Abstract
Introduction
Most HIV-positive persons in sub-Saharan Africa initiate antiretroviral therapy (ART) with advanced infection (late ART initiation). Intervening on the drivers of late ART initiation is a critical step towards achieving the full potential of HIV treatment scale-up. This study aimed to identify modifiable factors associated with late ART initiation in Ethiopia.
Methods
From 2012 to 2013, Ethiopian adults (n=1180) were interviewed within two weeks of ART initiation. Interview data were merged with HIV care histories to assess correlates of late ART initiation (CD4+ count <150 cells/µL or World Health Organization Stage IV).
Results
The median CD4 count at enrolment in HIV care was 263 cells/µL (interquartile range (IQR): 140 to 390) and 212 cells/µL (IQR: 119 to 288) at ART initiation. Overall, 31.2% of participants initiated ART late, of whom 85.1% already had advanced HIV disease at enrolment. Factors associated with higher odds of late ART initiation included male sex (vs. non-pregnant females; adjusted odds ratio (aOR): 2.02; 95% CI: 1.50 to 2.73), high levels of psychological distress (vs. low/none, aOR: 1.96; 95% CI: 1.34 to 2.87), perceived communication barriers with providers (aOR: 2.42, 95% CI: 1.24 to 4.75), diagnosis via provider initiated testing (vs. voluntary counselling and testing, aOR: 1.47, 95% CI: 1.07 to 2.04), tuberculosis (TB) treatment prior to ART initiation (aOR: 2.16, 95% CI: 1.43 to 3.25) and a gap in care of six months or more prior to ART initiation (aOR: 2.02, 95% CI: 1.10 to 3.72). Testing because of partner illness/death (aOR: 0.64, 95% CI: 0.42 to 0.95) was associated with lower odds of late ART initiation.
Conclusions
Programmatic initiatives promoting earlier diagnosis, engagement in pre-ART care, and integration of TB and HIV treatments may facilitate earlier ART initiation. Men and those experiencing psychological distress may also benefit from targeted support prior to ART initiation.
Keywords: HIV-positive adults, antiretroviral therapy initiation, tuberculosis treatment, Ethiopia, antiretroviral therapy guidelines, implementation science
Introduction
Although HIV care services have been increasingly scaled up [1], most HIV-positive persons in sub-Saharan Africa start treatment only after developing advanced infection, which leads to high early mortality [2], complex and expensive clinical management [3], blunted immune response [4] and missed opportunities to prevent HIV transmission [5]. The problem of late antiretroviral therapy (ART) initiation in sub-Saharan Africa has improved only slightly since the start of HIV scale-up in the region [6,7], making it important to identify its determinants, as well as necessary programmatic adjustments and policy changes.
Studies of factors associated with advanced HIV infection prior to ART initiation (i.e. at diagnosis and/or presentation to care) in sub-Saharan Africa have found that sex, pregnancy, age, family composition, living arrangements, education level, employment status, competing priorities, disclosure status, emotional health and alcohol use were important correlates [8–13]. Patients’ perceptions of stigma, medication side effects and healthcare access barriers may also contribute [8,10]. Demographic and clinical factors associated with a higher likelihood of advanced HIV disease at ART initiation have included male sex, being already beyond the point of ART eligibility at the time of enrolment into HIV care, TB treatment at ART initiation and a ≥12 month gap in care between enrolment and ART initiation [7,14]. A qualitative study from Uganda additionally reported patient concerns about stigma, lack of confidentiality, low social support and misconceptions about ART as barriers to ART initiation [15]. However, most studies have not examined factors beyond those routinely collected in HIV clinical records or the role of the pre-ART phase of care, limiting the ability to inform the development of interventions.
Ethiopia, with an estimated HIV prevalence among adults of 1.3% (590,000 persons) and 4.2% in urban areas [1,16], began scaling up comprehensive HIV services (including ART) in 2005. In 2012, however, only 68% of adult Ethiopians eligible for ART were receiving it [1]. In order to help maximize the individual and public health benefit from the treatment eligibility guideline expansion [17], the present study was designed to identify the major drivers of late ART initiation, particularly those that are amenable to intervention.
Methods
Study setting
We enrolled 1180 patients initiating ART at six HIV clinical care sites in the Oromia region of Ethiopia, and part of the Ethiopian National ART Program, under the jurisdiction of the Oromia Regional Health Bureau. The combined catchment areas of these clinics cover 9.3 million people, with background HIV prevalence estimates ranging from 3.2 to 11.5%, according to antenatal clinic (ANC) data at the sites. The clinics have been supported by the Ethiopian Ministry of Health with technical assistance from ICAP at Columbia University since 2005, via funding from the President's Emergency Plan for AIDS Relief. All six sites are secondary health facilities in urban areas, with on-site CD4+ testing and ART pharmacies. All offer voluntary counselling and testing (VCT), conduct provider initiated counselling and testing (PITC) and have ANC and labour and delivery wards. In January 2013, during the course of this study, the national guidelines for ART initiation were expanded from World Health Organization (WHO) Stage IV or CD4+ count <200 cells/µL or WHO Stage III with CD4+ <350 cells/µL to include all patients with CD4+ <350 cells/µL.
Participant recruitment and data collection
Interviews
All non-institutionalized patients aged 18 years and older initiating ART between June 2012 and April 2013 were eligible for inclusion in the study. Such persons were referred by providers to the study staff on the day of ART initiation. Study staff administered the structured questionnaire (lasting 45 to 60 minutes) with questions related to psychosocial factors and HIV care. The interviews were conducted within two weeks of the day of ART initiation, and participants were given a snack and money for transportation home from the clinic (20 Ethiopian birr, or approximately 1 USD). Among the eligible patients referred to the study, 95% consented and completed the interview.
