Abstract
Purpose:
To review the surgical outcomes of intravitreal bevacizumab (IVB) along with subretinal fluid drainage with cryotherapy in patients with stage 3B Coats’ disease.
Materials and Methods:
A retrospective study of seven cases of stage 3B Coats’ disease, who underwent subretinal fluid drainage with cryopexy, from May 2011 to March 2014. Five eyes received additional IVB at the end of surgery. Green laser therapy was performed on telangiectatic vessels postoperatively.
Results:
The mean age was 34 months (range, 10-84 months). Mean follow-up was 19 months. Six patients (85.7%) had an attached retina at final follow-up. Three out of four patients (75%) that received IVB developed tractional retinal detachments (TRDs). Two eyes that did not receive bevacizumab did not develop any traction. None progressed to neovascular glaucoma or phthisis bulbi.
Conclusion:
Simultaneous injection of bevacizumab along with subretinal drainage and cryotherapy for advanced Coats’ disease could not avoid TRD.
Keywords: Advanced Coats’ Disease, Bevacizumab, Tractional Retinal Detachment
INTRODUCTION
Coats’ disease was first described by Coats in 1908.1 This idiopathic condition is usually unilateral and is characterized by telangiectatic retinal vessels with massive subretinal exudation. It most commonly affects young males in the first or second decades of life. Patients often present with leukocoria or strabismus. Shields et al.2 proposed a classification of Coats disease based on their observation. Prior to the advent of ablative techniques, enucleation was common in cases with advanced Coats’ disease, in order to prevent or manage painful neovascular glaucoma. Over time, enucleation has been replaced by subretinal fluid drainage along with laser photocoagulation and cryotherapy. However, diffuse infiltrating retinoblastoma needs to be ruled out prior to attempting any surgical intervention.3
Intravitreal bevacizumab (IVB)4 and, recently, ranibizumab5 have both been used in advanced Coats’ disease with good results. We describe the surgical outcome of subretinal fluid drainage with cryotherapy and bevacizumab in stage 3B Coats’ disease in Indian eyes.
MATERIALS AND METHODS
A retrospective chart review was performed of seven patients, who presented with stage 3B Coats’ disease who underwent subretinal fluid drainage with cryotherapy from May 2011 to March 2014 were included in the study. This study adhered to the tenets of the Declaration of Helsinki and Institutional Review Board approval was granted. Age and symptom at presentation, gender, and presenting visual acuity (VA) were recorded. A detailed anterior and posterior segment evaluation and measurement of intraocular pressure was performed. In addition, tests included ultrasonography and fundus documentation with RetCam (Clarity Medical Systems, Pleasanton, California, USA) for all patients under general anesthesia. Magnetic resonance imaging (MRI) or computerized tomography (CT) was performed in all patients to exclude diffuse infiltrating type of retinoblastoma.
The surgical procedure was performed by a single surgeon (PKS) in all patients under general anesthesia. After performing a 360° conjunctival peritomy, bridle sutures were applied to all four recti muscles. Subretinal fluid drainage was performed using either a 24 or 26G needle attached to a 2cc syringe without the plunger. In all cases, 1 ml of subretinal fluid was sent for histopathological confirmation of Coats’ disease. After draining the fluid, globe was formed with intravitreal injection of balanced salt solution followed by cryotherapy of the telangiectatic blood vessels. The end point of cryotherapy was considered as the blanching of the telangiectatic vessels. The conjunctiva was closed with 8-0 vicryl sutures. Intravitreal injection of bevacizumab (Avastin, Genentech, San Francisco, CA, USA) 1.25 mg/0.05 ml was administered in five cases. The other two cases underwent subretinal fluid drainage with cryotherapy only. The patients were evaluated at regular intervals following surgery and were examined under anesthesia as warranted. Additional laser photocoagulation was performed to the telangiectatic blood vessels, using Aurolase 532 (Aurolab, Madurai, India) laser delivery system, when necessary. Anatomic success was defined as attached retina with resolving or resolution of exudation at the final visit.
RESULTS
From May 2011 to March 2014, ten patients presented with stage 3B Coats’ disease. Three eyes underwent primary enucleation due to the refusal of parental consent for globe-conserving surgery. Histopathology of all these three eyes confirmed Coats’ disease. Seven eyes of seven patients, whose parents consented to globe-conserving surgery, were included in the study. The mean age of presentation was 34 months (range, 10-84 months). There were 5 male and 2 female patients.
