Abstract
Background
Cohorting patients in dedicated hospital wards or wings during infection outbreaks reduces transmission of organisms, yet frequently, this may not be feasible because of inadequate capacity, especially in the intensive care unit (ICU). We hypothesized that cohorting isolation patients in one geographic location in a single ICU and using enhanced isolation procedures (“superisolation”) can prevent the further spread of highly multi-drug-resistant organisms (MDRO).
Methods
Six patients dispersed throughout our Surgical Trauma Burn ICU had infections with carbapenem-resistant, non-clonal gram-negative MDRO, namely Klebsiella pneumoniae, Citrobacter freundii, Stenotrophomonas maltophilia, Aeromonas hydrophilia, Proteus mirabilis, Pseudomonas aeruginosa, and Providencia rettgeri. Five of the six patients also had simultaneous isolation of vancomycin-resistant enterococci (VRE). Under threat of unit closure and after all standard isolation procedures had been enacted, these six patients were moved to the front six beds of the unit, the front entrance was closed, and all traffic was redirected through the back entrance. Nursing staff were assigned to either two isolation or two non-isolation patients. In accordance with the practice of Semmelweis, rounds were conducted so as to end at the rooms of the patients with the most highly-resistant bacterial infections.
Results
A few months after these interventions, all six patients had been discharged from the ICU (three alive and three dead), and no new cases of infection with any of their pathogens (based on species and antibiogram) or VRE occurred. The mean ICU stay and overall hospital length of stay for these six patients were 78.3 days and 117.2 days respectively, with a mortality rate of 50%.
Conclusion
Cohorting patients to one area and altering work routines to minimize contact with patients with MDRO (essentially designating a “high-risk” zone) may be beneficial in stopping patient-to-patient spread of highly resistant bacteria without the need for a dedicated isolation unit.
Enhanced infection control practices often are required to prevent the spread of multi-drug-resistant organisms (MDRO), including expanded isolation precautions, closing units to new admissions, universal screening of new admissions, isolating individual patients, or using isolation procedures for all patients, whether infected or colonized with a resistant pathogen or not [1,2]. In addition, cohorting patients in dedicated hospital wards or wings during infectious outbreaks reduces the transmission of infection [3–7]. Whereas individual patient isolation, screening, and other common infection control measures are possible in almost any hospital, cohorting depends on the availability of similar wards for infected and non-infected patients. These resources may not be available in many hospitals, particularly those that have a consistently high census or a small number of intensive care unit (ICU) beds.
In the fall of 2009, the Surgical Trauma Burn ICU (STBICU) in our tertiary-care hospital experienced an unprecedented number of patients infected with one or more MDROs. This epidemic continued despite the introduction of universal gowning and gloving for all patient contact and various programs to increase compliance with hand hygiene, gowning, gloving, and other infection control practices. No other ICU was available to care for these patients, so traditional cohorting, requiring different wards, was not possible. It was our hypothesis that cohorting patients infected with MDROs in one geographic location in the single ICU and initiating enhanced isolation procedures for infected patients (“superisolation”) could prevent the further spread of the highly resistant MDRO.
Patients and Methods
Disease outbreak
At one time during autumn 2009, the 12-bed STBICU at the University of Virginia Health System housed six patients being treated for infections with non-clonal gram-negative MDRO: Klebsiella pneumoniae (including carbapenemase-producing K. pneumoniae [KPC]), Citrobacter freundii, Stenotrophomonas maltophilia, Aeromonas hydrophilia, Proteus mirabilis, Pseudomonas aeruginosa, and Providencia rettgeri. Five of the six patients also were colonized with vancomycin-resistant enterococci (VRE). Three were admitted to the ICU following complications from liver transplantation, one with a 55% total body surface area burn, one after the development of multiple enterocutaneous fistulae, and one following a shotgun wound to the chest and abdomen.
