Abstract
Obstructive sleep apnea (OSA) is an independent risk factor for cardiovascular morbidity and death. Little information is available regarding the relationship between the severity of OSA and myocardial performance in OSA patients who have normal ejection fractions. We prospectively investigated this relationship, using the tissue-Doppler myocardial performance index (TD-MPI).
We conducted overnight, full-laboratory polysomnographic examinations of 116 patients, and calculated the left and right ventricular TD-MPIs. Patients were classified into 3 groups in accordance with their apnea-hypopnea index (AHI) levels: AHImild (≥5 to <15), AHImoderate (≥15 to <30), and AHIsevere (≥30).
Left and right ventricular TD-MPI values were higher in the AHIsevere group than in the AHImild and AHImoderate groups (all P <0.05). In addition, right ventricular TD-MPI values in the AHImoderate group were higher than those in the AHImild group (P <0.05). Right ventricular TD-MPI was significantly associated with AHI (β=0.468, P <0.001), left ventricular TD-MPI, and right ventricular early-to-late filling velocities (E/A ratio) in multiple linear regression analysis. On the other hand, left ventricular TD-MPI was significantly associated with right ventricular TD-MPI and left ventricular E/A ratio (both P <0.05).
Our results show that OSA severity, determined by means of AHI, is independently associated with impaired right and left ventricular function as indicated by TD-MPI in patients who have OSA and normal ejection fractions.
Keywords: Cardiovascular diseases/etiology; echocardiography, Doppler; heart-function tests/methods; predictive value of tests; prospective studies; risk factors; sleep apnea, obstructive/classification/complications/physiopathology; ventricular dysfunction, left/diagnosis/etiology/physiology; ventricular dysfunction, right/diagnosis/etiology/physiology
Obstructive sleep apnea (OSA) affects 4% to 6% of middle-aged men and 2% to 4% of middle-aged women, and its prevalence increases with age.1,2 This syndrome is characterized by excessive sleepiness and cognitive-behavioral, respiratory, cardiac, metabolic, and inflammatory disorders secondary to repeated episodes of upper-airway obstruction during sleep.3 Moreover, OSA is an independent risk factor for cardiovascular morbidity and death.4 Obstructive sleep apnea affects both ventricles and is associated with impaired ventricular function.5,6
Noninvasive echocardiographic indices of systolic and diastolic ventricular function are of great clinical importance in the diagnosis and treatment of patients who have heart disease. The tissue-Doppler myocardial performance index (TD-MPI) has been proposed as a measure of overall cardiac function.7–9 The TD-MPI, which combines values of both systolic and diastolic ventricular function, is easily obtainable and has been clinically useful to evaluate global ventricular function noninvasively.10,11 In addition, TD-MPI level is an independent risk factor for left ventricular (LV) systolic asynchrony in patients who have coronary artery ectasia.12 Although OSA has been associated with impaired ventricular function, we found no information regarding the relationship between OSA severity and LV and right ventricular (RV) myocardial function in patients who have normal ejection fractions (EFs). We therefore used the TD-MPI to investigate this.
Patients and Methods
Our study included 116 patients with symptoms of nocturnal snoring or excessive daytime sleepiness who were admitted to the sleep clinic of our hospital's Department of Pulmonology for OSA screening from January through August 2014. Detailed sleep and cardiovascular histories of the patients were recorded. All patients underwent physical examination.
Exclusion criteria were any known cardiac or lung disease, diabetes mellitus, hyperthyroidism or hypothyroidism, chronic renal or hepatic disease, or systemic hypertension. We also excluded patients who had abnormal electrocardiographic (ECG) recordings, positive cardiac stress tests, or left ventricular EFs (LVEF) <0.55, and patients who were taking any medications. The Institutional Ethics Committee approved the study protocol, and each participant provided written informed consent.
After evaluating the medical histories and the results of the physical examinations, we recorded the baseline characteristics of the included patients.