Leukocoria was the most common presenting symptom followed by strabismus. All the patients had uneventful birth history and normal developmental milestones. On evaluation under anesthesia, all seven patients had total retinal detachment involving macula, with telangiectatic retinal vessels (more than 3 clock h) and massive subretinal exudation suggestive of stage 3B. None had preoperative subretinal fibrosis. Intraocular pressure was normal in all eyes. One female patient presented with bilateral disease with the better eye having stage 2B, which was treated with green laser to the telangiectatic vessels along with IVB. MRI of all cases showed the presence of retinal detachment with no evidence of mass lesion. Subretinal fluid drainage with cryotherapy was done in all seven patients. Five patients received an additional intravitreal injection of bevacizumab at the end of surgery. All seven patients received laser photocoagulation 4 weeks after the initial procedure. Five patients needed one more sitting. None required additional cryotherapy. At mean follow-up of 19 months (range, 8-43 months), six patients (85.7%) showed anatomical success whereas one patient showed persistent shallow retinal detachment. Tractional retinal detachment (TRD) in the form of retinal folds was present in 3 of 4 eyes (75%) that received IVB [Figure 1]. Two eyes that did not receive bevacizumab did not develop any traction [Figure 2]. None of the patients developed neovascular glaucoma nor progressed to phthisis bulbi. None of the patients needed enucleation for a painful blind eye. Though anatomical improvement was noted, VA remained finger counting close to face or worse in all cases [Table 1]. Partial patching was attempted for all the six cases.
Figure 1.
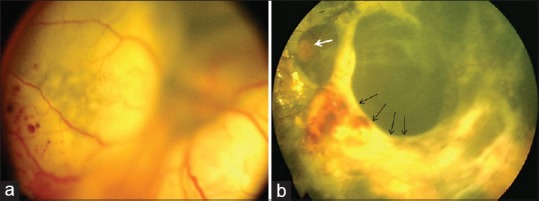
(a) Fundus photo of a left eye with total exudative retinal detachment suggestive of stage 3B Coats’ disease. (b) Fundus photo of the same eye at 33 months after subretinal fluid drainage with cryotherapy and intravitreal bevacizumab showing extensive subretinal fibrosis and tractional retinal detachment (black arrows). The optic disc is seen (white arrow)
Figure 2.
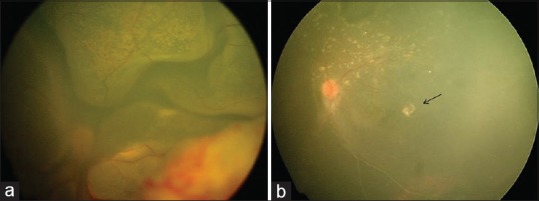
(a) Fundus photo of a left eye with total exudative retinal detachment suggestive of stage 3B Coats’ disease. (b) Fundus photo of the same eye at 13 months after subretinal fluid drainage with cryotherapy showing attached retina with resolving subretinal exudates and macular scar (black arrow). This eye did not receive intravitreal bevacizumab
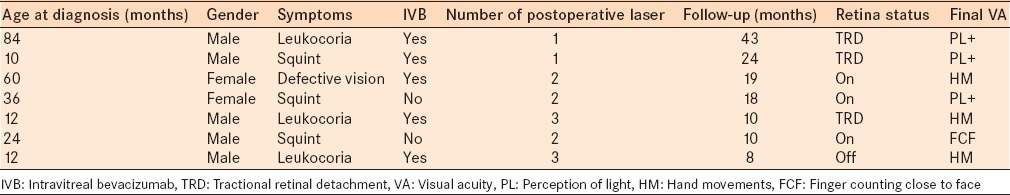
Table 1.
Characteristics of the patients with advanced Coats’ disease who underwent subretinal fluid drainage with cryopexy
DISCUSSION
In surgical management of advanced Coats’ disease, the first step is a correct clinical diagnosis and rule out diffuse infiltrating retinoblastoma with the help of clinical examination, ultrasound, and CT or MRI scan. The second goal is to prevent a painful blind eye due to a chronic retinal detachment. This can be achieved by ablation of the telangiectatic blood vessels. However, in a setting of bullous detachment, drainage of subretinal fluid followed by cryotherapy to the telangiectatic vessels allows better ablation of the abnormal vessels. This technique was first described in 1970 by Harris.6 Further modifications such as the addition of infusion cannula has been published. Silodor et al.7 reported 13 cases of Coats’ disease, of which seven cases underwent intraocular infusion, subretinal fluid drainage, and cryopexy. None of the seven eyes developed neovascular glaucoma.7 Four of the remaining six eventually developed painful neovascular glaucoma and necessitating enucleation.7 Needle subretinal fluid drainage followed by cryopexy provides a useful alternative to more aggressive surgeries in cases with total, bullous retinal detachment.8
Sun et al.9 reported elevated levels of vascular endothelial growth factor (VEGF) in Coats’ disease which reduced rapidly after injection of pegaptanib sodium. They9 suggested a disregulation of VEGF mediated angiogenesis in Coats’ disease. Although intravitreal injection of bevacizumab can cause regression of the abnormal vessels,4 in a setting of excessive cryotherapy, it can also cause traction and fibrosis leading to tractional detachment. Ramasubramanian and Shields10 saw this side effect in 3 of 8 (38%) cases compared to 3 of 4 (75%) cases in our series. The two control cases that did not receive bevacizumab in our series did not develop any traction. Gaillard et al.5 reported fibrotic vitreoretinopathy in 5/9 eyes and TRD in only 1 eye treated with ranibizumab. They5 concluded that better outcome in their series could be because local treatment (cryotherapy or photocoagulation) was not performed in combination with the ranibizumab injection, thus avoiding the increased risk of inflammation and subsequent fibrosis.
