Abstract
The gastrointestinal mucosa is exposed to numerous chemical substances and microorganisms, including macronutrients, micronutrients, bacteria, endogenous ions, and proteins. The regulation of mucosal protection, digestion, absorption and motility is signaled in part by luminal solutes. Therefore, luminal chemosensing is an important mechanism enabling the mucosa to monitor luminal conditions, such as pH, ion concentrations, nutrient quantity, and microflora. The duodenal mucosa shares luminal nutrient receptors with lingual taste receptors in order to detect the five basic tastes, in addition to essential nutrients, and unwanted chemicals. The recent ‘de-orphanization’ of nutrient sensing G protein-coupled receptors provides an essential component of the mechanism by which the mucosa senses luminal nutrients. In this review, we will update the mechanisms of and underlying physiological and pathological roles in luminal nutrient sensing, with a main focus on the duodenal mucosa.
Introduction
The duodenum is located at a strategic crossroads between the acid-secreting stomach and the nutrient absorbing jejunum and ileum. The duodenal mucosa contains a large number of enteroendocrine cells (EEC), a variety of gut hormones, unique defense mechanisms against gastric acid, and chemosensory systems.
Duodenal function is orchestrated via local signaling pathways within the gastrointestinal (GI) tract (Figure 1) and via remote pathways that originate in the central nervous system, including neural and endocrine mediators. A major advance in the past few years is the improved understanding of underlying mechanisms involved in gut sensing and handling of luminal contents, and the signaling pathways involved, including how the gut–brain axis controls food intake, energy and glucose metabolism.
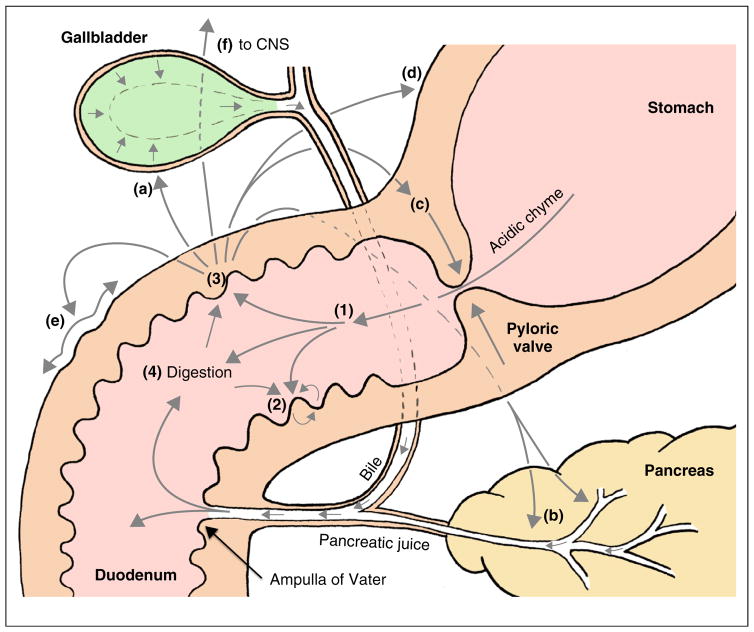
Figure 1.
The ‘master controller’ role of the duodenum. There are multiple physiological processes involving long and short regulatory loops with primary sensors in the duodenum. Acidic chyme entering the duodenum (1) evokes local mucosal autocrine and paracrine mechanisms involved in epithelial defense, that is, safe handling and absorption of large amounts of gastric acid (2) and stimulates a series of additional actions (3) related to the luminal digestion of nutrients (4) including secretion of bile from gallbladder (a), production and release of pancreatic secretions (b), activation of the ‘duodenal brake’ that inhibits gastric emptying and acid secretion (c,d), and increases duodenal motility (e). There are also signaling pathways to the brain (f). Appropriate handling and response to molecules is based on specific sensory information of luminal contents.
In this review, we will summarize recent findings regarding the duodenal response to luminal small molecules with a main focus on the chemosensors in the duodenal mucosa and the related signaling pathways predominantly underlying food-induced gut hormone release.
Duodenal sensing and handling — an overview
Duodenal transit time varies with meal composition, although a burst of chyme moves rapidly through duodenum with propagation velocities of up to 28 ± 20 cm s−1 [1]. Subsequent pulses combined with retrograde peristalsis and mixing ensures that the duodenum will be exposed to chyme for several hrs after a meal and until the stomach is empty.
Duodenal chemosensing encompasses monitoring the luminal content or detection of luminal substances after transmucosal transport. In some cases, sensing can be indirect but still related to the luminal concentration of target molecules. The chemical sensor systems mainly include G protein coupled receptors (GPCR) and transporters (Figure 2). Many of the sensors and related signaling systems have been localized to EEC, although brush cells and enterocytes sense nutrients as well. More than ten EEC types have been described, based on morphology and GI peptide expression, with distinct distribution patterns throughout the gut [2] (Figure 2). Nonetheless, the paradigm of ‘one cell — one hormone’ for EEC is no longer true since a lineage of mature EEC co-expresses a group of functionally related GI peptides up to six [3•,4•].