Sleep Test and Apnea-Hypopnea Index
All sleep data were recorded with use of E-Series equipment (Compumedics Limited; Abbotsford, Australia), including electroencephalography, electrooculography, submental electromyography, oxygen saturation (by means of pulse oximetry), respiratory movements (by means of inductance plethysmography), nasal and oral airflow (with use of thermistors), and nasal pressure (with use of nasal cannulas and pressure sensors). Sleep stages and sleep-disordered breathing were then scored in accordance with standard criteria.13 Apnea was defined as total obstruction of airflow for >10 s, and hypopnea was defined as a reduction in ventilation of ≥50% that results in a decrease in arterial saturation of ≥4% due to partial airway obstruction. The average number of apnea and hypopnea episodes per hour of sleep was defined as the apnea-hypopnea index (AHI). Patients with AHI values ≥5 were diagnosed to have OSA.
Echocardiography
Standard 2-dimensional, M-mode, and tissue-Doppler echocardiographic examinations were performed with use of the Vivid 7® cardiac ultrasonography system (GE Medical Systems; Horten, Norway) and a 2.5–3.5-MHz transducer. Simultaneous ECG recordings were obtained. One echocardiographer (blinded to the patients' clinical and laboratory data) interpreted each echocardiographic examination independently. All patients were examined while at rest in the left lateral decubitus position. M-mode echocardiograms were recorded from the parasternal window, to determine left and right atrial and ventricular dimensions. Left atrial diameter, LV end-systolic diameter, LV end-diastolic diameter, and LVEF were determined in apical 2- and 4-chamber views with use of the Simpson biplane formula, in accordance with the suggestions of the American Society of Echocardiography.14 Tracings of endocardial borders in end-diastole and end-systole were made in the technically best cardiac cycle, and the mean of 3 measurements was used.
After acquisition adjustments were made, LV and RV tissue-Doppler echocardiographic evaluations were performed from the apical 4-chamber position by placing the pulsed-wave Doppler beam on the part of the mitral annulus that is close to the LV lateral wall and inter-ventricular septum (for the LV), and on the part of the tricuspid annulus that is close to the RV lateral wall (for the RV). During recording, special attention was given to placing the Doppler beam in the myocardium, not the endocardium or epicardium. Measurements were made for 3 consecutive heartbeats in all positions, and their average was taken. Doppler measurements were made at a recording rate of 100 mm/s. The isovolumetric relaxation time (IVRT), isovolumetric contraction time (IVCT), and ejection time (ET) of both ventricles were measured in all patients. The left and right TD-MPIs were defined as the sum of the IVRT and IVCT divided by ET (Fig. 1). All echocardiograms were recorded and interpreted online on hard disks for offline analysis by another observer who was blinded to the patients' data. Mean intraobserver variability (3.9% ± 2.4%) and interobserver variability (4.2% ± 3.2%) were evaluated in data from 12 randomly selected patients and were calculated as the absolute difference divided by the average of the 2 observations.
Fig. 1.
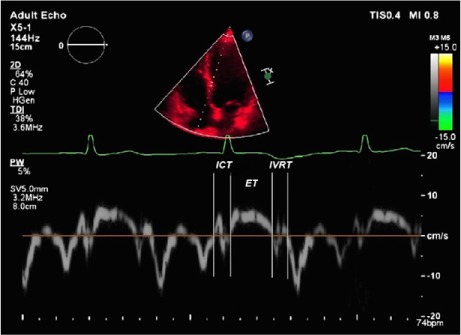
Example of a tissue-Doppler echocardiogram used to calculate the tissue-Doppler myocardial performance index, which is defined as the sum of isovolumetric relaxation time (IVRT) and isovolumetric contraction time (ICT), divided by ejection time (ET).
Statistical Analysis
All analyses were conducted with use of SPSS for Windows version 17.0 (IBM Corporation; Armonk, NY). Data were expressed as mean ± SD. Categorical variables between the groups were compared with use of the χ2 test. Analysis of variance was used on continuous variables among the AHI groups. Analysis of normality was performed with use of the Kolmogorov-Smirnov test. Associations between other variables in RV TD-MPI and LV TD-MPI were evaluated by means of the Pearson correlation coefficient. Multiple linear regression analysis was performed to identify the independent associations of RV TD-MPI and LV TD-MPI by including the values that were correlated with RV TD-MPI and LV TD-MPI in bivariate analysis. Standardized β regression coefficients and their significance from multiple linear regression analysis were reported. A P value <0.05 was considered statistically significant.
