Abstract
Background:
Phyllodes tumor (PT) of the breast can be categorized into benign, borderline and malignant subgroups depending on various histopathological factors. Although malignant PTs may be indolent and controlled by local excision, they frequently show local and distant relapses. Literature reveals local recurrence to be the predominant pattern of failure and thus emphasizes the importance of adjuvant radiation in these tumors. The role of systemic chemotherapy has remained doubtful.
Materials and Methods:
We have analyzed details of all patients of PT (n = 33) treated with adjuvant multi-modality approach in our institute since 1994–2009. The demographic data, treatment details, recurrence patterns and salvage treatment options were documented.
Results:
All patients received adjuvant radiation. Seven patients received adjuvant chemotherapy. The mean survival of the entire cohort was 150.618 months. There was a trend for better overall survival with borderline grade (193.6 vs. 160.2 months; P = 0.08, log rank). The disease free survival (DFS) favored borderline grade (193.6 months vs. 82.9 months for high grade; P = 0.02, log rank). The DFS was significantly better in tumors having negative margins on postoperative histopathological examination (DFS rate at 5 years being 100% vs. 69.2% for positive or close margins; P = 0.015). The mode of surgery did not have any impact on survival.
Conclusion:
Adjuvant Radiation should be discussed taking into account surgical margins, size and various pathological factors of the primary. Adjuvant radiation may be utilized in high risk patients to enhance loco-regional control. Systemic chemotherapy is an option, worth exploring, in cases of systemic failure.
Keywords: Adjuvant radiation, borderline, malignant, phyllodes tumors, systemic chemotherapy
Introduction
Phyllodes tumor (PT) is a relatively uncommon variant of breast neoplasms, accounting for <1% of all breast neoplasm.[1] It is a biphasic tumor consisting of both stromal and epithelial elements.[2] These tumors are categorized into benign, borderline and malignant subgroups depending on five histopathological features like infiltrating or circumscribed margins, mitotic figures (<4, 4–10 or >10 mitotic figures per 10 high power fields, i.e. HPF), cytological atypia, hypercellularity and stromal overgrowth. The majority of these lesions behave in a completely benign fashion. Over the years simple mastectomy has remained the standard of care for them. The treatment of choice for borderline and malignant PT, in the past, was simple or radical mastectomy. Currently, most authors favor conservative surgery. However local and distant relapses are not uncommon and these relapses occur usually within the first 5 years after treatment.[3] The recent literature has shown local recurrence (LR) to be the predominant pattern of failure and thus emphasizes the importance of postoperative radiation in these tumors.[4] The role of systemic chemotherapy has remained doubtful.
Materials and Methods
All the breast tumor cases (n = 4123) were reviewed from the departmental archive (1994-2009) and 33 were found to be having malignant and borderline PT. The risk stratification was done depending on five histopathological features: Nature of margins, number of mitotic figures per 10 HPF, cellular atypia, cellularity and stromal overgrowth. The demographic data, treatment details and recurrence patterns were noted and documented from the charts.
Baseline evaluation and management decision
The presurgical evaluation for all patients consisted of mammography of the breasts, trucut biopsy of the mass, chest X-ray, two-dimensional-echo-cardiography and laboratory studies including complete blood count, kidney and liver function tests.
Treatment policy
In properly selected cases with an appropriate breast: Tumor ratio, lumpectomy was done. Simple mastectomy was opted for the rest.
Uniform adjuvant treatment policy was adopted in our institute to treat the patients of PT of the breast with different high risk features like recurrent tumor, tumor size 5 cm or more, presence of margin <10 mm, malignant PT (stratification based on factors present in postoperative histopathology report) and after breast conservation surgeries. Postmastectomy chest wall adiation was delivered (50 Gy/25 fractions/5 weeks) in patients with high risk features. Whole breast radiation (50 Gy/25 fractions/5 weeks) followed by lumpectomy cavity boost with electron (16 Gy/8 fractions) was delivered after lumpectomy. Six cycles of adjuvant chemotherapy using ifosfamide (1.8 g/m2 BSA; day 1–4) and epirubicin [60 mg/m2; day 1, 2) was used in patients who had malignant histology and large tumor size (>10 cm in maximum dimension).