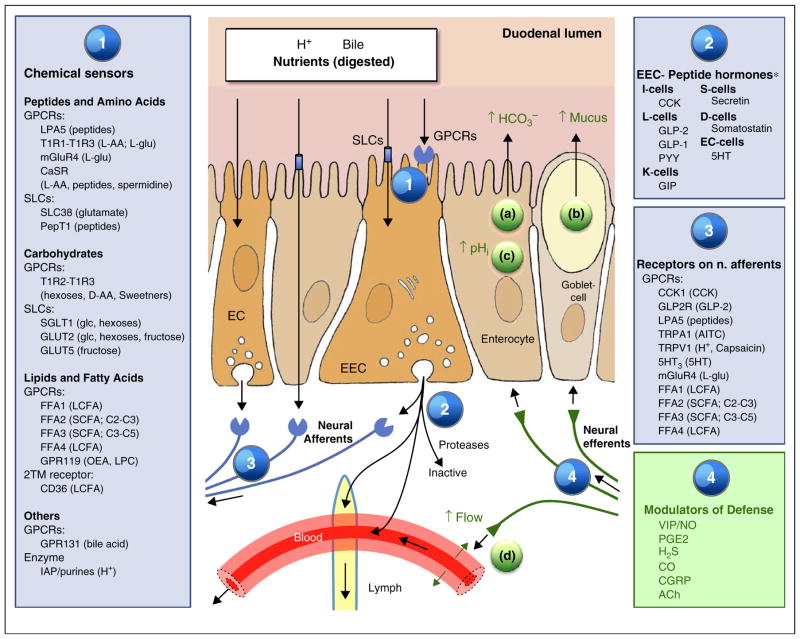
Figure 2.
Sensing, signaling pathways and duodenal epithelial defense. Sensing of luminal contents relies on G protein-coupled receptors (GPCR) and solute carriers (SLC; transporters) many of which are located at the apical brush border membrane (1) GPCR also known as seven-transmembrane receptors are cell surface receptors activated by a diverse range of inputs and ligands. Ligands bound to the extracellular face of the receptor activate intracellular G proteins, generating cascade-like downstream signaling pathways. SLCs exchange small solutes across plasma membranes or transfers solutes coupled to the transmembrane electrochemical gradient of ions such as Na+ or H+. Sensing is often linked to electrogenic activity that alters the transmembrane potential which subsequently enhances voltage-gated Ca2+ influx and subsequent Ca2+-induced stimulation of peptide hormone secretion. Binding of EEC sensors (1) in turn activates intracellular signaling pathways eventuating in the secretion of GI peptides or aromatic amines into the submucosal space (2). Released hormones act as paracrine mediators, can circulate systemically via blood flow or lymphatic flow, or are rapidly degraded. The release of GI peptides evokes local mucosal autocrine and paracrine mechanisms. Most of these signals are mediated through receptors in vagal, splanchnic and intrinsic afferent nerves (3). Mediators in efferent nerves evoke some or all factors in the duodenal mucosal defense system, including (a) stimulation of HCO3− secretion, (b) stimulation of mucus exocytosis resulting in a thicker mucus gel layer, (c) higher cellular buffering effort to protect against acid damage and (d) hyperemic response. For abbreviations, see Table 1. *Displayed to show classical EEC cell categories. Recent work suggests EEC may comprise a single cell type [3•,4•].
Since released GI peptides are rapidly degraded by proteases such as the serine protease dipeptidyl peptidase IV (DPPIV), the most important functions of GI peptides are usually proximate to their release site (autocrine or paracrine action). For example, DPPIV degrades incretins, glucagon-like peptide-1 (GLP-1) and gastric inhibitory peptide/glucose-dependent insulinotropic peptide (GIP), ensuring the short lifetime of incretins. Receptors expressed on afferent (vagal and splanchnic) nerves mediate signals from the GI tract to the brain, including the initial post-prandial satiety signals. In addition to this indirect activation of sensory nerves, peptides may also act in an endocrine (classical) fashion via the systemic circulation, activating receptors in brain areas accessible to blood-borne hormones (hypothalamus or the brainstem) or remote organs.
Peptide and amino acid sensing
The mechanisms underlying protein sensing remain controversial. It appears unlikely that intact proteins are sensed, since prior digestion is required for sensing of small peptides and amino acids (AA) [5,6], although oligopeptides also activate sensing pathways [7••].
The duodenal mucosa contains many AA sensors (Figure 2) such as LPA5 (lysophospholipid 5 receptor; also named GPR92 or GPR93), which reportedly acts like a taste receptor for luminal protein hydrolysates and peptone (partially digested protein) in the small intestine [8,9], and which senses peptides by an unclear mechanism [10]. Recently, Poole et al. [11••] identified LPA5 expression on sensory nerves in the jejunal mucosa, in submucosal and myenteric plexuses, and in primary afferent neurons of dorsal root ganglia of mouse, rat, and human. These authors suggested a novel pathway where molecules, including peptides, in the mesenteric lymphatic fluid (MLF) directly activate LPA5 receptors on sensory nerves. MLF may provide signals to comprise a ‘neurolymphocrine’ system in which nerve fibers of the lacteal are part of a visceral sensory system that may function separately from those of the vagus, perhaps through primary spinal afferent nerves [11••]. Irrespective of this, peptone-stimulated GLP-1 release was not reduced in GPR92/93 knockout mice [7••], suggesting that this alternative sensory pathway is less important, than the canonical sensing mechanism, at least in mice. The taste receptor type 1 heterodimer, T1R1/T1R3 (umami taste receptor) is expressed in duodenal brush cells and L-cells. T1R1/T1R3 is the luminal sensor for natural isoforms of aliphatic-nonessential L-AA and glutamate in the monosodium form (MSG) [12]. In humans T1R1/T1R3 only responds to MSG and Asp, whereas in rats and mouse T1R1/T1R3 senses Phe, Leu, and Glu but not Trp [12]. Sensing by the T1R1/T1R3 receptor is defined by its allosteric modulation by the nucleotides inosine monophosphate (IMP) or guanosine monophosphate (GMP). In mouse proximal intestinal explants and in the cultured L-cell line STC-1, Phe, Leu, and Glu, but not Trp stimulated an IMP enhanced secretion of cholecystokinin (CCK) [13].
T1R1/T1R3 is involved in the crosstalk between nutrients with reciprocal modification of absorption through convergent signaling pathways: T1R1/T1R3 activation by glutamate inhibits di-peptide transport by downregulating PepT1 expression at the apical membrane of enterocytes [14]; For T1R1/T1R3, this involves a shared cytosolic pool of protein kinase C (PKC) βII.
