Abstract
Introduction:
Breast carcinoma is the most prevalent tumors among women. Transformation of inflated cells in immune response leads to increase in inflammatory cells such as macrophages, mast cells (MC) and fibroblasts. The aim of this study was to determine the relationship between grades of invasive carcinoma of the breast ducts and MC infiltration around tumoral cells.
Methods:
During the present study, 75 female patients suffering from invasive ductal carcinoma who underwent surgery or diagnostic biopsy during 2010 and 2013 in Educational-Medical centers of Tabriz University of Medical Sciences, were included in the study. Based on Bloom-Richardson grading system, 25 cases were selected from each grade. To better observe of MCs, samples were stained by Toluidine blue and MCs were counted in 10 40 × 10 fields.
Results:
The mean age was 47.56 ± 10.84 and the number of MCs was between 6 and 96 and their overall average was 43.01. Average count of MCs in grade 1, 2 and 3 were 15.92 ± 10.07, 45.32 ± 10.47, and 67.8 ± 20.70, respectively. There was a significant relationship between the number of MCs and increase in disease grade (P < 0.001). With increasing grade of malignancy, the number of MCs had grown. No significant relationship was observed between age and grade of disease or age and number of MC.
Conclusion:
According to obtained results, number of MC around tumoral cells increased significantly with an increase in the grade of disease. In order to treat in the first stages of the disease, recognizing primary changes in the stroma of cells could be helpful.
Keywords: Breast cancer, histopathological grade, invasive ductal carcinoma, mast cell
Introduction
Breast carcinoma is the most prevalent tumor among women and constitutes one-third of women's cancers and is the second reason of mortality after lung cancer. Of all histological types of breast carcinomas, invasive ductal carcinoma (IDC), is the most prevalent type with the frequency of about 83%. Due to increase in early mammography prevalence rate, early diagnosis and proper treatment mortality and morbidity has been decreased.[1] One of the important prognostic factors is a grade of disease. Tumoral cells cause the emergence of antigens which are identified by immune system, so immune response causes an increase in inflammatory cells such as mast cells (MC), macrophages and fibroblasts around tumoral cells.[2,3] Increasing evidences indicate that a unique immune cell, the MC, accumulates in the stroma surrounding certain tumors, especially mammary and pancreatic adenocarcinoma as well as melanoma.[4]
Different studies have been conducted about the relation between MC activity and angiogenesis and other inflammatory cells. Some of breast carcinoma tumoral cells have hormonal receptors, it was shown that increased number of MC was around the cells with receptor which has been announced as activity against tumoral cells.[5,6] According to the analysis of clinical data it has been concluded that specific types of tumor infiltrating leukocytes have a significant impact on the clinical course of malignant diseases.[7,8]
The aim of this study was to investigate relationship between histologic grades of invasive carcinoma of breast ducts and MC infiltration to see if it is possible to present counting MCs in breast tumor in different grades of disease, as an independent prognostic factor.
Methods
During a present Descriptive-Analytic study in which 75 female patients suffering from IDC were enrolled in the study. Twenty-five patients from each histological grade were selected, and the relation between the number of MCs and histopathological grade of cancer was investigated.
Studied population were selected among patients who were hospitalized and underwent total or modified mastectomy surgery or open biopsy during 2010 (June) to 2013 (June) in Educational-Medical centers of Tabriz University of Medical Sciences (Tabriz, Iran), the most important referral center of Northwest Iran, because of previously diagnosed IDC. Data about age and IDC grade without age limitation were extracted from patient's medical documents.
Acquired samples were fixed with 10% formalin then they were sent to pathology ward. In pathology ward, they were cut and put in solutions according to the algorithm shown in Figure 1.[9] Then they were stained by Toluidine blue and MCs were counted in 10 × 40 × 10 fields.
Figure 1.
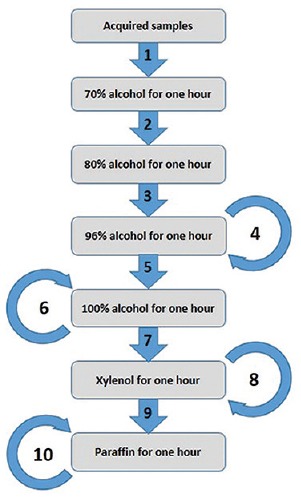
Required steps for fixing sample with 10% paraffin
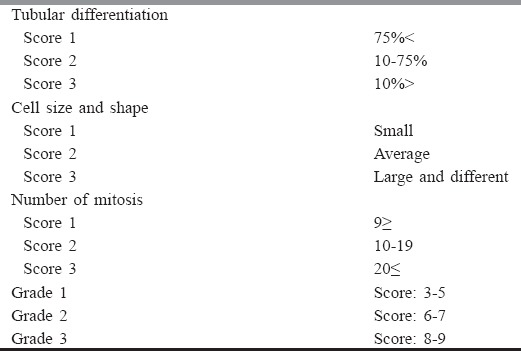
Determining histological category was based on Bloom-Richardson system, which was registered in a document by a pathology unit in Pathology Department of Tabriz University of Medical Sciences (Tabriz, Iran).[10] This grading score is shown in Table 1. According to grade and diagnosis of registered disease in folder, 25 cases were selected from each grade, which finally formed 75 cases.