Routine clinical information
Data obtained from patients at the time of HIV care enrolment and each subsequent clinic visit were recorded on national forms. In accordance with routine clinic procedures, clerks entered medical chart data into electronic databases. Assessments of data entry completeness and accuracy were done regularly. Interview data were linked with clinic medical records for the period between HIV care enrolment and ART initiation and then de-identified for analysis.
Measures
Outcome
Advanced HIV infection at ART initiation was defined as having a CD4+ count <150 cells/µL or WHO Stage IV. CD4+ count or WHO stage at ART initiation included those measurements taken three months before or one month after ART initiation. In instances where CD4+ count or WHO stage were missing in the above window, we used the highest stage preceding ART initiation and any prior CD4+ count <150 cells/µL.
Psychosocial variables
Questionnaire design and variable selection were guided by the framework of health service use developed by Aday and Andersen [18]. Predisposing factors (i.e. pre-existing propensity to access care) examined included sex, age, education, relationship status, having ever had children, alcohol use, psychological distress, enacted and internalized stigma, and history of holy water use for HIV. Alcohol use frequency was divided into high (at least twice a week), moderate (at least monthly) and low/none (never or not in the last three months). Psychological distress was assessed through the Kessler-10 scale, with three categories of distress: high (score of 30 to 50), mild/moderate (20 to 29) and low/none (10 to 19) [19]. Internalized and enacted stigma measures (alpha=0.99 with statements such as “you felt completely worthless,” and alpha=0.94 with statements such as “someone scolded you”) had answer options ranging from “most of the time” to “never” and were drawn from the instrument developed by Holzemer et al. [20]. For analysis, average scores on internalized stigma were grouped into tertiles and those on enacted stigma into “any stigma” or “none,” based on the respective score distributions. We also examined beliefs related to ART through a set of 10 items (alpha=0.74), for example, “AIDS no longer kills everyone because of the ARV medicines,” with responses on a 4-point Likert scale (ranging from 1, “strongly disagree,” to 4, “strongly agree”). Mean scores were categorized into high accuracy (if the score corresponded to “agree” or “strongly agree”) and low accuracy [21].
Enabling factors (i.e. means available to access care) examined included urban/rural residence; food insecurity; knowing someone on ART; HIV status disclosure; social support (9 questions; Cronbach's alpha=0.92, adapted from previous work by Wortman [22] with questions such as “Would someone be available to talk to you if you were upset, nervous or depressed?”); and communication barriers with providers (difficulties understanding information about HIV care or ART side effects).
Having been tested for HIV because of partner's sickness, death or HIV diagnosis was examined within the “need” component of the framework.
Correlates from routine clinical information
CD4+ count at enrolment in HIV care was defined as the earliest recorded CD4+ measurement in the first six months in care. A gap in care was defined as not having had a clinic visit for at least six months prior to ART initiation. Enrolment point of entry captured the source of the patient's referral to HIV care. Receipt of TB treatment at any point prior to ART initiation was also recorded.
Statistical methods
We compared the distribution of key sample characteristics between male and female participants using chi-square and Mann-Whitney tests of statistical significance. Potential correlates of late ART initiation were examined through bivariate and multivariable logistic regression. To assess added information on correlates of late ART initiation that may be gained from psychosocial data, two separate regression models were constructed using only psychosocial data and then combining them with routine clinical variables. Multivariable regression models initially included all variables that had p-values <0.20 in bivariate analyses. Covariates with the highest p-values were eliminated through backward stepwise regression until all remaining variables had p-values <0.05. Eliminated variables were then added individually back to this model in case of negative confounding (i.e. associations observed only in the presence of other variables). Sex and age group were retained in multivariable models in order to produce sex and age-adjusted odds ratios. Sex-specific models were also constructed to elucidate possible differences in correlates by gender. Median CD4+ counts at ART initiation were plotted by month and sex to assess the trend over time and possible impact of expanded guidelines on ART uptake. Analyses were conducted in SAS 9.3 (SAS Institute, Cary, NC, USA) using conditional logistic models with clinical sites as strata.
Ethical considerations
The study was approved by the Institutional Review Boards of the Oromia Regional Health Bureau, Columbia University Medical Center and the City University of New York. Written, informed consent for the interview and extraction of medical record data was obtained from each patient prior to study enrolment.
Results
Characteristics of persons initiating ART
The majority of participants were women (61.2%) and the median age was 34 (interquartile range (IQR): 28 to 40). Most patients were Ethiopian Orthodox (69.9%), urban residents (78.0%) and had no more than primary school education (71.8%). Women were more likely than men to have received no education (39.1% vs. 20.1%, p<0.001). More than half of the participants were in a relationship at the time of ART initiation and a quarter had been widowed. The largest proportion (55.7%) was first diagnosed with HIV in a PITC setting (Table 1).
Table 1.