The CaSR (calcium sensing receptor; CaR) also detects luminal aromatic AA such as Phe and Trp and some aliphatic and polar AA. While Ca2+ is the primary ligand for the CaSR, L-Phe allosterically modulates its activity. CaSR can also bind di-peptides, tri-peptides and oligo-peptides. In primary (colonic) L-cells, peptone-triggered GLP-1 secretion is sensitive to CaSR antagonists, while the CaSR agonist calindol increases peptone-induced GLP-1 secretion [7••]. Yet, the physiological relevance for CaSR as a sensor for larger peptides remains unclear.
CaSR is present in enteroendocrine D, G, I, and L cells, although its localization in intestinal epithelial cells is unclear. According to qPCR of isolated CCK-eGFP cells and immunochemical staining, CaSR is expressed in duodenal CCK-secreting enteroendocrine cells but not in absorptive epithelial cells. Administration of L-Phe increased CCK plasma concentrations, decreasing food intake in humans, which is likely mediated by CaSR [15•], in combination with T1R1/T1R3 [13]. Additionally, in STC-1, and in native intestinal I-cells, aromatic amino acids stimulated CCK and GLP-1 release via CaSR, respectively. In isolated rat intestinal loops, luminal AA also release incretins, further enhanced by the CaSR agonist [16•].
Sensing of luminal AA is also linked to solute carriers (SLC), a family of transmembrane proteins that facilitate transmembrane solute movement. Electrogenic AA transport depolarizes the plasma membrane, initiating a signaling cascade that includes Ca2+ influx via the voltage-gated Ca2+ ion channels (Ca2+V) and the final secretion of GI hormones. For example, glutamine, transported through the Na+ coupled transporter SLC38A family, stimulates GLP-1 secretion via voltage-gated Ca2+ ion channels [17].
The H+ coupled oligopeptide transporter, PepT1 may also serve as an intestinal peptide chemosensor, stimulating CCK [15•] and GLP-1 secretion [7••], in response to peptides. Peptone stimulates CCK and GLP-1 secretion through PepT1-dependent electrogenic uptake as well as by activation of CaSR by oligopeptides. Both of these pathways, highly expressed in L cells, may contribute to the observed ability of ingested protein to elevate plasma GLP-1 concentrations. Luminal perfusion of peptone stimulated a PepT1-dependent CCK-responsive vagal afferent discharge that inhibited gastric motility [15•].
Carbohydrate and monosaccharide sensing
Duodenal sensing of carbohydrates requires the presence of dimers or monomers. Luminal glucose evokes incretin (GLP-1 and GIP) release, although the details of the signaling pathways still are debated.
The sweet taste receptor heterodimer T1R2/T1R3 may be important for duodenal sugar chemosensing since it has a broad sensitivity for naturally occurring sweet substances, including sucrose, sweet-tasting D-amino acids, and commonly used artificial sweeteners. T1R2/T1R3, expressed in brush and EEC cells, may be linked to GLP-1 and GIP release from EEC. Duodenal T1R2 expression is regulated by luminal glucose in healthy subjects according to glycemic status but is disordered in type 2 diabetes during acute hyperglycemia [18]. Artificial sweeteners, which activate T1R2/T1R3 but are not transported by monosaccharide transporters, fail to trigger incretin release from EEC in vivo, casting doubt on the contribution of T1R2/T1R3 to direct intestinal glucose sensing.
A much higher concentration of GLP-1 is present in the intestinal lymphatic fluid than in the venous blood [19•]. Using parallel approaches, Sato et al. collected lymph samples from the thoracic duct after bolus administration of dietary nutrients into the duodenum of rats, demonstrating that sweetener-induced and fatty acid-induced GLP-2 secretion are mediated by distinct pathways, with T1R3 involved in the regulation of the former [20].
The sodium-dependent glucose transporter 1 (SGLT-1) is a key transporter for transport of glucose across the BBM [21,22,23•] and is highly expressed in the duodenum [24]. SGLT-1 is also targeted as the L-cell sweet sensor [22,25] where it may potentially act through two pathways: electrogenic, where Na+ directly depolarizes the plasma membrane and opens voltage-gated Ca2+ channels or, metabolic, where increased cellular glucose increases metabolism and closure of ATP-sensitive K+ (KATP) channels. Deletion of SGLT1 in mice reduced blood glucose elevations and abolished GIP and GLP-1 secretion in response to luminal glucose [23•], supporting the hypothesis that apically localized SGLT1 mediates glucose-induced incretin secretion [22,25], and also that this sensory mechanism is intrinsic to EEC, with no involved coupling through neighboring enterocytes [25]. Other electrogenic glucose transporters like SGLT3 also depolarize the plasma membrane, activating voltage-gated Ca2+ channels, but the chemosensory function of SGLT3 is believed to be minor [25].
GLUT2 is a facilitative glucose transporter expressed in the upper GI tract. There is conflicting evidence regarding glucose sensing, incretin secretion, and epithelial absorption of glucose by this transporter [26,27]. The sensory mechanism is based on elevated intracellular glucose concentrations increasing KATP channel closure: in isolated loops of rat small intestine, inhibition of GLUT2 completely abolished incretin secretion due to sucralose, a model dipeptide (glycylsarcosine), a lipid (oleoylethanolamine), a short chain fatty acid (propionate), and a bile acid (taurocholate), all nutrient-sensing GPCR ligands [14]. On the basis of these data, the authors proposed that GLUT2 is important in the control of GIP, GLP-1 and peptide YY (PYY) secretion through its effect on the KATP channels in K-cells and L-cells. This view was challenged by Röder et al. [23•] who reported no differences in glucose uptake, transporter abundance in apical membrane fractions, or plasma GIP, GLP-1 and insulin levels between GLUT2-deficient mice and controls.