The addition of laser photocoagulation in the postoperative period once subretinal fluid is reduced is needed to further reduce exudation.11 All our cases were given laser during subsequent postoperative visits. None of the patients in our study progressed to neovascular glaucoma, and none needed enucleation. Similar to previous reports, we found that despite satisfactory anatomical results, functional improvement remains poor in these patients.5,12
The drawbacks of our study included the retrospective nature and a very small sample size. In conclusion, IVB along with subretinal fluid drainage followed by cryotherapy is a good surgical option in advanced Coats’ disease with total retinal detachment. However, bevacizumab should not be delivered at the same sitting to avoid traction-related complications. We recommend initiating the treatment with anti-VEGF agents in advanced Coats’ disease followed by surgery, cryotherapy, or laser after exudation decreases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Coats G. Forms of retinal diseases with massive exudation. R Lond Ophthalmol Hosp Rep. 1908;17:440–525. [Google Scholar]
- 2.Shields JA, Shields CL, Honavar SG, Demirci H, Cater J. Classification and management of Coats disease: The 2000 proctor lecture. Am J Ophthalmol. 2001;131:572–83. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]
- 3.Grabowska A, Calvo JP, Fernandez-Zubillaga A, Rios JC, Gómez JA. A magnetic resonance imaging diagnostic dilemma: Diffuse infiltrating retinoblastoma versus Coats’ disease. J Pediatr Ophthalmol Strabismus. 2010;47 Online:e1–3. doi: 10.3928/01913913-20100818-10. [DOI] [PubMed] [Google Scholar]
- 4.Villegas VM, Gold AS, Berrocal AM, Murray TG. Advanced Coats’ disease treated with intravitreal bevacizumab combined with laser vascular ablation. Clin Ophthalmol. 2014;8:973–6. doi: 10.2147/OPTH.S62816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaillard MC, Mataftsi A, Balmer A, Houghton S, Munier FL. ranibizumab in the management of advanced Coats disease stages 3B and 4: Long-term outcomes. Retina. 2014;34:2275–81. doi: 10.1097/IAE.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 6.Harris GS. Coats’ disease, diagnosis and treatment. Can J Ophthalmol. 1970;5:311–20. [PubMed] [Google Scholar]
- 7.Silodor SW, Augsburger JJ, Shields JA, Tasman W. Natural history and management of advanced Coats’ disease. Ophthalmic Surg. 1988;19:89–93. [PubMed] [Google Scholar]
- 8.Mrejen S, Metge F, Denion E, Dureau P, Edelson C, Caputo G. Management of retinal detachment in Coats disease. Study of 15 cases. Retina. 2008;28(3 Suppl):S26–32. doi: 10.1097/IAE.0b013e31816b3158. [DOI] [PubMed] [Google Scholar]
- 9.Sun Y, Jain A, Moshfeghi DM. Elevated vascular endothelial growth factor levels in Coats disease: Rapid response to pegaptanib sodium. Graefes Arch Clin Exp Ophthalmol. 2007;245:1387–8. doi: 10.1007/s00417-007-0559-8. [DOI] [PubMed] [Google Scholar]
- 10.Ramasubramanian A, Shields CL. Bevacizumab for Coats’ disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol. 2012;96:356–9. doi: 10.1136/bjophthalmol-2011-300141. [DOI] [PubMed] [Google Scholar]
- 11.Schefler AC, Berrocal AM, Murray TG. Advanced Coats’ disease. Management with repetitive aggressive laser ablation therapy. Retina. 2008;28(3 Suppl):S38–41. doi: 10.1097/IAE.0b013e318163cd7c. [DOI] [PubMed] [Google Scholar]
- 12.Budning AS, Heon E, Gallie BL. Visual prognosis of Coats’ disease. J AAPOS. 1998;2:356–9. doi: 10.1016/s1091-8531(98)90034-9. [DOI] [PubMed] [Google Scholar]