Characteristics of patients initiating ART, by sex, at six Ethiopian clinics (June 2012 to April 2013)
| Total | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Men | Women | |||||
| n | % | n | % | n | % | pa | |
| Total | 1180 | 100.0 | 458 | 38.8 | 722 | 61.2 | – |
| Sex | |||||||
| Male | 458 | 38.8 | – | – | – | – | – |
| Female, not pregnant | 669 | 56.7 | – | – | – | – | |
| Female, pregnant | 53 | 4.5 | – | – | – | – | |
| Age | |||||||
| Median (IQR) | 34 (28 to 40) | 37 (32 to 43) | 30 (26 to 38) | <0.001 | |||
| 18 to 29 | 374 | 31.7 | 78 | 17.0 | 296 | 41.0 | <0.001 |
| 30 to 39 | 482 | 40.8 | 205 | 44.8 | 277 | 38.4 | |
| 40 to 49 | 238 | 20.2 | 131 | 28.6 | 107 | 14.8 | |
| 50 + | 86 | 7.3 | 44 | 9.6 | 42 | 5.8 | |
| Religion | |||||||
| Ethiopian Orthodox | 825 | 69.9 | 323 | 70.5 | 502 | 69.5 | 0.549 |
| Protestant | 239 | 20.3 | 85 | 18.6 | 154 | 21.3 | |
| Muslim | 108 | 9.2 | 47 | 10.3 | 61 | 8.4 | |
| Other Christian | 8 | 0.7 | 3 | 0.7 | 5 | 0.7 | |
| Highest education level | |||||||
| No school | 374 | 31.7 | 92 | 20.1 | 282 | 39.1 | <0.001 |
| Primary school or vocational/other | 484 | 41.0 | 206 | 45.0 | 278 | 38.5 | |
| Secondary school | 239 | 20.3 | 111 | 24.2 | 128 | 17.7 | |
| University | 83 | 7.0 | 49 | 10.7 | 34 | 4.7 | |
| Area of residence | |||||||
| Urban area | 920 | 78.0 | 335 | 73.1 | 585 | 81.0 | 0.002 |
| Rural area | 259 | 21.9 | 122 | 26.6 | 137 | 19.0 | |
| Unknown | 1 | 0.1 | 1 | 0.2 | 0 | 0.0 | |
| Relationship status | |||||||
| In a relationship | 673 | 57.0 | 309 | 67.5 | 364 | 50.4 | <0.001 |
| Not in a relationship | 507 | 43.0 | 149 | 32.5 | 358 | 49.6 | |
| Ever widowed | |||||||
| Yes | 293 | 24.8 | 75 | 16.4 | 218 | 30.2 | <0.001 |
| No | 874 | 74.1 | 377 | 82.3 | 497 | 68.8 | |
| Unknown | 13 | 1.1 | 6 | 1.3 | 7 | 1.0 | |
| Original diagnosis unit | |||||||
| PITC | 647 | 54.8 | 245 | 53.5 | 402 | 55.7 | 0.763 |
| VCT | 513 | 43.5 | 205 | 44.8 | 308 | 42.6 | |
| Unknown | 20 | 1.7 | 8 | 1.7 | 12 | 1.7 | |
| Site | |||||||
| Ambo Hospital | 241 | 20.4 | 109 | 23.8 | 132 | 18.3 | 0.027 |
| Bishoftu Hospital | 309 | 26.2 | 133 | 29.0 | 176 | 24.4 | |
| Fitche Hospital | 166 | 14.1 | 53 | 11.6 | 113 | 15.7 | |
| Goba Hospital | 129 | 10.9 | 45 | 9.8 | 84 | 11.6 | |
| Nekemte Hospital | 194 | 16.4 | 70 | 15.3 | 124 | 17.2 | |
| Shashemene Hospital | 141 | 11.9 | 48 | 10.5 | 93 | 12.9 | |
ART, antiretroviral therapy; IQR, interquartile range; PITC, provider-initiated testing and counselling; VCT, voluntary counselling and testing
chi-squared test for categorical variables, Mann-Whitney U test for continuous variables.
Clinical and immunological characteristics at enrolment and ART initiation
Of the 97.4% of participants with available CD4+ counts and/or WHO stage at enrolment in HIV care, 27.3% had enrolled in HIV care with advanced HIV infection (CD4 <150 cells/µL or WHO Stage IV). The median enrolment CD4+ count among the 94.0% of participants with available data was 263 cells/µL (IQR: 140 to 390), with significantly higher values among women (median=296; IQR: 172 to 423) than men (median=206; IQR: 108 to 344, p<0.001). Overall, 47.8% of participants were already beyond the point of ART eligibility at enrolment in HIV care, including 57.9% of men and 41.4% of women (p<0.001). Median time between enrolment in care and ART initiation was 2.9 months (IQR: 15 days to 2.6 years). This period was significantly shorter among men than women (median 1.1 months vs. 5.3 months, respectively; p<0.001). At ART initiation, 31.2% of participants were classified as having late-stage HIV infection, most of whom (85.1%) already had advanced HIV disease at enrolment in care. Among the 91.2% of participants with available data, the median CD4+ count at treatment initiation was 212 cells/µL (IQR: 119 to 288) and was significantly higher among women than men (median=232; IQR: 152 to 302; vs. median=178; IQR: 98 to 251, respectively; p<0.001). After HIV care enrolment and prior to ART initiation, 12.7% of patients underwent TB treatment (Table 2). Among these patients, the median delay between initiation of TB treatment and ART was 27 days (IQR: 14 to 97 days), with 65% starting ART within eight weeks.
Table 2.