Results
Patients were classified into 3 groups in accordance with AHI level: ≥5 to <15 was AHImild (26 patients; mean age, 44.1 ± 10.2 yr), ≥15 to <30 was AHImoderate (41 patients; mean age, 44.2 ± 10 yr), and ≥30 was AHIsevere (49 patients; mean age, 46.4 ± 11.2 yr).
Baseline and Laboratory Findings
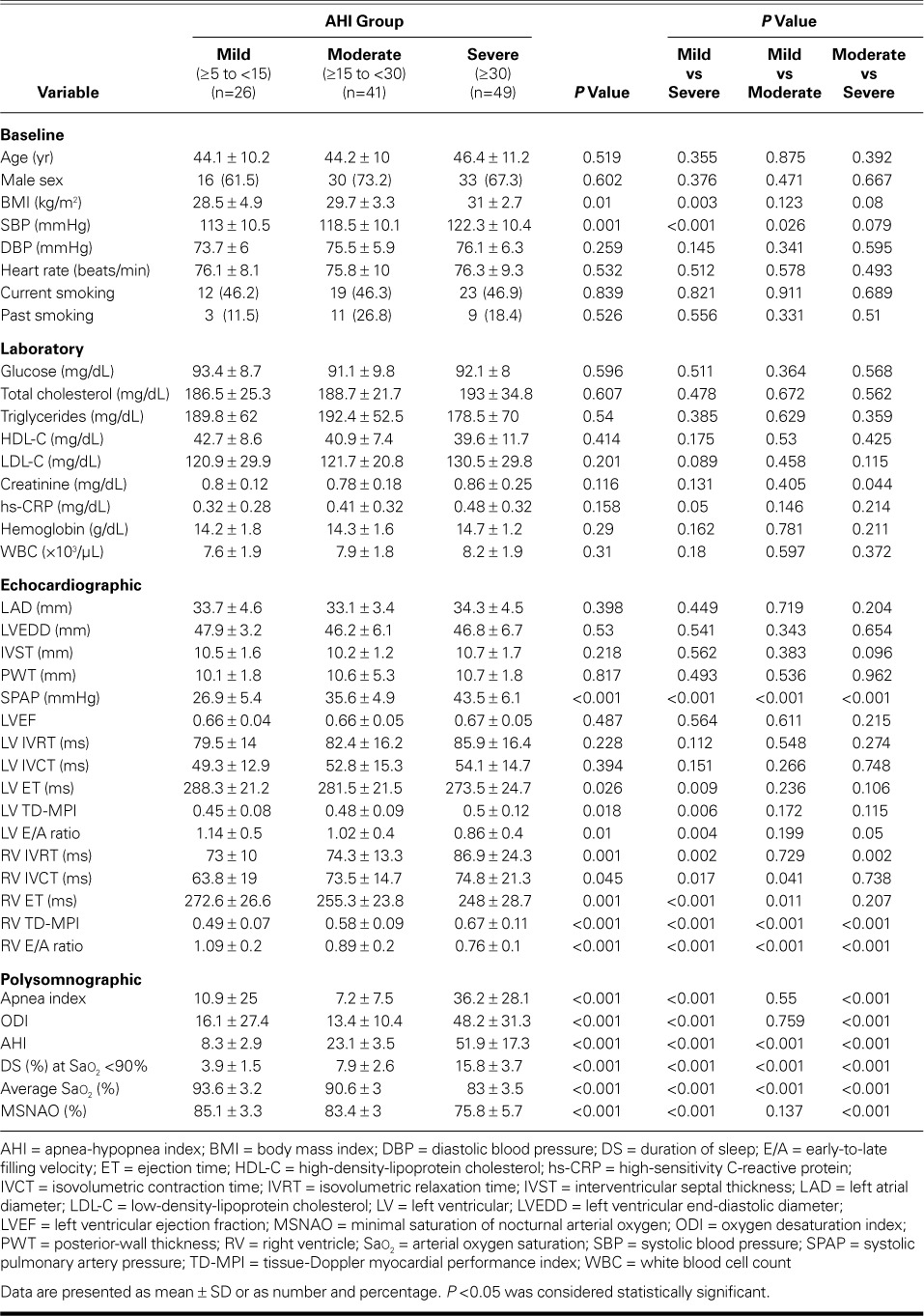
Table I shows the baseline characteristics and laboratory findings. Body mass index (BMI) values were significantly higher in the AHIsevere group than in the AHImild group (P <0.05). The systolic blood pressures in the AHIsevere and AHImoderate groups were significantly higher than those in the AHImild group (both P <0.05). The creatinine levels in the AHImoderate group and the levels of high-sensitivity C-reactive protein in the AHImild group were significantly lower than those in the AHIsevere group (both P <0.05).