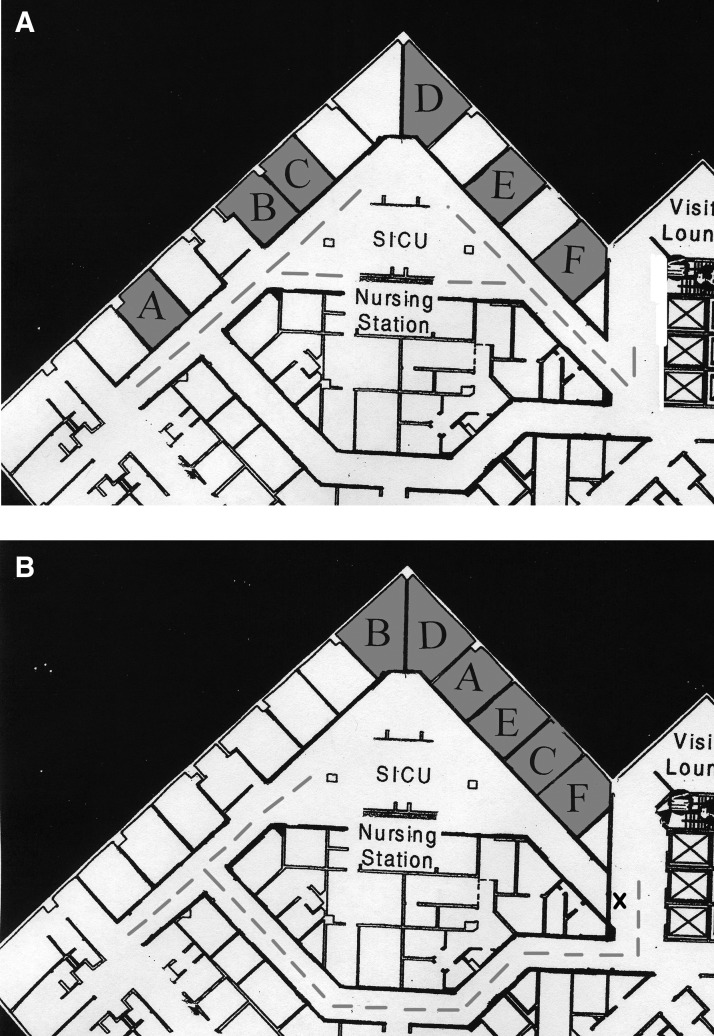
Over a ten-week period, these six patients were admitted to the STBICU and subsequently developed multiple infections, as summarized in Table 1. Initially, the six patients were interspersed throughout the 12-bed ICU, and each had a nurse also providing care for a non-isolation patient, as seen in Fig. 1A.
Table 1.
Organisms Cultured from Patients
| Date of culture | Site of isolation | Microorganism |
|---|---|---|
| Patient A | ||
| 8/13 | BAL | Serratia marcescens |
| Klebsiella pneumoniae | ||
| 8/21 | Blood | Pseudomonas aeruginosa |
| 8/24 | Peri-rectal swab | VRE |
| 9/5 | BAL | Stenotrophomonas maltophilia (MDRO) |
| 9/7 | Blood | Enterococcus faecalis |
| Enterococcus faecium (VRE) | ||
| Methicillin-resistant Staphylococcus aureus | ||
| Urine | Candida krusei | |
| 9/16 | Urine | Candida parapsilosis |
| 10/7 | Cohort day | |
| No further positive cultures | ||
| 10/28 | Death | |
| Patient B | ||
| 5/31 | Peritoneal fluid | Candida albicans |
| 6/8 | Peri-rectal swab | VRE |
| Blood | E. faecium (VRE) | |
| 6/25 | Wound | K. oxytoca (MDRO) |
| 7/10 | Blood | S. epidermidis |
| 7/16 | Peritoneal fluid | C. glabrata |
| S. maltophilia (MDRO) | ||
| 8/12 | Peritoneal fluid | Aeromonas hydrophilia (MDRO) |
| 8/21 | Peritoneal fluid | C. parapsilosis |
| 10/7 | Cohort day | |
| 10/21 | Peritoneal fluid | P. aeruginosa |
| 11/17 | Death | |
| Patient C | ||
| 9/14 | Peri-rectal swab | VRE |
| 9/20 | Abdominal abscess | C. albicans |
| Citrobacter freundii (MDRO) | ||
| K. pneumoniae (MDRO, KPC) | ||
| 9/24 | Blood | E. faecalis |
| 10/7 | Cohort day | |
| 10/14 | Peritoneal fluid | P. aeruginosa |
| 10/16 | Discharge from ICU | |
| 10/28 | Discharge to home | |
| Patient D | ||
| 9/28 | Blood | A. hydrophilia (MDRO) |
| Escherichia coli | ||
| 9/30 | Peritoneal fluid | K. oxytoca |
| Proteus mirabilis | ||
| E. faecium (VRE) | ||
| 10/7 | Cohort day | |
| 10/15 | Discharge from ICU | |
| 11/1 | Sputum | P. aeruginosa (MDRO) |
| 11/6 | Peritoneal fluid | Providencia rettgeri |
| 5/3 | Discharge to home | |
| Patient E | ||
| 8/17 | Blood | C. glabrata |
| 8/22 | Wound abscess | C. albicans |
| 8/24 | Peri-rectal swab | VRE |
| 9/12 | Peritoneal fluid | K. pneumoniae (MDRO) |
| Enterobacter cloacae | ||
| 9/24 | BAL | E. faecium (VRE) |
| 9/26 | Pseudocyst aspirate | P. mirabilis (MDRO) |
| 10/7 | Cohort day | |
| 10/10 | Blood | C. parapsilosis |
| 10/16 | Peritoneal fluid | C. freundii (MDRO) |
| P. aeruginosa (MDRO) | ||
| E. faecium | ||
| 11/30 | Death | |
| Patient F | ||
| 9/14 | Urine | E. coli |
| 9/23 | Urine | C. glabrata |
| 9/28 | Peri-rectal | K. oxytoca (MDRO) |
| 10/7 | Cohort day | |
| 10/8 | BAL | K. oxytoca |
| 11/3 | Discharge from ICU | |
| 11/5 | Urine | C. freundii (MDRO) |
| 11/24 | Discharge to home | |
BAL=bronchoalveolar lavage fluid; ICU=intensive care unit; KPC=cCarbenapenase-producing Klebsiella pneumoniae; MDRO=multi-drug-resistant organism; VRE=vancomycin-resistant Enterococcus.
FIG. 1.
Patient locations before (A) and after (B) cohorting. During cohorting, front unit entrance (X) was closed to all traffic. Dashed line represents traffic pattern.
Cohorting