Clinical and immunological characteristics at enrolment in care and ART initiation at six Ethiopian clinics (June 2012 to April 2013)
| Enrolment in care | ART initiation | |||||
|---|---|---|---|---|---|---|
| n (%) | % among men | % among women | n (%) | % among men | % among women | |
| Total | 1180 (100) | 1180 (100) | ||||
| Advanced HIV diseasea | ||||||
| Data available | 1149 (97.4) | 98.0 | 96.8 | 1180 (100) | 100 | 100 |
| Yes | 313 (27.3) | 38.3 | 20.1 | 368 (31.2) | 43.2 | 23.6 |
| No | 835 (72.7) | 61.7 | 79.9 | 812 (68.8) | 56.7 | 76.4 |
| CD4+ count, cells/µL | ||||||
| Data available | 1109 (94.0) | 6.1 | 6.0 | 1076 (91.2) | 93.9 | 89.5 |
| Median (IQR) | 263 (140 to 390) | 206 (108 to 344) | 296 (172 to 423) | 212 (119 to 288) | 178 (98 to 251) | 232 (152 to 302) |
| Clinical WHO disease stage | ||||||
| Data available | 1136 (96.3) | 96.9 | 95.7 | 1177 (99.7) | 99.8 | 99.6 |
| Stage I | 381 (33.6) | 22.5 | 40.6 | 257 (21.8) | 14.9 | 26.2 |
| Stage II | 307 (27.0) | 30.2 | 25.0 | 335 (28.5) | 28.5 | 28.5 |
| Stage III | 418 (36.8) | 43.9 | 32.2 | 540 (45.9) | 52.7 | 41.6 |
| Stage IV | 30 (2.6) | 3.4 | 2.2 | 45 (3.8) | 3.9 | 3.8 |
| ART eligibility at enrolmentb | ||||||
| Not eligible | 585 (49.6) | 40.1 | 55.5 | – | – | |
| Eligible | 564 (47.8) | 57.9 | 41.4 | – | – | |
| Unknown | 31 (2.6) | 2.0 | 3.1 | – | – | |
| History of TB treatment in HIV care | ||||||
| Yes | – | – | 150 (12.7) | 15.9 | 10.7 | |
| No | – | – | 1030 (87.3) | 84.1 | 89.3 | |
| Time between diagnosis and ART initiation | ||||||
| Median (IQR) | 8.3 months (1.1 months to 3.4 years) | |||||
| Men | 3.6 months (27 days to 2.4 years) | |||||
| Women | 1.1 years (1.2 months to 4 years) | |||||
| Time between enrolment in care and ART initiation | ||||||
| Median (IQR) | 2.9 months (15 days to 2.6 years) | |||||
| Men | 1.1 months (14 days to 1.4 months) | |||||
| Women | 5.3 months (18 days to 3.1 years) | |||||
ART, antiretroviral therapy; IQR, interquartile range; TB, tuberculosis; WHO, World Health Organization
advanced HIV disease at enrolment or ART initiation is defined as having a CD4+ cell count <150 cells/µL or WHO Stage IV
eligibility based on national Ethiopian HIV guidelines.
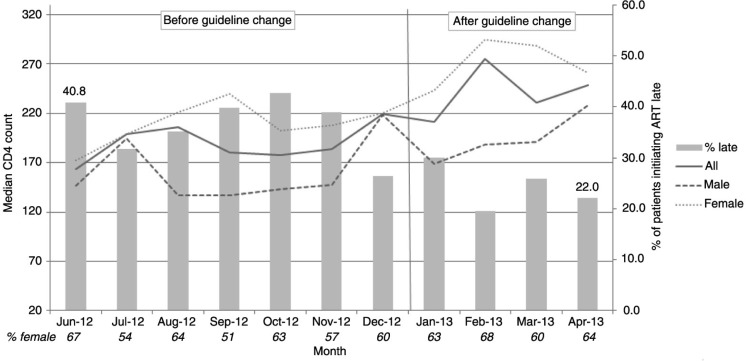
During the study period, there was an appreciable increase in the median CD4+ count at ART initiation. An increase in CD4+ counts was observed among both men (average of 30 cells) and women (average of 78 cells) after the January 2013 guideline expansion (p=0.003 and p<0.001, respectively). The proportion of patients starting treatment with advanced HIV infection decreased significantly, from 40.8% in June 2012 to 22.0% in April 2013 (p trend<0.001; Figure 1).
Figure 1.
Median CD4+ count at antiretroviral therapy (ART) initiation and proportion of patients initiating ART late, by month and sex.
Factors associated with late ART initiation
Table 3 presents bivariate associations, a multivariable model including only the psychosocial variables and a final multivariable model incorporating the clinical variables.
Table 3.