TABLE I.
Comparison of Baseline, Laboratory, Polysomnographic, and Echocardiographic Characteristics
Echocardiographic Findings
Table I shows the echocardiographic comparisons. Among findings of statistical significance, the LV TD-ET values were lower and the LV TD-MPI values were higher in the AHIsevere group than in the AHImild group (both P <0.05). The LV late-to-early filling (E/A) ratio was lower in the AHIsevere group than in the other groups (both P <0.05).
The RV TD-IVRT values in the AHIsevere group were significantly higher than those in the other groups (both P <0.05). The RV TD-IVCT values were lower and the RV ET values were higher in the AHImild group than in the other groups (all P <0.05).
The highest RV TD-MPI and lowest E/A values were observed in the AHIsevere group (P <0.05 vs the other groups). The RV TD-MPI values in the AHImoderate group were higher than those in the AHImild group (P <0.05).
Bivariate and Multivariate Relationships between RV TD-MPI and Variables
Table II shows the bivariate associations of RV TD-MPI with age (r=0.23, P=0.013), BMI (r=0.265, P=0.004), AHI (r=0.61, P <0.001), LV TD-MPI (r=0.467, P <0.001), RV E/A ratio (r= −0.486, P <0.001) and systolic blood pressure (r=0.194, P=0.037). Figure 2 shows the relationship between AHI and RV TD-MPI. Upon multivariate linear regression analysis, RV TD-MPI was independently associated with AHI (β=0.468, P <0.001), LV TD-MPI (β=0.289, P <0.001), and RV E/A ratio (β= −0.23, P=0.007).
TABLE II.
Bivariate and Multivariate Relationships between RV TD-MPI and Variables
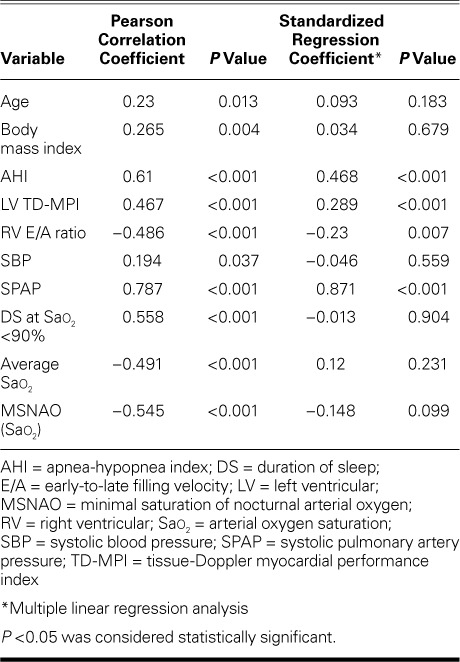
Fig. 2.
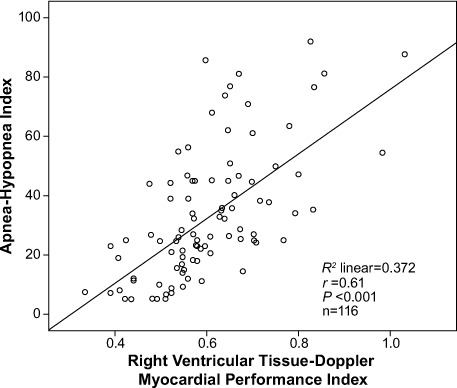
Scatter plot shows a positive correlation between the apnea-hypopnea index and the right ventricular tissue-Doppler myocardial performance index in patients with obstructive sleep apnea.
P <0.05 was considered statistically significant.
