Santin JR, Lemos M, Klein Júnior LC, Couto AG, da Silva Bittencourt CM. et al. Gastroprotective activity of methanol extract and marrubiin obtained from leaves of Marrubium vulgare L. (Lamiaceae) J Pharm Pharmacol. 2011;63(9):1230–7. doi: 10.1111/j.2042-7158.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- 18.Prasanth KG, Anandbabu A, Venkatanarayanan R, Dineshkumar B, Sankar V. HPTLC technique: Determination of flavonoid from Clerodendrum viscosum vent roots. Der Pharma Chemica. 2012;4(3):926–9. [Google Scholar]
- 19.Ghasemi K, Ghasemi Y, Ebrahimzadeh MA. Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci. 2009;22(3):277–81. [PubMed] [Google Scholar]
- 20.Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Dehpour AA. Antioxidant activity of hydroalcholic extract of Ferula gummosa boiss roots. Eur Rev Med Pharmacol Sci. 2011;15(6):658–64. [PubMed] [Google Scholar]
- 21.Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY. et al. Antioxidant activity and free radical scavenging capacity between korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002;163(6):1161–8. doi: 10.1016/S0168-9452(02)00332-1. [DOI] [Google Scholar]
- 22.Skerget M, Kotnik P, Hadolin M, Hras AR, Simonic M, Knez Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005;89(2):191–8. doi: 10.1016/j.foodchem.2004.02.025. [DOI] [Google Scholar]
- 23.Burton KP, McCord JM, Ghai G. Myocardial alterations due to free-radical generation. Am J Physiol. 1984;246(6 Pt 2):H776–83. doi: 10.1152/ajpheart.1984.246.6.H776. [DOI] [PubMed] [Google Scholar]
- 24.Burton KP. Evidence of direct toxic effects of free radicals on the myocardium. Free Radic Biol Med. 1988;4(1):15–24. doi: 10.1016/0891-5849(88)90006-8. [DOI] [PubMed] [Google Scholar]
- 25.Fugh-Berman A. Herbs and dietary supplements in the prevention and treatment of cardiovascular disease. Prev Cardiol. 2000;3(1):24–32. doi: 10.1111/j.1520-037X.2000.80355.x. [DOI] [PubMed] [Google Scholar]