Sensing of fructose appears to be different than that of glucose. In healthy humans, rats, and mice, fructose elicits similar patterns of CCK and GLP-1 secretion, but without simultaneous release of GIP or PYY [28]. In GLUTag cells, a murine L cell model, fructose was metabolized and stimulated GLP-1 secretion dose-dependently by KATP channel closure and cell depolarization [28]. The signaling pathway is unknown, but may be linked to the fructose transporter GLUT5 present in L cells and GLUTag cells [25]. A putative, but unknown, sensing pathway has also been proposed for maltose, based on the expression response of sucrase-isomaltase to different substrates in a Caco-2 cell model [29].
Lipid and fatty acid sensing
Fat is a strong stimulus for the release of several GI peptides, including CCK, GIP and GLP-1 [10]. The sensory mechanisms involved, at least for triacylglycerol (TAG), appear reliant of prior hydrolysis [30], with little information regarding signaling pathways for other lipid components such as phospholipids and cholesterol.
Digestion of TAG from most food sources mainly releases long-chain fatty acids (LCFA), which stimulate CCK secretion, with the secretory response correlating to non-esterified FA (NEFA) chain length between C12–22 and the degree of saturation [31]. A possible function for chylomicrons underlying CCK release by an unclear, but yet essential mechanism have been proposed, although lack of observed chylomicrons makes this less likely as an important pathway in the duodenum. Luminal perfusion of LCFA oleate (C18) activated mouse intestinal afferents in vitro in a concentration-dependent fashion. A L-type calcium channel blocker reduced the effect of oleate [32•]. Vagotomy significantly reduced (>60%) whereas a CCK-1 receptor antagonist nearly abolished the responses to CCK and oleate. This suggests that oleate activates vagal afferents but non-vagal fibers also contribute.
Short-chain fatty acids (SCFA), including, acetate, propionate, and butyrate, though present at high concentrations in the hindgut [33], are also involved in foregut chemosensing and signaling. The salivary concentration of SCFA produced by oral flora is 4–6 mM. Duodenal juice contains 0.1–0.4 mM, mainly acetate, even after overnight fasting [34–36]. SCFA are also present in foods such as vinegar and in a range of fermented products. Chyme entering the duodenum may thus contain SCFA in sub-mM concentrations as part of luminal contents of normal physiology.
As described above, most receptors for long chain fatty acids are activated by NEFA. The GPCR fat-sensitive receptors, highly expressed in EEC, are categorized according to their sensitivity to NEFA chain length (Figure 2). Free fatty acid receptors (FFAs), FFA1 (GPR40) and FFA4 (GPR120), activated by medium-chain FA and LCFA, mediate some of the observed hormonal responses to luminal FA [37•,38]. Both receptors transduce their signals through the Gq-phosphatidylinositol pathway. FFA2 (GPR43) and FFA3 (GPR41) are NEFA receptors that selectively respond to SCFA with FFA2 activated by C2–C3 FFAs, whereas FFA3 responds to C3–C5. FFA2 signals are linked with the Gq and Gi/o pathways whereas FFA3 is linked with the Gi/o pathway [38,39]. Both receptors are expressed in duodenum. FFA2 is expressed in EEC and in lamina propria leukocytes, while FFA3 is expressed in most EEC and also in the enteric plexus [40•].
Most foregut FFA2-immunoreactive cells co-express with serotonin (5-HT) [41], strongly suggesting that enterochromaffin (EC) cells sense SCFA. Yet, in terminal ileum and in large intestine, FFA2 is colocalized with L cell-derived peptides [42]. FFA3 transcripts are expressed in EEC producing CCK, GIP and secretin in the upper GI tract and also in EC cells [40•]. SCFA receptors are also expressed in the submucosal and myenteric plexus with varying expression profiles. FFA3, but not FFA2 is expressed in dorsal root ganglion (DRG) neurons, whereas FFA2 and FFA3 are expressed in nodose ganglion neurons [41]. FFA2 transcripts are also present in leukocytes [40•]. These reports support the premise that FFA2 and FFA3 have differential function regarding nutrient sensing and downstream signaling pathways in the upper GI tract. How FFA2 and FFA3 affect neural signaling remains controversial. Expression of FFA3 in sympathetic ganglia supports its contribution to autonomic metabolism regulation [43,44].
GPR119 is not activated by FFA, but rather by lipid derivatives like oleoylethanolamide (OEA) and lysophosphatidyl choline [10]. GPR119, expressed in L and K cells, also correlates with elevated plasma concentrations of the incretins GIP and GLP-1 after administration of these lipid substrates in rats, further supported by impairment of incretin release in GPR119 knockout mice [10].
CD36, a LCFA transporter proposed to act as a sensor in lingual taste papillae, is also expressed in duodenal EEC (secretin and CCK positive cells) BBM [45•]. Since CD36 transports LCFA into the cytoplasm, CD36 may indirectly facilitate chemosensing by supplying cytoplasmic LCFA that are converted to OEA that then activate GPR119 [10]. Data obtained in vivo and in vitro suggest that CD36 also facilitates FA-induced CCK and secretin release [45•].
Sensing and epithelial defense systems
As part of its normal function, the duodenum is exposed to strong gastric acid. Acid-sensing mechanisms underlie a well-described system for increased post-prandial epithelial and pancreaticobiliary HCO3− (equimolar) secretion that neutralizes acid and strengthens epithelial defense (see recent reviews [46–48]). During continuous acid exposure, as occurs during and after a meal, all components of the duodenal defense system, including a thicker mucus gel layer, higher secretion of HCO3−, elevated mucosal blood perfusion, and a higher cellular buffering effort to protect against acid damage are augmented (Figure 2a–d) [47]. In addition to luminal acid/CO2 sensing, other small molecules present in the post-prandial duodenum such as amino acids, bile acids and fatty acids enhance duodenal mucosal defenses through some of the signaling pathways described above. Recent research in the field has focused on continued elucidation of molecular mechanisms involved in this protection, including TRPV (transient receptor potential vanilloid)-1 and TRPA1 [49], T1R1–T1R3 and GLP-2 receptors [50], and bile acid receptor TGR5 (GPR131) [51] (Figure 2).