Under threat of unit closure and long after all standard isolation procedures had been enacted, including universal gowning and gloving for all patients in the ICU and strict antimicrobial use policies, the patients with MDRO were cohorted into the front six beds of the unit on our “Cohort Day,” as designated in Table 1 and shown in Fig. 1B. The main front entrance was closed, and all traffic was redirected through the back entrance of the unit, decreasing unnecessary flow in the “high-risk” area. Nursing staff were assigned to provide care for either two isolation or two non-isolation patients, and no nurse provided care to both an isolation and a non-isolation patient. Staff rounds in the ICU were modified according to the recommendations of Semmelweis, who altered his daily routine after attributing a high incidence of puerperal fever to “cadaverous particles” acquired during his autopsies. Daily rounds were conducted so as to end at the rooms of patients cohorted in the front of the unit, who had the most highly resistant bacterial infections. Procedures and diagnostic tests were performed at the bedside without transportation out of the unit unless absolutely necessary. When a procedure outside the unit was necessary for an isolated patient, his or her exit from the unit was through the previously closed front door to avoid passage in front of the rooms of non-isolated patients. Nurses and physicians without responsibility for the isolated patients were discouraged from using computers, tables, and counter space, in the area of the isolated patients. Lastly, all patient families were instructed to comply with standard isolation precautions, donning gowns and gloves prior to entry.
Among the isolated patients, no effort was made to place patients with similar organisms next to each other. Instead, patients projected to have the longest ICU stays were placed farthest from the non-isolated patients. As isolated patients were discharged from the ICU, their beds were not filled with non-isolated patients. Instead, the remaining isolated patients were shifted farther back into the isolated area so that there was always an exact interface between the isolated and non-isolated areas of the unit. Although some studies have implicated environmental surfaces and objects as vectors in infection transmission, we did not specifically implement any additional cleaning procedures to exclude these possible transmission routes [8]. No extra sanitation measures were carried out directly, although an attempt was made to leave the “superisolation” beds unfilled as long as possible. The Hospital Infection Control Practices Advisory Committee (HICPAC) recommends focused environmental cultures be performed electively to ensure adequate disinfection, but this should be done under the supervision of an infection control program officer [1].
Results
Following closure of the main front entrance, all traffic was redirected through the back entrance, reducing all unintentional flow around the superisolation rooms. Compliance with this intervention was high, as a physical sign and barrier prevented entrance through the main doors. A secondary effect of closure of the front entrance was lack of congregation in this area. Most students, residents, and consultants worked at computer stations in regions away from the previous front entrance, as it was now at the far end of the ICU.
Two months after these interventions, all six patients had been discharged from the ICU (three alive and three deceased). During this period, there were no new cases of infection with any of the previously isolated MDRO pathogens (based on species and antibiogram) or any new cases of VRE within the ICU.