Table 1.
Bloom-Richardson grading system
Statistical analysis was performed by SPSS software package version 16.0 for windows (SPSS Inc., Chicago, USA). Quantitative data are presented as mean ± standard deviation while qualitative data are demonstrated as frequency and percent (%). Normal distribution of data was tested using Kolmogorov-Smirnov test (P > 0.05), then One-way ANOVA test was used to compare groups. P < 0.05 was considered statistically significant in all steps.
Results
Number of MCs were counted in biopsy samples in all groups. MCs as round and purple cells were widely observed around tumoral cells. Variables under study were MCs count and grade of IDC. Mean age of patients was 47.65 ± 10.84 years. The minimum age was 27 years, and the maximum was 88 years. Statistical difference between age and grade was not significant (P = 0.03). The highest age group of studied patients was in grade II.
Age group between 40 and 50 constituting 40% of the studied population was the most prevalent age, which was in accordance with global statistics of disease prevalence rate. 60% of these people were in grade II and remaining were in grades I and III. Frequent age in this study was 47 years constituting 10.88% of the population.
Average number of MCs was 43.01 ± 25.65, which had no statistically significant relationship with age [Figure 2], (P = 0.41). Number of MCs varied from 6 to 96. Average count of MCs is shown in Table 2.
Figure 2.
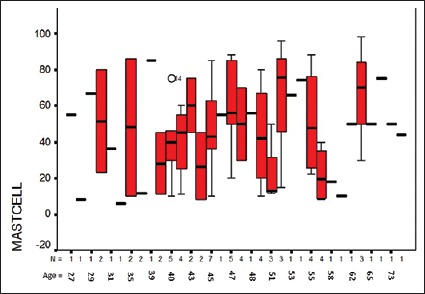
Relationship between age and mast cell in breast ductal carcinoma patients
Table 2.
Average count of MCs in IDC samples
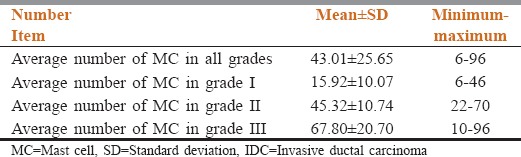
A significant relationship was seen between the number of MCs and increasing the grade of disease (P < 0.001).
Discussion
In the present study according to findings, MCs count reflect a grade of IDC (P < 0.001). In Iran, prevalence of IDC, tubular carcinoma, and lobular carcinoma are 78%, 4.8% and 3.3% respectively. In accordance with statistics of other countries, IDC is the most prevalent one. Of all patients with breast carcinoma in Iran, Age group containing patients 40–50 years old has the most frequency in comparison to other age groups,[11] this is just similar to the most affected age group in present study.
Investigating the number of MCs in various tumors, and potential checking of increased factors in cells’ stroma has been conducted. All results show an increase in the number of MCs but relationship between the number of MCs, the prognosis of disease and metastasis in these studies is different.[12,13] In a study where relationship between the number of MC and prognosis of breast IDC has been studied, it was concluded that stromal MC infiltration in invasive breast carcinoma is an independent good prognostic marker and demonstrates the outstanding role of local inflammatory reactions in breast cancer progression.[14]
In the current study, the relationship of the number of MC and grades of disease was investigated. According to this study, like conducted studies, number of MC around tumoral cells increased significantly with an increase in the grade of disease.
In a study about the significance of MCs in basal cell carcinoma and its subtypes by comparing their numbers in the peritumoral stroma to those in uninvolved adjacent normal skin. It was concluded that no significant relationship was found between MC number and the degree of peritumoral inflammation, patient age, or gender.[15] Also in the current study, relation of age and number of MC was not statistically significant.
In the present study, among patients with grade III IDC age varied from 27 to 73 years old, Therefore possibility of high-grade disease also exists in low ages. Identifying effective factors in tumor development and other effective factors in disease procedure could be helpful in therapy and finding a basic strategy. The practice should be based on diagnosing the first intra-cellular changes and during the disease and after therapeutic acts such as chemotherapy.
According to MC's possible role in tumor generation, wide studies are needed to discover available mechanism. According to diagnostic importance and usage of various techniques, available knowledge to the accurate evaluation of disease is increasing.
In conclusion, most of the studies have reported increased number of MC around tumoral cells as disease grade increases. However it has not been used as an independent factor to diagnose and therapeutic acts yet, but by inspiration of increase in factors affected by MC around tumor with its possible origin, it has been the basis for other studies to help pre and postsurgical treatment. Considering easy staining, MC could be stated as a prognostic factor. It is possible to use anti-MC and MC abductor materials in the treatment of disease to reduce number of tumoral cells and to reduce angiogenesis. To determine the relationship with other factors effective in diagnosis and prognosis of disease, studies with a wide spectrum of patients are suggested.