Clinical perspectives
Since GLP-2 is an intestinotrophic peptide via the actions of insulin-like growth factors, is released by luminal nutrients, and is rapidly degraded by DPPIV, one can assume that DPPIV inhibition combined with luminal nutrients may be useful therapy for small intestinal injury. Exogenous GLP-2 or the DPPIV inhibitor prevented the formation of indomethacin-induced small intestinal ulcers [52]. Furthermore, DPPIV inhibition combined with umami receptor ligands accelerated small intestinal ulcer healing via the GLP-2 pathway. Activation of GLP-2 pathway by luminal nutrient sensing may be a novel therapeutic for the treatment of small intestinal injury and the short bowel syndrome.
Loss of gut luminal nutrient sensing contributes to intestinal mucosal atrophy and loss of epithelial barrier function (EBF). These conditions, often associated with intravenous feeding or parental nutrition (PN), are ameliorated by oral L-Glu [53••]. In the mouse model, atrophy prevention was associated with upregulation of the signaling molecules pAkt/mTOR, but independent of T1R3 and mGluR5 signaling. L-Glu protected EBF via T1R3 signaling, whereas mGluR5 was associated with EBF loss. T1R3, mGluR5 and α-gustducin were downregulated by PN and upregulated by luminal L-Glu. Luminal L-Glu may improve the absorption of luminal nutrients during PN, by also stimulating expression of multiple transporters such as excitatory amino acid transporter 2 and 3, and SGLT-1 [53••].
Duodenal chemosensing may contribute to the pathogenesis of functional dyspepsia [54]. For example, duodenal acidification is implicated in the onset of epigastric pain in healthy subjects [55], suggesting that duodenal chemosensing may control epigastric sensation via neuro-hormonal pathways [56].
Despite the importance of duodenal luminal nutrient sensing, bypassing the duodenum improves metabolic disorders, including obesity and type 2 diabetes. Gastric bypass improves glucose tolerance [57,58] and bariatric surgery improves type 2 diabetes in obese patients [59]. Possible explanations for this observation are that direct exposure of glucose to the distal small intestine, where L-cells are more abundant than in proximal intestine, releases more GLP-1, improving glycemic control. Nevertheless, bypassing the duodenum may eliminate proximal nutrient sensing with associated gut hormone release. Duodenal feeding in piglets was superior to jejunal feeds in terms of more weight gain and less fat malabsorption and diarrhea [60], supporting the importance of duodenum for nutrient absorption. Therefore the primary duodenal function is likely to maximize nutrient absorption, which was evolutionarily important when nutrients were scarce, but may be unnecessary in the era of nutrient overabundance for most of the world.
Conclusions
The upper GI mucosa ‘tastes’ small luminal solutes such as H+, CO2, amino acids, bile acids, and fatty acids, which enhance mucosal defense mechanisms through specific signaling cascades. Novel and known signaling pathways are described in high resolution and provide more insight into the mechanisms that underlie release of gut hormones and stimulation of nerves and how this affects mucosal protection, appetite, satiety, and systemic metabolism. Further knowledge of each chemosensory mechanism may guide most efficient therapies by suggesting combinations of the multiple pathways used to treat disease conditions.
Table 1.
| Abbreviations | |
|---|---|
| 5-HT | 5-Hydroxy tryptamin, serotonin |
| AA | Amino acid |
| ACh | Acetylcholine |
| AITC | Allyl isothiocyanate |
| cAMP | Cyclic adenosine monophosphate |
| CaSR | Calcium-sensing receptor |
| CCK | Cholecystokinin |
| CCK1 | Cholecystokinin 1 receptor |
| CD36 | Cluster of differentiation 36 |
| CGRP | Calcitonin gene-related peptide |
| COX | Cyclooxygenase |
| DPPIV | Dipeptidyl peptidase IV |
| EAAT2 | Excitatory amino acid transporter 2 |
| EBF | Epithelial barrier function |
| EC cell | Enterochromaffin cell |
| EEC | Enteroendocrine cell |
| FFA | Free fatty acid |
| FFA1 | Free fatty acid receptor (type given with numbers) |
| GI | Gastrointestinal |
| GIP | Glucose-dependent insulinotropic peptide |
| GLP | Glucagon-like peptide |
| GLP2R | Glucagon-like peptide2 receptor |
| GLUT | Glucose transporter (type given with numbers 1−) |
| GPCR | G protein-coupled receptor |
| GPR | G protein-coupled receptor |
| IAP | Intestinal alkaline phosphatase |
| IP3 | Inositol 3,4,5 triphosphate |
| KATP | ATP-sensitive K channel |
| LCFA | Long-chain fatty acids |
| LPA5 | Lysophosphatidic acid receptor 5 (LPAR5) |
| mGluR | Metabotropic glutamate receptor (member given by number) |
| MLF | Mesenteric lymphatic fluid |
| NEFA | Non esterified fatty acid |
| NO | Nitric oxide |
| OEA | Oleoylethanolamide |
| PepT1 | Peptide transporter 1 |
| PGE2 | Prostaglandin E2 |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PLCβ2 | Phospholipase C beta 2 |
| PYY | Peptide YY |
| SCFA | Short-chain fatty acid |
| SGLT-1 | Sodium-glucose cotransporter 1 |
| SLC | Solute carrier transporters |
| STC-1 | A mouse enteroendocrine-like cell line |
| T1R1 | Taste receptor type 1 member 1 (member given by numbers) |
| T2Rs | Taste receptor type 2, Bitter receptors |
| TRPA1 | Transient receptor potential cation channel, subfamily A, member 1 |
| TRPV1 | Transient receptor potential cation channel, subfamily V, member 1 |
| VIP | Vasoactive intestinal polypeptide |
Acknowledgments
Supported by a research grant from Ajinomoto, Inc., Japan (Y Akiba), Department of Veterans Affairs Merit Review Award, NIH-NIDDK R01 DK54221 (J Kaunitz), the funding from a Grant-in-Aid for Japan Society for the Promotion of Science Fellow (I Kaji), and funding from Research Council of Norway, 208352; 199482, and University of Bergen, Sabbatical Grant (I Rønnestad).