Bivariate and multivariable associations between participants’ characteristics and late ART initiationa
| Bivariate | Multivariable Model 1 (N=1171)b | Multivariable Model 2 (N=1174)c | ||
|---|---|---|---|---|
| n | OR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Predisposing factors | ||||
| Sex (ref: non-pregnant women, n=669) | ||||
| Men | 458 | 2.34 (1.81 to 3.03) | 2.29 (1.73 to 3.02) | 2.02 (1.50 to 2.73) |
| Pregnant women | 53 | 0.44 (0.19 to 1.00) | 0.41 (0.18 to 0.97) | 0.53 (0.21 to 1.33) |
| Age at ART initiation (ref: 18 to 29, n=374) | ||||
| 30 to 39 | 482 | 1.63 (1.21 to 2.19) | 1.27 (0.92 to 1.75) | 1.25 (0.88 to 1.77) |
| 40 to 49 | 238 | 1.29 (0.90 to 1.85) | 0.91 (0.61 to 1.35) | 0.92 (0.60 to 1.40) |
| 50 + | 86 | 1.14 (0.67 to 1.93) | 0.72 (0.41 to 1.26) | 0.60 (0.33 to 1.10) |
| Education level (ref: primary school or vocational/other, n=484) | ||||
| No school | 374 | 0.78 (0.57 to 1.05) | ||
| Secondary school | 239 | 1.09 (0.78 to 1.51) | ||
| University | 83 | 1.16 (0.71 to 1.91) | ||
| Relationship status (ref: in a relationship, n=673) | ||||
| Not in a relationship | 507 | 1.15 (0.90 to 1.48) | ||
| Has ever had children (ref: no, n=253) | ||||
| Yes | 926 | 0.78 (0.58 to 1.05) | ||
| Alcohol use (ref: low or none, n=916) | ||||
| High | 162 | 1.59 (1.12 to 2.26) | ||
| Moderate | 102 | 1.06 (0.68 to 1.67) | ||
| Psychological distress (ref: low or none, n=480) | ||||
| Medium | 348 | 1.93 (1.41 to 2.66) | 1.76 (1.27 to 2.46) | 1.61 (1.12 to 2.31) |
| High | 347 | 2.91 (2.08 to 4.07) | 2.70 (1.90 to 3.85) | 1.96 (1.34 to 2.87) |
| Enacted stigma (ref: none, n=983) | ||||
| Any | 192 | 1.05 (0.74 to 1.48) | ||
| Internalized stigma (ref: top tercile/least stigma, n=469) | ||||
| Bottom tercile (=most stigma) | 395 | 1.44 (1.06 to 1.94) | ||
| Middle tercile | 310 | 1.24 (0.90 to 1.70) | ||
| History of holy water use for HIV (ref: no, n=840) | ||||
| Yes | 189 | 0.69 (0.48 to 0.99) | ||
| Treatment beliefs (ref: low accuracy, n=118) | ||||
| High accuracy | 1061 | 0.86 (0.56 to 1.32) | ||
| Enabling factors | ||||
| Area of residence (ref: rural, n=259) | ||||
| Urban | 920 | 0.79 (0.59 to 1.07) | ||
| Trouble satisfying food needs in last year (ref: never, n=422) | ||||
| Sometimes | 502 | 0.91 (0.68 to 1.22) | ||
| Often | 252 | 0.84 (0.59 to 1.19) | ||
| Knows someone on ART (ref: no, n=368) | ||||
| Yes | 809 | 0.73 (0.56 to 0.95) | 0.77 (0.57 to 1.04) | |
| Number of people disclosed to (ref: 0, n=167) | ||||
| 1 | 422 | 0.79 (0.55 to 1.15) | 1.00 (0.67 to 1.49) | |
| ≥ 2 | 591 | 0.57 (0.40 to 0.82) | 0.69 (0.46 to 1.02) | |
| Social support (ref: bottom quarter/least support; n=298) | ||||
| Second quarter | 305 | 0.91 (0.64 to 1.28) | ||
| Third quarter | 292 | 0.84 (0.59 to 1.19) | ||
| Top quarter (= most support) | 285 | 0.90 (0.62 to 1.30) | ||
| Communication barriers with providers (ref: no, n=1127) | ||||
| Yes | 53 | 2.27 (1.30 to 3.97) | 2.43 (1.32 to 4.48) | 2.42 (1.24 to 4.75) |
| Perceived need | ||||
| Tested for HIV because partner sick/dead/HIV+ (ref: no, n=930) | ||||
| Yes | 250 | 0.38 (0.27 to 0.55) | 0.47 (0.32 to 0.69) | 0.64 (0.42 to 0.95) |
| Clinical characteristics | ||||
| Enrolment point of entry (ref: VCT, n=493) | ||||
| PMTCT | 45 | 0.31 (0.11 to 0.88) | – | 0.56 (0.18 to 1.72) |
| PITC | 491 | 1.80 (1.36 to 2.39) | – | 1.47 (1.07 to 2.04) |
| Other, including TB clinic | 32 | 2.15 (1.03 to 4.49) | – | 1.85 (0.73 to 4.71) |
| Unknown | 119 | 2.22 (1.45 to 3.42) | – | 1.70 (1.04 to 2.77) |
| History of TB treatment in HIV care (ref: no, n=1030) | ||||
| Yes | 150 | 2.93 (2.07 to 4.16) | – | 2.16 (1.43 to 3.25) |
| Gap in care of six months or more prior to ART (ref: no, n=260) | ||||
| Yes | 260 | 2.41 (1.35 to 4.33) | – | 2.02 (1.10 to 3.72) |
| Initiated ART during first six months in care | 659 | 11.64 (7.04 to 19.25) | – | 8.98 (5.34 to 15.08) |
aOR, adjusted odds ratio; ART, antiretroviral therapy; OR, odds ratio; VCT, voluntary counselling and testing; PMTCT, prevention of mother-to-child transmission; PITC, provider-initiated testing and counselling; TB, tuberculosis
late ART initiation is defined as having a CD4+ cell count <150 cells/µL or WHO Stage IV. Analyses account for site-level clustering. Statistically significant associations (p<0.05) in bold
multivariable Model 1 includes only psychosocial factors
multivariable Model 2 is final, combining psychosocial and clinical factors.
Psychosocial variables
In the final multivariable model, men had twice the odds of late ART initiation as non-pregnant women (aOR: 2.02; 95% CI: 1.50 to 2.73). Psychological distress (high vs. low/none, aOR: 1.96; 95% CI: 1.34 to 2.87) and perceived communication barriers with providers (vs. no barriers, aOR: 2.42; 95% CI: 1.24 to 4.75) were associated with increased odds of late ART initiation, whereas testing for HIV because of a partner's death or illness (vs. not, aOR: 0.64; 95% CI: 0.42 to 0.95) was associated with lower odds. In the multivariable model containing only psychosocial variables, patients who knew someone on ART (vs. not, aOR: 0.77; 95% CI: 0.57 to 1.04) and those who had disclosed their status to two or more people (vs. 0, aOR: 0.69; 95% CI: 0.46 to 1.02) were also marginally less likely to initiate treatment with advanced HIV disease, but these associations were no longer significant after adjustment for clinical factors.