The significance of the MDRO infections, especially in patients who have concomitant secondary infections (VRE and other nosocomial infections) is the prolonged treatment and additional resources required. The mean ICU stay and overall hospital length of stay for these six patients were 78.3 days and 117.2 days, respectively. These are significantly longer than for patients with infections not associated with an MDRO. The overall mortality rate was 50%.
The final patient of this superisolation cohort had chronic ventilator dependence. He died 113 days after his ICU admission, which marked the discharge of the last patient of the cohort. Following this event, the main front entrance of the unit was reopened, with previous traffic patterns and other standards of care being reinstituted.
Discussion
For hundreds of years, people have been isolating or “cohorting” patients for various diseased, as well as natural, states of health in order to reduce patient-to-patient transmission. Most instances are performed for a particular disease state and are well-documented in the literature. Most reports describe gram-positive, monoclonal organism outbreaks such as VRE or methicillin-resistant Staphylococcus aureus (MRSA) [3–5,9,10–13]. An early example occurred in the 1960s, where for five years, an MRSA outbreak persisted in a district hospital of 1,000 beds following institution of standard practices for infection control, including barrier precautions and ward closures. It was decided in 1973 that an isolated ward would be created, complete with controlled ventilation, private facilities, and a permanent staff, for the infected patients. Following establishment of the isolation (cohorting) unit, there was a significant reduction in the number of patients infected with MRSA [3]. Successful cohorting for isolated infectious outbreaks are numerous, and two reports are reviewed.
In a small community hospital, an outbreak was reported after persistent acquisition of VRE despite implementation of Hospital Infection Control Practices Advisory Committee (HICPAC) recommendations. Following a U.S. Centers for Disease Control and Prevention epidemiologic investigation and a one-year study period, the institution executed a massive cohort system to interrupt the transmission of VRE [5]. Prior to cohorting, direct observations revealed that full compliance with contact isolation measures was practiced only 22% of the time. Most of the isolation failures were absence of glove use, improper disposal of gowns and gloves, and failure to wash the hands after patient contact.
All VRE-infected or –colonized patients were cohorted into a single hospital ward despite differences in the required level of care, from rehabilitation to intensive care. Dedicated nursing staff as well as equipment was designated for the cohort ward. Compliance within this cohort improved significantly, 88% of observed interactions being compliant with isolation techniques compared with 22% in the first study period (p<0.001) [5]. The overall success in decreasing VRE prevalence and infections and markedly increased compliance with isolation procedures was attributed to a designated cohort ward and better compliance with isolation techniques.
Gram-negative, non-clonal organism outbreaks are far less frequently described; however, one report has illustrated cohorting of extended-spectrum β-lactamase-producing (ESBL) K. pneumoniae [6]. An 858-bed university hospital with 31 ICU beds found an increasing incidence of ESBL-producing K. pneumoniae that peaked at 11.57 over the baseline of 0.44 cases per 1,000 patient-days. Infection control methods were reviewed and reinforced but failed to disrupt the outbreak. In addition to increasing surveillance cultures, all patients with ESBL-producing K. pneumoniae were cohorted into a single isolated six-bed ICU with dedicated staff. Approximately two months after implementation of these infection control strategies, the incidence fell to 0.08 cases per 1,000 patient-days. The genotype of the bacteria in this outbreak revealed a polyclonal ESBL-producing K. pneumoniae. This outbreak was controlled with cohorting into a dedicated region of the hospital, as has been described for numerous monoclonal outbreaks [6].
At our institution, in addition to standard isolation precautions, we were required to take further action when five of the six patients infected with multiple non-clonal MDRO became infected simultaneously with VRE. The establishment of a high-risk superisolation area provided a way to cohort patients within a single ICU where a private ward or ICU was not feasible. The success of this cohorting, as demonstrated by no new cases of MDRO or VRE within the unit in the ensuing two months, was attributable to a number of factors.
Providing a physical barrier, such as closure of a front entrance, stresses the importance of cohorting. The creation of a high-risk area reinforces sometimes imperfectly executed isolation precautions. Designated nursing staff was essential to our cohorting. Nurse cohorting specifically improves hand hygiene and reduces exogenously acquired infections [14,15]. Each nurse in our cohorting provided care to two isolation patients and had no interaction with non-isolation patients, thereby reducing the likelihood of patient-to-patient transmission of the MDRO. This is similar to the results in an outbreak of Serratia marcescens in a neonatal care unit, in which both nursing staff and respiratory therapists were cohorted to care only for an infected or an uninfected infant [16]. The success of our nurse cohorting was evidenced by the absence of simultaneous positive cultures of the same organism in multiple patients. For instance, S. maltophilia was isolated from Patient B in peritoneal fluid and subsequently in Patient A in a bronchoalveolar lavage two months thereafter. Citrobacter freundii (ESBL) was cultured in Patients C, E, and F in an abdominal abscess, peritoneal fluid, and urine, respectively. Each of these cultures was isolated no less than three weeks apart. This time between infections in various patients suggests that although transmission of MDROs may have occurred, this event was relatively uncommon.