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Worsoe J, Fynne L, Gregersen T, Schlageter V, Christensen L, Dahlerup J, Rijkhoff N, Laurberg S, Krogh K. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol. 2011;11:145. doi: 10.1186/1471-230X-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjölund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–1130. [PubMed] [Google Scholar]
- 3•.Habib AM, Richards P, Cairns LS, Rogers GJ, Bannon CAM, Parker HE, Morley TCE, Yeo GSH, Reimann F, Gribble FM. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology. 2012;153:3054–3065. doi: 10.1210/en.2011-2170. The data presented indicate a strong overlap between EEC in the upper small intestine L-cells, K-cells, and I-cells and suggest they may comprise a single cell type. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4•.Egerod KL, Engelstoft MS, Grunddal KV, Nohr MK, Secher A, Sakata I, Pedersen J, Windelov JA, Fuchtbauer EM, Olsen J, et al. A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology. 2012;153:5782–5795. doi: 10.1210/en.2012-1595. The dataset published by a different research group than [3•] independently supports broad EEC cell co-expression of functionally related GI-tract peptide hormones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabriel AS, Uneyama H. Amino acid sensing in the gastrointestinal tract. Amino Acids. 2013;45:451–461. doi: 10.1007/s00726-012-1371-2. [DOI] [PubMed] [Google Scholar]
- 6.Gribble FM. The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc Nutr Soc. 2012;71:456–462. doi: 10.1017/S0029665112000705. [DOI] [PubMed] [Google Scholar]
- 7••.Diakogiannaki E, Pais R, Tolhurst G, Parker HE, Horscroft J, Rauscher B, Zietek T, Daniel H, Gribble FM, Reimann F. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia. 2013;56:2688–2696. doi: 10.1007/s00125-013-3037-3. A thorough description of two nutrient sensing pathways highly expressed in native L cells that contribute to the ability of ingested protein to elevate plasma GLP-1 levels. They reported that oligopeptides stimulate GLP-1 secretion through PepT1-dependent electrogenic uptake and activation of CaSR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi S, Lee M, Shiu AL, Yo SJ, Hallden G, Aponte GW. GPR93 activation by protein hydrolysate induces CCK transcription and secretion in STC-1 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1366–G1375. doi: 10.1152/ajpgi.00516.2006. [DOI] [PubMed] [Google Scholar]
- 9.Choi S, Lee M, Shiu AL, Yo SJ, Aponte GW. Identification of a protein hydrolysate responsive G protein-coupled receptor in enterocytes. Am J Physiol Gastrointest Liver Physiol. 2007;292:G98–G112. doi: 10.1152/ajpgi.00295.2006. [DOI] [PubMed] [Google Scholar]
- 10.Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol. 2012:309–335. doi: 10.1007/978-3-642-24716-3_14. [DOI] [PubMed] [Google Scholar]
- 11••.Poole DP, Lee M, Tso P, Bunnett NW, Yo SJ, Lieu TM, Shiu A, Wang JC, Nomura DK, Aponte GW. Feeding-dependent activation of enteric cells and sensory neurons by lymphatic fluid: evidence for a neurolymphocrine system. Am J Physiol Gastrointest Liver Physiol. 2014;306:G686–G698. doi: 10.1152/ajpgi.00433.2013. Sensory nerves in the mesenteric lymphatic fluid may provide a means for constituents of the interstitial fluid to act as signals and thereby comprise a ‘neurolymphocrine’ system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 13.Daly K, Al-Rammahi M, Moran A, Marcello M, Ninomiya Y, Shirazi-Beechey SP. Sensing of amino acids by the gut-expressed taste receptor T1R1–T1R3 stimulates CCK secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G271–G282. doi: 10.1152/ajpgi.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, Helliwell P, Bronk JR, Kellett GL, Meredith D, Boyd R, et al. An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol. 2009;587:195–210. doi: 10.1113/jphysiol.2008.159616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Liou AP, Chavez DI, Espero E, Hao S, Wank SA, Raybould HE. Protein hydrolysate-induced cholecystokinin secretion from enteroendocrine cells is indirectly mediated by the intestinal oligopeptide transporter PepT1. Am J Physiol Gastrointest Liver Physiol. 2011;300:G895–G902. doi: 10.1152/ajpgi.00521.2010. PepT1 does not directly mediate CCK secretion in response to PepT1 specific substrates. Instead, the authors suggest PepT1 may have an indirect role in protein sensing in the intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Mace OJ, Schindler M, Patel S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J Physiol. 2012;590:2917–2936. doi: 10.1113/jphysiol.2011.223800. The facilitative glucose transporter, GLUT2, can act as a glucose sensor whereas CaSR is involved in intestinal sensing of amino acids. The data for GLUT2 confirm some earlier studies, but differ from others emphasizing the need for more studies are needed to fully clarify the in vivo pathways involved in glucose sensing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reimann F, Maziarz M, Flock G, Habib AM, Drucker DJ, Gribble FM. Characterization and functional role of voltage gated cation conductances in the glucagon-like peptide-1 secreting GLUTag cell line. J Physiol. 2005;563:161–175. doi: 10.1113/jphysiol.2004.076414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young RL, Chia B, Isaacs NJ, Ma J, Khoo J, Wu T, Horowitz M, Rayner CK. Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes. Diabetes. 