Clinical variables
Participants referred to HIV care from PITC (vs. VCT, aOR: 1.47; 95% CI: 1.07 to 2.04) and those with history of TB treatment while in HIV care (vs. no treatment, aOR: 2.16; 95% CI: 1.43 to 3.25) had higher odds of initiating treatment with advanced HIV infection. Having a gap in pre-ART care of six months or more was also associated with late ART initiation (aOR: 2.02; 95% CI: 1.10 to 3.72), as was initiation in the first six months after enrolment in care (aOR: 8.98; 95% CI: 5.34 to 15.08). Inclusion of the gap in care variable eliminated the disclosure variable from the final model (Table 3).
Sex-specific models
When psychosocial and clinical data were analyzed separately for men and women, sex differences included marginally lower odds of late ART initiation by men if they had disclosed their HIV status to at least two people (vs. none, aOR: 0.61; 95% CI: 0.36 to 1.05) and higher odds of late ART initiation if referred to HIV care from PITC settings (vs. VCT, aOR: 1.96; 95% CI: 1.21 to 3.18). In contrast, women had higher odds of late treatment initiation if they reported experiencing communication barriers with providers (vs. none, aOR: 2.51; 95% CI: 1.10 to 5.75) (models not shown).
Discussion
In our study, conducted with a large sample of patients initiating ART in a mature national HIV programme in East Africa, 31.2% of patients started ART with advanced HIV infection (CD4+ <150 cells/µL or WHO Stage IV), of whom 85.1% already had advanced HIV infection at the time of enrolment in care. This finding underscores the need to promote and expand testing coverage in the community to provide opportunities for earlier diagnosis with timely linkage to care, particularly for men. Additionally, we identified a number of factors that could represent potentially important targets for interventions aimed at reducing the persistently high rates of late ART initiation.
In multivariable analysis, consistent with prior research [7,14], male sex was a strong correlate of late ART initiation, with men having twice the odds of starting treatment with advanced HIV infection as non-pregnant women. This disparity is increasing over time [7] and has been at least partly attributed to women's increasing access to PMTCT services [23,24], as well as differences in health-seeking behaviour resulting in delayed diagnosis and care entry among men [25].
Patients with a gap in care of six months or greater prior to ART initiation had twice the odds of initiating treatment with advanced HIV infection. This finding is consistent with the results of a recent large-scale analysis of data from sub-Saharan Africa conducted by our team [7]. Others have noted that fewer than a third of patients not eligible for treatment at the time of enrolment in care are retained in pre-ART care in the region [26]. In one study conducted in Ethiopia, those with less advanced HIV infection were more likely to be lost to follow-up prior to ART initiation than those with advanced HIV infection [12]. Patients enrolling in care at the early stages of HIV infection may therefore be more likely to remain engaged in care where there are services that could be perceived as beneficial to them such as free co-trimoxazole [27] and time-saving clinic-level efficiencies, such as reliable point-of-care CD4+ testing [28].
Additional support may be beneficial for patients undergoing TB treatment in HIV care, who had twice the odds of late ART initiation as those who did not undergo TB treatment; an association previously also reported elsewhere in sub-Saharan Africa [7]. Although TB disease is more common among persons with advanced HIV-related immunosuppression, both the disease itself and TB therapy can each be markers of and risk factors for late ART initiation. TB disease can accelerate CD4+ decline [29] and uptake of treatment guidelines in clinical practice can take time [30]. Although WHO guidelines recommend that TB treatment be started first, with ART added as soon as possible during the first eight weeks of TB therapy [17,31], only 65% of TB patients in our study initiated ART within that period. Concerns about drug interactions, additional side effects, immune reconstitution inflammatory syndrome and high pill burden often cause ART initiation to be delayed until TB treatment has been completed, despite evidence that integration of TB and HIV treatment may successfully extend AIDS-free survival of severely immunocompromised patients [32].
Alongside clinical variables, several psychosocial factors were identified as significant correlates of late ART initiation. The marginal association between disclosure of HIV status to at least two people and lower odds of late ART initiation suggests that practical and emotional support potentially enabled by disclosure may facilitate engagement in care and timely treatment initiation. A similar observation was previously reported in another Ethiopian study, where non-disclosure of HIV status was associated with late presentation to HIV care [10]. Although facilitation of disclosure and testing of family members, mainly partners, is recognized by HIV care providers as a critical element of pre-ART care, disclosure appears to be discussed less frequently with patients after initial enrolment visits [33], perhaps because most patients have by then disclosed their status to at least one person. However, our findings suggest that it may be worthwhile for clinic staff to continue encouraging disclosure, especially among men, even after a patient has disclosed to one other person. That the disclosure variable was eliminated from our final multivariable model when the gap in care variable was introduced suggests that time since enrolment in care partially confounds the association between disclosure and late ART initiation [34], but also that poor engagement in care may be one of the ways that under-disclosure influences the risk of delayed treatment initiation.
Enacted and internalized stigma were not significantly associated with late ART initiation, consistent with a prior ecological study from eight sub-Saharan African countries [35], but in contrast to qualitative research from the region [15]. Psychological distress, however, previously linked with late HIV diagnosis [8], was strongly associated with late ART initiation in our analysis. Although advanced HIV disease may be a cause of psychological distress, rather than the reverse, this association suggests that it is important for providers to screen for signs of distress at enrolment in HIV care and subsequent clinic visits, with referral to appropriate/available resources such as peer support or mental health services, where available.