Follow up policy
The patients were reviewed every 6 months for the initial 2 years followed by annual visits with for another 5 years. In cases of local or loco-regional failure patients were salvaged by surgery or irradiation. In cases with distant metastases palliative systemic therapy or focal radiation, as indicated, were used.
Statistical method
Overall survival (OS) was defined as period from the date of diagnosis to last date of follow-up or death due to any cause and disease free survival (DFS) was defined as period from the date of diagnosis to occurrence of any event such as progression, relapse, recurrence or death. OS and DFS were calculated using Kaplan–Meier method. Both univariate and multivariate analyses were performed for age (≤40 years vs. >40 years), tumor size (≤15 cm vs. >15 cm), grade (borderline vs. high), margin status (positive/close vs. negative) and type of surgery, wide local excision (WLE) versus modified radical mastectomy (MRM). The data was analyzed using SPSS 16 (IBM, Chicago, Ilinois).
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional) and with the Helsinki Declaration of 1975, as revised in 2000.
Results
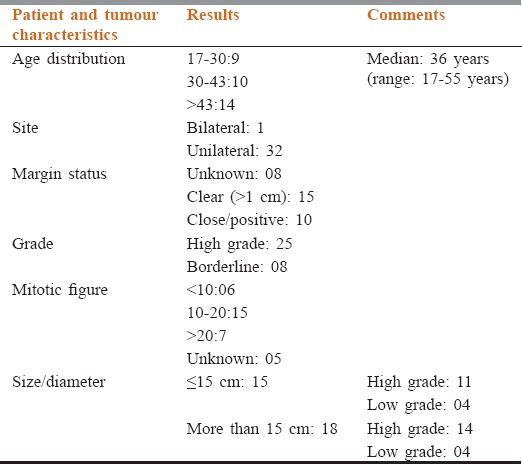
A total of 33 patients met the study criteria. Median age at presentation was 36 years (range: 17–55 years). Only one patient had bilateral disease. Seven patients (21.2%) underwent WLE or lumpectomy, 20 (60.60%) patients underwent simple mastectomy and six patients underwent MRM. Twenty five (75%) patients had high grade tumors while eight patients (25%) had borderline tumors. The median size of tumor was 13.6 cm (range: 3–24 cm). The margin status was found to be positive or close in 10 patients, margin status was unknown in eight patients. In six patients mitotic activity was <10 per 10 HPF; it was between 10 and 20 in 15 patients and more than 20 in seven patients. The mitotic activity was unknown in five patients [Table 1].
Table 1.
The demographics, tumour characteristics, management and outcome of the patients included in our cohort
All patients completed adjuvant radiation. Seven patients received adjuvant chemotherapy [Table 2]. Patterns of failure have been shown in Table 3.
Table 2.
Treatment protocol followed in the cohort
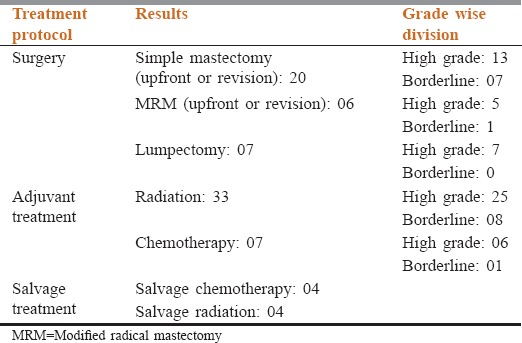
Table 3.