Bivariate and Multivariate Relationships between LV TD-MPI and Variables
Table III shows the bivariate associations of LV TD-MPI with age (r =0.207, P=0.026), BMI (r =0.207, P=0.026), AHI (r=0.349, P <0.001), RV TD-MPI (r=0.467, P <0.001), and LV E/A ratio (r= −0.179, P=0.113). Figure 3 shows the relationship between AHI and LV TD-MPI. Upon multivariate linear regression analysis, LV TD-MPI was associated with RV TD-MPI (β=0.258, P=0.005), LV E/A ratio (β= −0.234, P=0.03), and duration of sleep at arterial oxygen saturation <90% (β= −0.421, P=0.007).
TABLE III.
Bivariate and Multivariate Relationships between LV TD-MPI and Variables

Fig. 3.
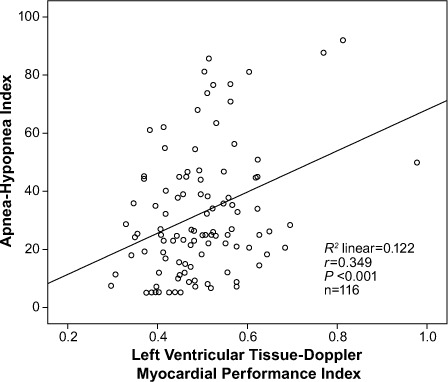
Scatter plot shows a positive correlation between the apnea-hypopnea index and the left ventricular tissue-Doppler myocardial performance index in patients with obstructive sleep apnea.
P <0.05 was considered statistically significant.
Discussion
Our results showed that OSA severity, determined by means of AHI, was independently associated with impairment of RV and LV functions as indicated by TD-MPI in patients who had OSA and normal EFs. An important finding of our study is that an increase in AHI affects RV global function more than it affects LV global function.
Previously, an inconsistent relationship between RV function and AHI was shown15; however, we found a strong correlation between AHI and RV TD-MPI, and in a larger population of patients who had isolated OSA. Previous investigators showed a correlation between OSA, LV mass, and LV global functions.16 Others found a relationship between OSA, LV mass, and LV diastolic functions.17 Obstructive sleep apnea might cause pulmonary hypertension and right-sided heart failure.18 Hammerstingl and colleagues19 said that the relationship of OSA to right-sided heart function was debatable. In their study, RV longitudinal strain values indicated that RV systolic functions decrease with disease severity; however, the authors observed no correlation between RV-MPI and AHI in their study and control groups.19 In a previous report, a correlation between AHI and biventricular TD-MPI was identified, similar to our findings.20 Obstructive sleep apnea (AHI >5) was inversely correlated with RV function when compared to that in control subjects.21,22 Therefore, in the current study, our main purpose was to show the relationship between biventricular TD-MPI and OSA severity as indicated by increases in AHI, rather than by the presence of OSA. To make our findings more reliable, we excluded patients who were taking medications or who had disorders that could have affected MPI values. To our knowledge, no previous investigators had evaluated the relationship between OSA severity and biventricular TD-MPI.
When we categorized our patients in accordance with AHI level (mild, moderate, and severe), the TD-MPI values of both ventricles varied significantly among the groups. We found that both LV and RV TD-MPI indicated global cardiac functional impairment that was proportional to the severity of OSA, even when patients had normal EFs. After we adjusted for BMI and age and repeated the correlation analysis, we observed that, although the relationship between AHI and LV TD-MPI was lost, the one between AHI and RV TD-MPI persisted. The LV TD-MPI correlated only with the RV TD-MPI and LV E/A ratio. Therefore, above all else, RV functions are impaired in patients who have OSA.
Previous investigators have disagreed with regard to the relationship between OSA and RV function.23–25 We excluded patients who had additional disorders or who were taking medications, and we directly evaluated the effects of AHI on biventricular functions. According to our results, AHI severity strongly affects RV function. Mechanisms that might cause RV dysfunction in OSA patients include repetitive nocturnal arterial oxygen desaturation and hypercapnia, large intrathoracic negative-pressure periods, and increases in pulmonary artery pressure.26,27 In animal models, intermittent hypoxia resulted in pulmonary artery remodeling and RV hypertrophy.28 Moreover, in OSA patients, hypoxic or hypercapnic periods and oxidative stress lead to pulmonary vasoconstriction and endothelial dysfunction, which causes pulmonary remodeling.29 Airway occlusion in OSA results in the generation of negative intra-thoracic pressure, which causes increased venous return, volume overload, and RV distention during apnea episodes.30 Primarily as a result of close anatomic association, the ventricles influence each other as a result of direct-injury extension, afterload changes, or ventricular interdependence. Accordingly, we speculate that, in OSA, RV dysfunction might lead to LV dysfunction.