Problems understanding HIV care providers, reported by nearly 5% of participants, can constitute a barrier to timely treatment initiation, especially among women, for whom this variable remained highly significant in final sex-specific models. This finding underscores the need for individualized, stage-appropriate counselling [36], as well as frequent assessments of patient understanding/comprehension during clinic visits.
The impact of gaps in care on late ART initiation may have been lessened by the January 2013 expansion of Ethiopia's national ART guidelines to include all patients with CD4+ counts ≤350 [17]. We observed a substantial decrease in the proportion of patients initiating treatment late and an increase in median CD4+ counts at initiation among both men and women during the study period (June 2012 to April 2013). This finding suggests that the expansion of ART initiation guidelines enabled the clinics to put more patients who were already diagnosed and engaged in pre-ART care on treatment earlier, before their health deteriorated further.
Major strengths of the study include the combination of longitudinal clinical data, including pre-ART care data, dating back to participants’ enrolment in care with self-reported information from interviews, as well as a high degree of data completeness. Our study also has some limitations. The clinics included in the study may not be representative of all settings in which Ethiopian patients receive HIV care and treatment, tempering our ability to further generalize our findings. A large proportion of persons initiating treatment with late-stage HIV already had advanced infection at enrolment in HIV care, and likely often at diagnosis, making it challenging to tease apart the correlates of late ART initiation from factors related to late enrolment or diagnosis. Additionally, our study did not include those patients who died prior to ART initiation, who may have had very advanced HIV disease. Finally, the cross-sectional nature of the interview relative to outcome measurement makes it difficult to sort out temporality of some exposures measured in the interviews in relation to the outcome.
Conclusions
A substantial number of patients who initiated ART in our study already had advanced HIV infection at the time of enrolment into care, pointing to the need to expand testing coverage with timely linkage to care. Although the proportion of patients initiating ART late decreased over time in our study, many patients still started treatment with low CD4 counts (Figure 1). Patient sub-groups (such as men, those who have not disclosed their HIV status, those experiencing distress and those reporting communication difficulties with providers) may be at increased risk of late ART initiation. Additionally, elements of clinical histories (referral from PITC settings, gaps in pre-ART care, TB treatment) can be used by providers to identify patients who may be in need of targeted support in preparation for retention and timely ART initiation. Clinic-based programmatic initiatives promoting patient-centred, stage-appropriate counselling, engagement in pre-ART care and smoother integration of TB and HIV treatments may also facilitate more timely ART initiation.
Acknowledgements and funding
The authors gratefully acknowledge the study participants and clinic and research staff who collected the data. The project was supported by a research grant from the US National Institute of Mental Health (R01MH089831).
Competing interests
The authors declare no competing interests.
Authors' contributions
DN, TG, SGK, SH, BE, RR, ML, SD, WE and ZM designed the study. SGK and MY coordinated the study. DN and OT analyzed the data and drafted the manuscript. All authors were responsible for, reviewed and approved the final manuscript.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013. [Google Scholar]
- 2.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krentz HB, Auld MC, Gill MJ. The high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infection. HIV Med. 2004;5(2):93–8. doi: 10.1111/j.1468-1293.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 4.Nash D, Katyal M, Brinkhof MW, Keiser O, May M, Hughes R, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008;22(17):2291–302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2012;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lahuerta M, Ue F, Hoffman S, Elul B, Kulkarni SG, Wu Y, et al. The problem of late ART initiation in Sub-Saharan Africa: a transient aspect of scale-up or a long-term phenomenon? J Health Care Poor Underserved. 2013;24(1):359–83. doi: 10.1353/hpu.2013.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahuerta M, Wu Y, Hoffman S, Elul B, Kulkarni SG, Remien RH, et al. Advanced HIV disease at entry into HIV care and initiation of antiretroviral therapy during 2006–2011: findings from four Sub-Saharan African countries. Clin Infect Dis. 2014;58(3):432–41. doi: 10.1093/cid/cit724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drain PK, Losina E, Parker G, Giddy J, Ross D, Katz JN, et al. Risk factors for late-stage HIV disease presentation at initial HIV diagnosis in Durban, South Africa. PLoS One. 2013;8(1):e55305. doi: 10.1371/journal.pone.0055305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;52(2):282–9. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abaynew Y, Deribew A, Deribe K. Factors associated with late presentation to HIV/AIDS care in South Wollo Zone Ethiopia: a case-control study. AIDS Res Ther. 2011;8:8. doi: 10.1186/1742-6405-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zango A, Dube K, Kelbert S, Meque I, Cumbe F, Chen PL, et al. Determinants of prevalent HIV infection and late HIV diagnosis among young women with two or more sexual partners in Beira, Mozambique. PLoS One. 2013;8(5):e63427. doi: 10.1371/journal.pone.0063427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulissa Z, Jerene D, Lindtjorn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS One. 2010;5(10):e13268. doi: 10.1371/journal.pone.0013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman S, Wu Y, Lahuerta M, Kulkarni SG, Nuwagaba-Biribonwoha H, Sadr WE, et al. Advanced disease at enrollment in HIV care in four sub-Saharan African countries: change from 2006 to 2011 and multilevel predictors in 2011. AIDS. 2014;28(16):2429–38. doi: 10.1097/QAD.