Pattern of failure in our study cohort
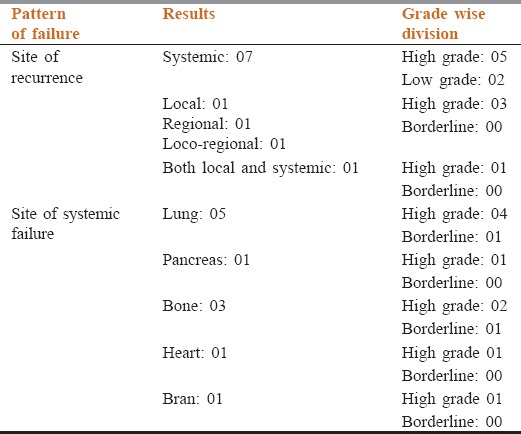
Four patients had received palliative radiation for local or distant relapse and four patients received palliative chemotherapy with ifosfamide and epirubicin based regimen. Three patients with lung metastasis achieved complete response with chemotherapy; one was having stable disease while one progressed after chemotherapy and developed brain metastasis.
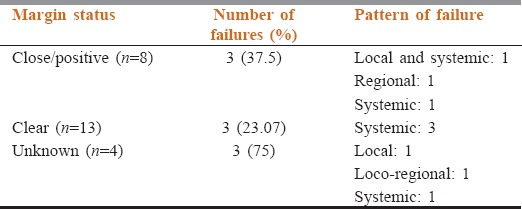
The median follow up duration was 23 months. Estimated 5 year OS and DFS were 85% and 77% respectively. On multivariate analysis only grade and margin status was found to correlate with survival. The DFS was significantly better in tumors having negative margins on postoperative histopathological examination than those having close or positive margins (DFS rate at 5 years being 100% vs. 69.2%; P = 0.015). Surgical treatment, margin status and pattern of failure in the patients of malignant PT have been elicited in Table 4 while the correlation of pattern of failure and margin status have been described in Table 5. There was a trend for better OS with borderline grade – 193.6 versus 160.2 months for high grade (P = 0.08). The DFS favored borderline grade – 193.6 versus 82.9 months for high grade (P = 0.02). The mode of surgery did not have any impact on survival. The DFS rate at 5 years for patients undergoing mastectomy was 75% versus 52.4% for those undergoing breast conservation surgery (P = 0.715).
Table 4.
The surgical treatment, margin status and pattern of failure in the patients of malignant phyllodes tumor
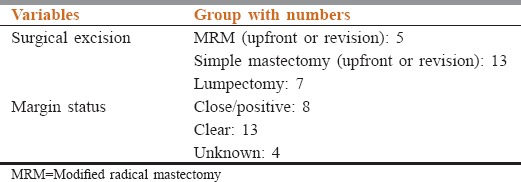
Table 5.
Pattern of failure in patients with different margin status among patients of malignant phyllodes tumor
Discussion
Phyllodes tumor is a comparatively rare malignancy with median age at presentation being 42–45 (range 10–82 years).[3,4] Literature shows that the tumor grade increases with age at diagnosis. In our series the median age was 36 years and more than half of patients were below 45 years of age. No co-relation between age and tumor grade was found. Pezner et al. showed that actuarial 5-year local control rates were 79.4% for lumpectomy patients and 91.2% for mastectomy patients treated by surgery alone. LR rates were 15% or greater for patients with tumors >2 cm treated by lumpectomy alone and tumors >10 cm treated by mastectomy alone. The authors recommended adjuvant radiation therapy for these patients.[5]
Study Hassouna et al. points out the impact of radical surgery for malignant PT. the 5-year overall and disease-free survivals were 28.5% and 15.6% versus 72.7% and 73.6% when the surgery was radical.[6] They found tumor size and margins status to be the independent predictors for LR. However study by Barth et al.[7] showed that WLE with postoperative radiotherapy has equivalent outcome in terms of local control when compared to radical surgery. The authors showed 100% local control in margin negative PT treated with WLE and radiotherapy. In another French multicentric study with a larger number of patients (n = 443) RT significantly decreased LR and total mastectomy had better results than WLE.[8] The current study failed to derive any impact of extent of surgery.