2013;62:3532–3540. doi: 10.2337/db13-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Lu WJ, Yang Q, Sun W, Woods SC, D’Alessio D, Tso P. The regulation of the lymphatic secretion of glucagon-like peptide-1 (GLP-1) by intestinal absorption of fat and carbohydrate. Am J Physiol Gastrointest Liver Physiol. 2007;293:G963–G971. doi: 10.1152/ajpgi.00146.2007. GLP-1 was assayed in response to nutrients using an intestinal lymph fistula model. The incretin response analyzed in lymph was slower, but with significant increase in levels in GLP-1. This is a novel and powerful means of studying the secretion of GLP-1 and potentially other gastrointestinal hormones in vivo. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Hokari R, Kurihara C, Sato H, Narimatsu K, Hozumi H, Ueda T, Higashiyama M, Okada Y, Watanabe C, et al. Dietary lipids and sweeteners regulate glucagon-like peptide-2 secretion. Am J Physiol Gastrointest Liver Physiol. 2013;304:G708–G714. doi: 10.1152/ajpgi.00282.2012. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Sarr MG. Effect of the artificial sweetener, acesulfame potassium, a sweet taste receptor agonist, on glucose uptake in small intestinal cell lines. J Gastrointest Surg. 2013;17:153–158. doi: 10.1007/s11605-012-1998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorboulev V, Schuermann A, Vallon V, Kipp H, Jaschke A, Klessen D, Friedrich A, Scherneck S, Rieg T, Cunard R, et al. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose-absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–196. doi: 10.2337/db11-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Röder PV, Geillinger KE, Zietek TS, Thorens B, Koepsell H, Daniel H. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLOS ONE. 2014;9:e89977. doi: 10.1371/journal.pone.0089977. SGLT1 is unequivocally the prime intestinal glucose transporter even at high luminal glucose concentrations. The authors conclude that SGLT1 mediates glucose-induced incretin secretion and could not provide any evidence for GLUT2 playing a role in either apical glucose influx or incretin secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshikawa T, Inoue R, Matsumoto M, Yajima T, Ushida K, Iwanaga T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol. 2011;135:183–194. doi: 10.1007/s00418-011-0779-1. [DOI] [PubMed] [Google Scholar]
- 25.Parker HE, Adriaenssens A, Rogers G, Richards P, Koepsell H, Reimann F, Gribble FM. Predominant role of active versus facilitative glucose transport for glucagon-like peptide-1 secretion. Diabetologia. 2012;55:2445–2450. doi: 10.1007/s00125-012-2585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellett GL. The facilitated component of intestinal glucose absorption. J Physiol. 2001;531:585–595. doi: 10.1111/j.1469-7793.2001.0585h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stumpel F, Burcelin R, Jungermann K, Thorens B. Normal kinetics of intestinal glucose absorption in the absence of GLUT2. evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2001;98:11330–11335. doi: 10.1073/pnas.211357698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhre RE, Gribble FM, Hartmann B, Reimann F, Windeløv JA, Rehfeld JF, Holst JJ. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol. 2014;306:G622–G630. doi: 10.1152/ajpgi.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng MW, Chegeni M, Kim KH, Zhang G, Benmoussa M, Quezada-Calvillo R, Nichols BL, Hamaker BR. Different sucrose-isomaltase response of Caco-2 cells to glucose and maltose suggests dietary maltose sensing. J Clin Biochem Nutr. 2014;54:55–60. doi: 10.3164/jcbn.13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster SR, Roura E, Thomas WG. Extrasensory perception: odorant and taste receptors beyond the nose and mouth. Pharmacol Ther. 2014;142:41–61. doi: 10.1016/j.pharmthera.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Harden CJ, Jones AN, Maya-Jimenez T, Barker ME, Hepburn NJ, Garaiova I, Plummer SF, Corfe BM. Effect of different long-chain fatty acids on cholecystokinin release in vitro and energy intake in free-living healthy males. Br J Nutr. 2012;108:755–758. doi: 10.1017/S0007114511006003. [DOI] [PubMed] [Google Scholar]
- 32•.Webster WA, Beyak MJ. The long chain fatty acid oleate activates mouse intestinal afferent nerves in vitro. Can J Physiol Pharmacol. 2013;91:375–379. doi: 10.1139/cjpp-2012-0138. Oleate activates intestinal afferents, mainly through vagal afferents but also that nonvagal fibers contribute. The activation is dependent on CCK release and activation of CCK-1 receptors on the afferent terminals. [DOI] [PubMed] [Google Scholar]
- 33.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1040. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 34.Botta GA, Radin L, Costa A, Schito G, Blasi G. Gas–liquid chromatography of the gingival fluid as an aid in periodontal diagnosis. J Periodontal Res. 1985;20:450–457. doi: 10.1111/j.1600-0765.1985.tb00827.x. [DOI] [PubMed] [Google Scholar]
- 35.Hoverstad T, Bjorneklett A, Fausa O, Midtvedt T. Short-chain fatty acids in the small-bowel bacterial overgrowth syndrome. Scand J Gastroenterol. 1985;20:492–499. doi: 10.3109/00365528509089686. [DOI] [PubMed] [Google Scholar]
- 36.Hoverstad T, Bjorneklett A, Midtvedt T, Fausa O, Bohmer T. Short-chain fatty acids in the proximal gastrointestinal tract of healthy subjects. Scand J Gastroenterol. 1984;19:1053–1060. [PubMed] [Google Scholar]