Awareness of superisolation and understanding of cohorting began to extend beyond patient care providers. Nurses in this designated area secondarily became isolation advocates, providing extensive teaching to families as well as staff from all areas of the hospital. As seen in the community hospital in Indiana, compliance with isolation precautions goes up significantly. Although in our unit, we did not quantify or track the data, it was clear that because of the heightened awareness of the severity of the isolated organisms, adherence to isolation precautions improved.
Although we did not observe any adverse effects from our isolation and cohorting directly, a number of articles report negative impacts and barriers to care with patient cohorting [17–20]. These studies revealed that barrier precautions created a disincentive to enter patients' rooms, and two reports found health-care workers were half as likely to enter the room or examine the patient [17,18]. The systematic review conducted by Abad et al. found numerous studies revealing a negative impact on patient well-being, satisfaction, and safety [20].
By cohorting all isolation patients to one region of the ICU, some normalcy was given to the procedures of isolation. With a single nurse assigned to two isolation patients, the process of donning gown and gloves was the norm, not the exception. As this entire section of the unit was in isolation, the disincentive to examine a patient was far less than it would have been for a single isolated patient in a unit full of non-isolation patients.
In countless literature reports, cohorting has been successful in dealing with a single organism outbreak, most commonly gram-positive organisms such as VRE or MRSA [3,5,9,12,13,15,21]. Far fewer articles describe outbreaks of single gram-negative organisms such as ESBL-producing K. pneumoniae and MDR Serratia marcescens [6,16,22,23]. We believe our study is the first to describe cohorting for numerous non-clonal organisms. Five of our six patients had VRE and simultaneous infections with other organisms, including various MDROs. This model of cohorting assigns a region of space within an ICU that reduces patient-to-patient transmission and may be used with multiple patients infected with non-clonal organisms.
Conclusion
Our study shows cohorting patients to one area and altering work routines to minimize contact with patients with MDRO (essentially designating a high-risk zone) may be beneficial in stopping patient-to-patient spread of highly resistant bacteria without the need for a dedicated isolation unit. The literature reveals numerous studies in which cohorting has been successful in diminishing outbreaks of a single monoclonal organism, whereas this study describes cohorting as a novel technique for isolating patients with multiple simultaneous infections, including those with MDRO, in one region within an ICU.
Author Disclosure Statement
Dr. Robert Sawyer is a consultant for Merck, Pfizer, Astellas, and Johnson & Johnson. No other authors have any disclosures to state.
References
- 1.Hospital Infection Control Practices Advisory Committee (HICPAC) Recommendations for preventing the spread of vancomycin resistance. Infect Control Hosp Epidemiol. 1995;16:105–113. doi: 10.1086/647066. [DOI] [PubMed] [Google Scholar]
- 2.Garner JS. Guideline for isolation precautions in hospitals. The Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1996;17:53–80. doi: 10.1086/647190. [DOI] [PubMed] [Google Scholar]
- 3.Selkon JB. Stokes ER. Ingham HR. The role of an isolation unit in the control of hospital infection with methicillin-resistant staphylococci. J Hosp Infect. 1980;1:41–46. doi: 10.1016/0195-6701(80)90030-4. [DOI] [PubMed] [Google Scholar]
- 4.Murray-Leisure KA. Geib S. Graceley D, et al. Control of epidemic methicillin-resistant Staphylococcus aureus. Infect Control Hosp Epidemiol. 1990;11:343–350. doi: 10.1086/646185. [DOI] [PubMed] [Google Scholar]
- 5.Jochimsen EM. Fish L. Manning K, et al. Control of vancomycin-resistant enterococci at a community hospital: Efficacy of patient and staff cohorting. Infect Control Hosp Epidemiol. 1999;20:106–109. doi: 10.1086/501598. [DOI] [PubMed] [Google Scholar]