Because of the limited time period among cross-sectional studies, confounding factors, lack of data such as early symptoms of disease, time interval between emergence of symptoms and diagnosis of disease, which had not been mentioned in patients’ documents, a prospective and comparative study is suggested. Further studies are required to determine the relationship between MC and other prognostic factors and factors effective on the number of MC.
Acknowledgment
This study was supported by Tabriz University of Medical Sciences (Tabriz, Iran).
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Fauci AS. Harrison's Principles of Internal Medicine. Vol. 2. New York: McGraw-Hill Medical; 2008. [Google Scholar]
- 2.Mohammadzadeh M, Pourzand A, Eftekhar-Sadat AT, Alikhah H, Naghavi-Behzad M. A case of concurrent several forms of thyroid cancer. Niger Med J. 2013;54:351–3. doi: 10.4103/0300-1652.122372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbas AK, Lichtman AH, Pillai S. Cellular and Molecular Immunology: With Student Consult Online Access. Philadelphia: Elsevier Health Sciences; 2011. [Google Scholar]
- 4.Theoharides TC, Alysandratos KD, Angelidou A, Zhang B. The Tumor Microenvironment. New York: Springer; 2010. Mast cells and tumor microenvironment; pp. 353–70. [Google Scholar]
- 5.Della Rovere F, Granata A, Familiari D, D’Arrigo G, Mondello B, Basile G. Mast cells in invasive ductal breast cancer: Different behavior in high and minimum hormone-receptive cancers. Anticancer Res. 2007;27:2465–71. [PubMed] [Google Scholar]
- 6.Fakhrjou A, Dastranj-Tabrizi A, Ghojazadeh M, Ghorashi S, Smaeili AB, Halimi M, et al. Diagnostic value of protein Ki67 (MIB-1) in atypical pap smears of postmenopausal women. Asian Pac J Cancer Prev. 2013;14:4815–8. doi: 10.7314/apjcp.2013.14.8.4815. [DOI] [PubMed] [Google Scholar]
- 7.Gutkin DW. The Tumor Immunoenvironment. New York: Springer; 2013. Tumor infiltration by immune cells: Pathologic evaluation and a clinical significance; pp. 39–82. [Google Scholar]
- 8.Ghojazadeh M, Mohammadi M, Azami-Aghdash S, Sadighi A, Piri R, Naghavi-Behzad M. Estimation of cancer cases using capture-recapture method in Northwest Iran. Asian Pac J Cancer Prev. 2013;14:3237–41. doi: 10.7314/apjcp.2013.14.5.3237. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamurthy S, Mathews K, McClure S, Murray M, Gilcrease M, Albarracin C, et al. Multi-institutional comparison of whole slide digital imaging and optical microscopy for interpretation of hematoxylin-eosin-stained breast tissue sections. Arch Pathol Lab Med. 2013;137:1733–9. doi: 10.5858/arpa.2012-0437-OA. [DOI] [PubMed] [Google Scholar]
- 10.Bansal C, Singh US, Misra S, Sharma KL, Tiwari V, Srivastava AN. Comparative evaluation of the modified Scarff-Bloom-Richardson grading system on breast carcinoma aspirates and histopathology. Cytojournal. 2012;9:4. doi: 10.4103/1742-6413.92550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Movahedi M, Haghighat S, Khayamzadeh M, Moradi A, Ghanbari-Motlagh A, Mirzaei H, et al. Survival rate of breast cancer based on geographical variation in Iran, a national study. Iran Red Crescent Med J. 2012;14:798–804. doi: 10.5812/ircmj.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabiri S, Huntsman D, Makretsov N, Cheang M, Gilks B, Bajdik C, et al. The presence of stromal mast cells identifies a subset of invasive breast cancers with a favorable prognosis. Mod Pathol. 2004;17:690–5. doi: 10.1038/modpathol.3800094. [DOI] [PubMed] [Google Scholar]
- 13.Gulubova M, Vlaykova T. Prognostic significance of mast cell number and microvascular density for the survival of patients with primary colorectal cancer. J Gastroenterol Hepatol. 2009;24:1265–75. doi: 10.1111/j.1440-1746.2007.05009.x. [DOI] [PubMed] [Google Scholar]
- 14.Rajput AB, Turbin DA, Cheang MC, Voduc DK, Leung S, Gelmon KA, et al. Stromal mast cells in invasive breast cancer are a marker of favourable prognosis: A study of 4,444 cases. Breast Cancer Res Treat. 2008;107:249–57. doi: 10.1007/s10549-007-9546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erkiliç S, Erbagci Z. The significance of mast cells associated with basal cell carcinoma. J Dermatol. 2001;28:312–5. doi: 10.1111/j.1346-8138.2001.tb00139.x. [DOI] [PubMed] [Google Scholar]