- 37•.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. GPR40 (FFA1) is expressed in endocrine cells of the proximal intestine, including cells expressing the incretin hormones GLP-1 and GIP, and that GPR40 mediates FA-stimulated incretin secretion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reimann F, Gribble FM. A tag to track short chain fatty acid sensors. Endocrinology. 2013;154:3492–3500. doi: 10.1210/en.2013-1789. [DOI] [PubMed] [Google Scholar]
- 40•.Nøhr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Seier Poulsen S, Han S, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154:3552–3564. doi: 10.1210/en.2013-1142. FFA3 and FFA2 act as short-chain fatty acids sensors in enteroendocrine cells, whereas FFA3 apparently has this role alone in enteric neurons and FFAR2 in enteric leukocytes. [DOI] [PubMed] [Google Scholar]
- 41.Kaji I, Akiba Y, Kaunitz JD, Karaki S, Kuwahara A. Differential expression of short-chain fatty acid receptor FFA2 and FFA3 in foregut. Gastroenterology. 2012;142:S494. [Google Scholar]
- 42.Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–142. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- 43.Won YJ, Lu VB, Puhl HL, III, Ikeda SR. Beta-hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J Neurosci. 2013;33:19314–19325. doi: 10.1523/JNEUROSCI.3102-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41) Proc Natl Acad Sci U S A. 2011;108:8030–8040. doi: 10.1073/pnas.1016088108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Sundaresan S, Shahid R, Riehl TE, Chandra R, Nassir F, Stenson WF, Liddle RA, Abumrad NA. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013;27:1191–1202. doi: 10.1096/fj.12-217703. CD36 is a major mediator of FA-induced release of CCK and secretin which contribute to how CD36 affects fat absorption and to its pleiotropic metabolic effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaunitz JD, Akiba Y. Purinergic regulation of duodenal surface pH and ATP concentration: implications for mucosal defence, lipid uptake and cystic fibrosis. Acta Physiol (Oxf) 2011;201:109–116. doi: 10.1111/j.1748-1716.2010.02156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akiba Y, Kaunitz JD. Duodenal luminal chemosensing: acid, ATP, and nutrients. Curr Pharm Des. 2014;20:2760–2770. doi: 10.2174/13816128113199990565. [DOI] [PubMed] [Google Scholar]
- 48.Seidler UE. Gastrointestinal HCO3− transport and epithelial protection in the gut: new techniques, transport pathways and regulatory pathways. Curr Opin Pharmacol. 2013;13:900–908. doi: 10.1016/j.coph.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Akiba Y, Kaunitz JD. Capsiate, a non-pungent capsinoid, enhances mucosal defenses via activation of TRPV1 and TRPA1 in rat duodenum. Gastroenterology. 2011;140:S32. [Google Scholar]
- 50.Wang JH, Inoue T, Higashiyama M, Guth PH, Engel E, Kaunitz JD, Akiba Y. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–473. doi: 10.1124/jpet.111.184788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue T, Wang JH, Higashiyama M, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–G816. doi: 10.1152/ajpgi.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Inoue T, Higashiyama M, Kaji I, Rudenkyy S, Higuchi K, Guth PH, Engel E, Kaunitz JD, Akiba Y. Dipeptidyl peptidase IV inhibition prevents the formation and promotes the healing of indomethacin-induced intestinal ulcers in rats. Dig Dis Sci. 2014;59:1286–1295. doi: 10.1007/s10620-013-3001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Xiao W, Feng Y, Holst JJ, Hartmann B, Yang H, Teitelbaum DH. Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. FASEB J. 2014;28:2073–2087. doi: 10.1096/fj.13-238311. Glutamate may prevent intestinal atrophy changes during in vivo feeding while increasing the expression of several nutrient receptors and transporters. Restoring luminal sensing via glutamate could be a strategy for patients receiving parenteral nutrition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Boxel OS, Ter Linde JJ, Siersema PD, Smout AJ. Role of chemical stimulation of the duodenum in dyspeptic symptom generation. Am J Gastroenterol. 2010;105:803–811. doi: 10.1038/ajg.2010.100. [DOI] [PubMed] [Google Scholar]
- 55.Tanimura T, Adachi K, Furuta K, Ohara S, Morita T, Koshino K, Miki M, Kinoshita Y. Usefulness of catheterless radiotelemetry pH monitoring system to examine the relationship between duodenal acidity and upper gastrointestinal symptoms. J Gastroenterol Hepatol. 2011;26:98–103. doi: 10.1111/j.1440-1746.2010.06468.x. [DOI] [PubMed] [Google Scholar]
- 56.Akiba Y, Kaunitz JD. Duodenal chemosensing: master control for epigastric sensation? J Gastroenterol Hepatol. 2011;26:6–7. doi: 10.1111/j.1440-1746.2010.06580.x. [DOI] [PubMed] [Google Scholar]
- 57.Hansen EN, Tamboli RA, Isbell JM, Saliba J, Dunn JP, Marks-Shulman PA, Abumrad NN. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol. 2011;300:G795–G802. doi: 10.1152/ajpgi.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kindel TL, Yoder SM, Seeley RJ, D’Alessio DA, Tso P. Duodenal-jejunal exclusion improves glucose tolerance in the diabetic, Goto-Kakizaki rat by a GLP-1 receptor-mediated mechanism. J Gastrointest Surg. 2009;13:1762–1770. doi: 10.1007/s11605-009-0912-9. [DOI] [PubMed] [Google Scholar]
- 59.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1570. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curet-Scott MJ, Meller JL, Shermeta DW. Transduodenal feedings: a superior route of enteral nutrition. J Pediatr Surg. 1987;22:516–518. doi: 10.1016/s0022-3468(87)80210-5. [DOI] [PubMed] [Google Scholar]