- 6.Laurent C. Rodriguez-Villalobos H. Rost F, et al. Intensive care unit outbreak of extended-spectrum β-lactamase-producing Klebsiella pneumoniae controlled by cohorting patients and reinforcing infection control measures. Infect Control Hosp Epidemiol. 2008;29:517–524. doi: 10.1086/588004. [DOI] [PubMed] [Google Scholar]
- 7.Gilroy SA. Stahl BM. Noonan C, et al. Reduction of hospital-acquired methicillin-resistant Staphylococcus aureus infection by cohorting patients in a dedicated unit. Infect Control Hosp Epidemiol. 2009;30:203–205. doi: 10.1086/593968. [DOI] [PubMed] [Google Scholar]
- 8.Livornese LL., Jr Dias S. Samel C, et al. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992;117:112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- 9.Sample ML. Gravel D. Oxley C, et al. An outbreak of vancomycin-resistant enterococci in a hematology-oncology unit: Control by patient cohorting and terminal cleaning of the environment. Infect Control Hosp Epidemiol. 2002;23:468–470. doi: 10.1086/502088. [DOI] [PubMed] [Google Scholar]
- 10.Handwerger S. Raucher B. Altarac D, et al. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993;16:750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- 11.Wells CL. Juni BA. Cameron SB, et al. Stool carriage, clinical isolation, and mortality during an outbreak of vancomycin-resistant enterococci in hospitalized medical and/or surgical patients. Clin Infect Dis. 1995;21:45–50. doi: 10.1093/clinids/21.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Hanna H. Umphrey J. Tarrand J. Mendoza M. Raad I. Management of an outbreak of vancomycin-resistant enterococci in the medical intensive care unit of a cancer center. Infect Control Hosp Epidemiol. 2001;22:217–219. doi: 10.1086/501892. [DOI] [PubMed] [Google Scholar]
- 13.Montecalvo MA. Jarvis WR. Uman J, et al. Infection-control measures reduce transmission of vancomycin-resistant enterococci in an endemic setting. Ann Intern Med. 1999;131:269–272. doi: 10.7326/0003-4819-131-4-199908170-00006. [DOI] [PubMed] [Google Scholar]
- 14.Beggs CB. Noakes CJ. Shepherd SJ, et al. The influence of nurse cohorting on hand hygiene effectiveness. Am J Infect Control. 2006;34:621–626. doi: 10.1016/j.ajic.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Karanfil LV. Murphy M. Josephson A, et al. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol. 1992;13:195–200. doi: 10.1086/646509. [DOI] [PubMed] [Google Scholar]
- 16.Maragakis LL. Winkler A. Tucker MG, et al. Outbreak of multidrug-resistant Serratia marcescens infection in a neonatal intensive care unit. Infect Control Hosp Epidemiol. 2008;29:418–423. doi: 10.1086/587969. [DOI] [PubMed] [Google Scholar]
- 17.Kirkland K. Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354:1177–1178. doi: 10.1016/S0140-6736(99)04196-3. [DOI] [PubMed] [Google Scholar]
- 18.Saint S. Higgins LA. Nallamothu BK. Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31:354–356. doi: 10.1016/s0196-6553(02)48250-8. [DOI] [PubMed] [Google Scholar]
- 19.Evans H. Shaffer MM. Hughes MG, et al. Contact isolation in surgical patients: A barrier to care? Surgery. 2003;134:180–188. doi: 10.1067/msy.2003.222. [DOI] [PubMed] [Google Scholar]
- 20.Abad C. Fearday A. Safdar N. Adverse effects of isolation in hospitalised patients: A systematic review. J Hosp Infect. 2010;76:97–102. doi: 10.1016/j.jhin.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guiguet M. Rekacewicz C. Leclercq B, et al. Effectiveness of simple measures to control an outbreak of nosocomial methicillin-resistant Staphylococcus aureus infections in an intensive care unit. Infect Control Hosp Epidemiol. 1990;11:23–26. doi: 10.1086/646074. [DOI] [PubMed] [Google Scholar]
- 22.Langer AJ. Lafaro P. Genese CA, et al. Using active microbiologic surveillance and enhanced infection control measures to control an outbreak of health care-associated extended-spectrum beta-lactamase-producing Klebsiella pneumonia infections–New Jersey, 2007. Am J Infect Control. 2009;37:73–75. doi: 10.1016/j.ajic.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Gregory CJ. Llata E. Stine N, et al. Outbreak of carbapenem-resistant Klebsiella pneumoniae in Puerto Rico associated with a novel carbapenemase variant. Infect Control Hosp Epidemiol. 2010;31:476–484. doi: 10.1086/651670. [DOI] [PubMed] [Google Scholar]