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng EH, Hunt PW, Diero LO, Kimaiyo S, Somi GR, Okong P, et al. Trends in the clinical characteristics of HIV-infected patients initiating antiretroviral therapy in Kenya, Uganda and Tanzania between 2002 and 2009. J Int AIDS Soc. 2011;14:46. doi: 10.1186/1758-2652-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhamadi L, Nsabagasani X, Tumwesigye MN, Wabwire-Mangen F, Ekstrom AM, Peterson S, et al. Inadequate pre-antiretroviral care, stock-out of antiretroviral drugs and stigma: policy challenges/bottlenecks to the new WHO recommendations for earlier initiation of antiretroviral therapy (CD<350 cells/microL) in eastern Uganda. Health Policy. 2010;97(2–3):187–94. doi: 10.1016/j.healthpol.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and Health Survey 2011. Addis Ababa, Ethiopia: Central Statistical Agency and ICF International; 2012. [Google Scholar]
- 17.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: WHO; 2013. [PubMed] [Google Scholar]
- 18.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208. [PMC free article] [PubMed] [Google Scholar]
- 19.Kessler RC, Andrews G, Colpe LJ, Hiripi E, Mroczek DK, Normand SL, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. 2002;32(6):959–76. doi: 10.1017/s0033291702006074. [DOI] [PubMed] [Google Scholar]
- 20.Holzemer WL, Uys LR, Chirwa ML, Greeff M, Makoae LN, Kohi TW, et al. Validation of the HIV/AIDS Stigma Instrument – PLWA (HASI-P) AIDS Care. 2007;19(8):1002–12. doi: 10.1080/09540120701245999. [DOI] [PubMed] [Google Scholar]
- 21.Tymejczyk O, Hoffman S, Kulkarni SG, Gadisa T, Lahuerta M, Remien RH, et al. HIV care and treatment beliefs among patients initiating antiretroviral treatment (ART) in Oromia, Ethiopia. AIDS Behav. 2015 [Epub ahead of print] doi: 10.1007/s10461-015-1184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovira D, Schwefel R, Leidl J, Drummond MF. Economic aspects of AIDS and HIV infection. Berlin, Germany: Springer-Verlag Berlin Heidelberg; 1990. [Google Scholar]
- 23.Chi BH, Adler MR, Bolu O, Mbori-Ngacha D, Ekouevi DK, Gieselman A, et al. Progress, challenges, and new opportunities for the prevention of mother-to-child transmission of HIV under the US President's Emergency Plan for AIDS Relief. J Acquir Immune Defic Syndr (1999) 2012;60(Suppl 3):S78–87. doi: 10.1097/QAI.0b013e31825f3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taha TE. Mother-to-child transmission of HIV-1 in sub-Saharan Africa: past, present and future challenges. Life Sci. 2011;88(21–22):917–21. doi: 10.1016/j.lfs.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Galdas PM, Cheater F, Marshall P. Men and health help-seeking behaviour: literature review. J Adv Nurs. 2005;49(6):616–23. doi: 10.1111/j.1365-2648.2004.03331.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosen S, Fox MP. Retention in HIV care between testing and treatment in Sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohler PK, Chung MH, McGrath CJ, Benki-Nugent SF, Thiga JW, John-Stewart GC. Implementation of free cotrimoxazole prophylaxis improves clinic retention among antiretroviral therapy-ineligible clients in Kenya. AIDS. 2011;25(13):1657–61. doi: 10.1097/QAD.0b013e32834957fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378(9802):1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 29.Del Amo J, Malin AS, Pozniak A, De Cock KM. Does tuberculosis accelerate the progression of HIV disease? Evidence from basic science and epidemiology. AIDS. 1999;13(10):1151–8. doi: 10.1097/00002030-199907090-00002. [DOI] [PubMed] [Google Scholar]
- 30.Vijayan T, Semitala FC, Matsiko N, Elyanu P, Namusobya J, Havlir DV, et al. Changes in the timing of antiretroviral therapy initiation in HIV-infected patients with tuberculosis in Uganda: a study of the diffusion of evidence into practice in the global response to HIV/AIDS. Clin Infect Dis. 2013;57(12):1766–72. doi: 10.1093/cid/cit654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Treatment of tuberculosis: guidelines. 4th ed. Geneva, Switzerland: World Health Organization; 2009. [PubMed] [Google Scholar]
- 32.Naidoo K, Baxter C, Abdool Karim SS. When to start antiretroviral therapy during tuberculosis treatment? Curr Opin Infect Dis. 2013;26(1):35–42. doi: 10.1097/QCO.0b013e32835ba8f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarni S, Hoffman S, Gadisa T, Melaku Z, Fantehun M, Yigzaw M, et al. Identifying perceived barriers along the HIV care continuum: findings from providers, peer educators, and observations of provider-patient interactions in ethiopia. J Int Assoc Provid AIDS Care. 2015 [Epub ahead of print] doi: 10.1177/2325957415593635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gadisa T, Tymejczyk O, Kulkarni SG, Hoffman S, Lahuerta M, Remien RH, et al. Disclosure history among persons initiating antiretroviral treatment at Six HIV clinics in Oromia, Ethiopia, 2012–2013. AIDS Behav. 2016 [Epub ahead of print] doi: 10.1007/s10461-016-1290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nash D, Wu Y, Elul B, Hoos D, El Sadr W. Program-level and contextual-level determinants of low-median CD4+ cell count in cohorts of persons initiating ART in eight sub-Saharan African countries. AIDS. 2011;25(12):1523–33. doi: 10.1097/QAD.0b013e32834811b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nozaki I, Kuriyama M, Manyepa P, Zyambo MK, Kakimoto K, Barnighausen T. False beliefs about ART effectiveness, side effects and the consequences of non-retention and non-adherence among ART patients in Livingstone, Zambia. AIDS Behav. 2013;17(1):122–6. doi: 10.1007/s10461-012-0221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]