Study Limitations
Coronary angiography was not performed in our patients; however, the diagnosis of coronary artery disease was excluded by means of clinical characteristics, medical histories, ECG results, and results of treadmill exercise tests. It would have been more instructive had we recorded data about ventricular interaction as an indicator of ventricular dysfunction. During some patients' echocardiographic examinations, we observed evidence of ventricular interaction such as ventricular septal shift or paradoxical movement; however, we did not record this, because it was not included in our study design.
Conclusion
In patients who have OSA, we found that RV and LV functions are impaired as AHI level increases; however, we attribute this impairment chiefly to RV dysfunction.
Footnotes
From: Departments of Cardiology (Drs. Akyol, Baykan, and Seker) and Pulmonology (Dr. Cortuk), Adana Numune Training and Research Hospital, 01170 Adana; Department of Pulmonology (Dr. Kiraz), Antalya Training and Research Hospital, 07030 Antalya; Department of Cardiology (Drs. Borekci and Gur), Kafkas University School of Medicine, 36270 Kars; and Department of Cardiology (Dr. Cayli), Dicle University School of Medicine, 21280 Diyarbakir; Turkey
References
- 1.Duran J, Esnaola S, Rubio R, Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. Am J Respir Crit Care Med. 2001;163(3 Pt 1):685–9. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 3.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) [published erratum appears in Circulation 2009;119(12):e380] Circulation. 2008;118(10):1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 4.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 5.Laaban JP, Pascal-Sebaoun S, Bloch E, Orvoen-Frija E, Oppert JM, Huchon G. Left ventricular systolic dysfunction in patients with obstructive sleep apnea syndrome. Chest. 2002;122(4):1133–8. doi: 10.1378/chest.122.4.1133. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Hino M, Mizuno K, Gemma A. Assessment of the relationship between right ventricular function and the severity of obstructive sleep-disordered breathing. Clin Respir J. 2014;8(2):145–51. doi: 10.1111/crj.12051. [DOI] [PubMed] [Google Scholar]
- 7.Eidem BW, Tei C, O'Leary PW, Cetta F, Seward JB. Nongeometric quantitative assessment of right and left ventricular function: myocardial performance index in normal children and patients with Ebstein anomaly. J Am Soc Echocardiogr. 1998;11(9):849–56. doi: 10.1016/s0894-7317(98)70004-5. [DOI] [PubMed] [Google Scholar]
- 8.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, Seward SB. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9(6):838–47. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 9.Tei C, Nishimura RA, Seward JB, Tajik AJ. Noninvasive Doppler-derived myocardial performance index: correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10(2):169–78. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]
- 10.Tei C, Dujardin KS, Hodge DO, Kyle RA, Tajik AJ, Seward JB. Doppler index combining systolic and diastolic myocardial performance: clinical value in cardiac amyloidosis. J Am Coll Cardiol. 1996;28(3):658–64. doi: 10.1016/0735-1097(96)00202-1. [DOI] [PubMed] [Google Scholar]
- 11.Tei C, Ling LH, Hodge DO, Bailey KR, Oh JK, Rodeheffer RJ et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function--a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26(6):357–66. [PubMed] [Google Scholar]
- 12.Ozturk S, Ayhan S, Aslantas Y, Erdem A, Ozlu MF, Ekinozu I, Yazici M. Detection of left ventricular asynchrony and its relationship with the Tei index in patients with coronary artery ectasia. Exp Clin Cardiol. 2013;18(1):e8–e11. [PMC free article] [PubMed] [Google Scholar]
- 13.The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Darien (IL): American Academy of Sleep Medicine; 2007. [Google Scholar]