The distant metastases rate in malignant PT varies considerably, ranging from 6.6% to 70%. Most of them occur in lung (84.5%) and bone (39%).[1,9] We had an isolated distant failure rate of 21% and the most common site of distant metastasis was lung. Studies by Kapiris et al.[9] and West et al.[10] showed that among patients having metastases, 60% to 85% had already developed LRs. We had two patients who had both local relapse and distant metastasis.
The current study observed a superior loco-regional control. Such improved loco-regional control can be attributed to uniform use of adjuvant radiation in all the patients. Hence systemic failure emerged to be the predominant pattern of failure. Still no confirmatory conclusion can be drawn from our study due to lack of controls. We also admit the inherent limitations in the current study because of its retrospective nature. Some informations could not be furnished because of non-availability of the representative tissue blocks because of prolonged study duration. The sample size was small and the follow-up duration was also short in comparison to some of the other contemporary series.
Conclusion
Phyllodes tumor represents a heterogeneous group of tumors with an unpredictable outcome. The prognosis depends on the histological and biological characteristics of the tumors rather than their clinical behavior. Margin status and grade correlated with survival in the current study. Mode of surgery did not have any impact on disease control and survival. Our experience supports use of adjuvant radiation for select group of patients with adverse pathological features to optimize local control. Chemotherapy can be explored for metastatic disease.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Reinfuss M, Mitus J, Duda K, Stelmach A, Rys J, Smolak K. The treatment and prognosis of patients with phyllodes tumor of the breast: An analysis of 170 cases. Cancer. 1996;77:910–6. doi: 10.1002/(sici)1097-0142(19960301)77:5<910::aid-cncr16>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Fou A, Schnabel FR, Hamele-Bena D, Wei XJ, Cheng B, El Tamer M, et al. Long-term outcomes of malignant phyllodes tumors patients: An institutional experience. Am J Surg. 2006;192:492–5. doi: 10.1016/j.amjsurg.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Norris HJ, Taylor HB. Relationship of histologic features to behavior of cystosarcoma phyllodes. Analysis of ninety-four cases. Cancer. 1967;20:2090–9. doi: 10.1002/1097-0142(196712)20:12<2090::aid-cncr2820201206>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Khosravi-Shahi P. Management of non metastatic phyllodes tumors of the breast: Review of the literature. Surg Oncol. 2011;20:e143–8. doi: 10.1016/j.suronc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Pezner RD, Schultheiss TE, Paz IB. Malignant phyllodes tumor of the breast: Local control rates with surgery alone. Int J Radiat Oncol Biol Phys. 2008;71:710–3. doi: 10.1016/j.ijrobp.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 6.Ben Hassouna J, Damak T, Gamoudi A, Chargui R, Khomsi F, Mahjoub S, et al. Phyllodes tumors of the breast: A case series of 106 patients. Am J Surg. 2006;192:141–7. doi: 10.1016/j.amjsurg.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Barth RJ, Jr, Wells WA, Mitchell SE, Cole BF. A prospective, multi-institutional study of adjuvant radiotherapy after resection of malignant phyllodes tumors. Ann Surg Oncol. 2009;16:2288–94. doi: 10.1245/s10434-009-0489-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belkacémi Y, Bousquet G, Marsiglia H, Ray-Coquard I, Magné N, Malard Y, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys. 2008;70:492–500. doi: 10.1016/j.ijrobp.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 9.Kapiris I, Nasiri N, A’Hern R, Healy V, Gui GP. Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol. 2001;27:723–30. doi: 10.1053/ejso.2001.1207. [DOI] [PubMed] [Google Scholar]
- 10.West TL, Weiland LH, Clagett OT. Cystosarcoma phyllodes. Ann Surg. 1971;173:520–8. doi: 10.1097/00000658-197104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