- 14.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–67. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 15.Dursunoglu N, Dursunoglu D, Ozkurt S, Gur S, Ozalp G, Evyapan F. Effects of CPAP on right ventricular myocardial performance index in obstructive sleep apnea patients without hypertension. Respir Res. 2006;7:22. doi: 10.1186/1465-9921-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dursunoglu D, Dursunoglu N, Evrengul H, Ozkurt S, Kuru O, Kilic M, Fisekci F. Impact of obstructive sleep apnoea on left ventricular mass and global function. Eur Respir J. 2005;26(2):283–8. doi: 10.1183/09031936.05.00038804. [DOI] [PubMed] [Google Scholar]
- 17.Niroumand M, Kuperstein R, Sasson Z, Hanly PJ. Impact of obstructive sleep apnea on left ventricular mass and diastolic function. Am J Respir Crit Care Med. 2001;163(7):1632–6. doi: 10.1164/ajrccm.163.7.2007014. [DOI] [PubMed] [Google Scholar]
- 18.Bradley TD, Rutherford R, Grossman RF, Lue F, Zamel N, Moldofsky H, Phillipson EA. Role of daytime hypoxemia in the pathogenesis of right heart failure in the obstructive sleep apnea syndrome. Am Rev Respir Dis. 1985;131(6):835–9. doi: 10.1164/arrd.1985.131.6.835. [DOI] [PubMed] [Google Scholar]
- 19.Hammerstingl C, Schueler R, Wiesen M, Momcilovic D, Pabst S, Nickenig G, Skowasch D. Effects of untreated obstructive sleep apnea on left and right ventricular myocardial function. Int J Cardiol. 2012;155(3):465–9. doi: 10.1016/j.ijcard.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Romero-Corral A, Somers VK, Pellikka PA, Olson EJ, Bailey KR, Korinek J et al. Decreased right and left ventricular myocardial performance in obstructive sleep apnea. Chest. 2007;132(6):1863–70. doi: 10.1378/chest.07-0966. [DOI] [PubMed] [Google Scholar]
- 21.Altekin RE, Karakas MS, Yanikoglu A, Ozel D, Ozbudak O, Demir I, Deger N. Determination of right ventricular dysfunction using the speckle tracking echocardiography method in patients with obstructive sleep apnea. Cardiol J. 2012;19(2):130–9. doi: 10.5603/cj.2012.0024. [DOI] [PubMed] [Google Scholar]
- 22.Dursunoglu N, Dursunoglu D, Kilic M. Impact of obstructive sleep apnea on right ventricular global function: sleep apnea and myocardial performance index. Respiration. 2005;72(3):278–84. doi: 10.1159/000085369. [DOI] [PubMed] [Google Scholar]
- 23.Bradley TD. Right and left ventricular functional impairment and sleep apnea. Clin Chest Med. 1992;13(3):459–79. [PubMed] [Google Scholar]
- 24.Guidry UC, Mendes LA, Evans JC, Levy D, O'Connor GT, Larson MG et al. Echocardiographic features of the right heart in sleep-disordered breathing: the Framingham Heart Study. Am J Respir Crit Care Med. 2001;164(6):933–8. doi: 10.1164/ajrccm.164.6.2001092. [DOI] [PubMed] [Google Scholar]
- 25.Weitzenblum E, Chaouat A. Sleep and chronic obstructive pulmonary disease. Sleep Med Rev. 2004;8(4):281–94. doi: 10.1016/j.smrv.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Nishimura RA, Housmans PR, Hatle LK, Tajik AJ. Assessment of diastolic function of the heart: background and current applications of Doppler echocardiography. Part I. Physiologic and pathophysiologic features. Mayo Clin Proc. 1989;64(1):71–81. doi: 10.1016/s0025-6196(12)65305-1. [DOI] [PubMed] [Google Scholar]
- 27.Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis. 2009;51(5):363–70. doi: 10.1016/j.pcad.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Nattie EE, Bartlett D, Jr, Johnson K. Pulmonary hypertension and right ventricular hypertrophy caused by intermittent hypoxia and hypercapnia in the rat. Am Rev Respir Dis. 1978;118(4):653–8. doi: 10.1164/arrd.1978.118.4.653. [DOI] [PubMed] [Google Scholar]
- 29.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99(7):675–91. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 30.Bradley TD, Hall MJ, Ando S, Floras JS. Hemodynamic effects of simulated obstructive apneas in humans with and without heart failure. Chest. 2001;119(6):1827–35. doi: 10.1378/chest.119.6.1827. [DOI] [PubMed] [Google Scholar]


