Abstract
Randomized trials demonstrated the superiority of chemoimmunotherapy over chemotherapy in the frontline treatment of CLL. Based on favorable experience with the addition of mitoxantrone (M) to fludarabine (F) plus cyclophosphamide (C), we designed a pilot study testing the combination of FCM plus rituximab (R). Thirty patients with previously untreated, symptomatic CLL, < 70 years, and beta-2-microglobulin < twice upper limit of normal were evaluated. Treatment consisted of F 25 mg/m2/day on days 2 to 4, C 250 mg/m2/day on days 2 to 4, M 6 mg/m2 on day 2, and R 375 mg/m2 on day 1. For cycles 2 to 6, FCM started day 1 together with R 500 mg/m2. Pegfilgrastim was administered with each cycle. Cycles were repeated every 4 to 6 weeks. Complete remission (CR) was achieved in 83% of 30 patients, nodular partial response in 10%, and partial response in 3%. The overall response rate was 96%. Sixteen of 24 CR patients (67%) achieved a flow cytometry response with < 1% marrow CD5/CD19-positive cells and 13 of 21 CR patients (62%) were MRD-negative by molecular evaluation for clonal IgVH. With a median follow up of 38.5 months, the median time to treatment failure (TTF) has not been reached. A comparison with a historical group of FCR-treated patients showed no significant differences with respect to response and toxicities. FCM-R is highly active in patients < 70 years with favorable beta-2-microglobulin levels and previously untreated CLL. Outcome does not differ from FCR-treated patients.
Keywords: Chronic lymphocytic leukemia (CLL), chemoimmunotherapy, mitoxantroe, nucleoside analogs
Introduction
Commensurate to the discovery that CLL is not of a uniformly indolent nature, but can take a remarkably heterogeneous clinical course with short survival time in some patients, treatment has evolved from single-agent alkylator therapy, to purine nucleoside analogs, to combination therapies with alkylators and nucleoside analogs (e.g. fludarabine plus cyclophosphamide, FC), and eventually addition of monoclonal antibodies to chemotherapy (e.g. FC plus rituximab in “FCR”).1 Single institution phase II studies suggested that FCR (or similar chemoimmunotherapy regimens) improves outcome in both frontline and relapsed patients over chemotherapy alone.2,3 In fact, recently conducted large randomized phase III studies in both settings confirmed significantly higher response rates and progression-free survival (PFS) for FCR compared to FC.4,5 Furthermore, the rate of patients achieving molecular responses or low-level minimal residual disease (MRD) is higher, too, and has been associated with improved PFS.6 On the other hand, overall survival has not been impacted. Although these studies suggest that chemoimmunotherapy should become standard therapy in CLL, they are not curative. Hence efforts are ongoing to optimize combination therapy in CLL. Mitoxantrone was shown in vitro to trigger apoptosis in CLL cells and exhibit synergistic activity in combination with nucleoside analogs and alkylators.7 Combining mitoxantrone with FC (FCM), Bosch et al. in two separate studies demonstrated high response rates with durable response durations in relapsed/refractory and previously untreated patients, respectively.8,9 Responses included eradication of minimal residual disease in up to a third. Given the additional lead from the chemoimmunotherapy experience, we designed a clinical study combining FCR with mitoxantrone (FCM-R) for previously untreated, symptomatic patients with CLL. The objective was to assess clinical, flow cytometry, and molecular response rates and to compare these with a historical control of FCR-treated patients.
Patients and Methods
Study Group
Patients < 70 years and beta-2-microglobulin levels ≤ 2× the upper limit of normal (ULN) with untreated CLL and NCI-Working Group indications for therapy were eligible to participate in the study. Patients were excluded for ECOG performance status of ≥ 3, active hepatitis B, and either symptomatic heart disease (NYHA ≥ 3) or a left ventricular ejection fraction (as measured by either multigated cardiac acquisition scan [MUGA] or echocardiography) of < 40%. All patients signed informed consent according to institutional guidelines. The study was approved by the Institutional Review Board (IRB) of the University of Texas MD Anderson Cancer Center and was conducted in accordance with the basic principles of the Declaration of Helsinki.
Treatment Plan
Fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2 were administered on days 2 to 4 of course 1 and on days 1 to 3 of courses 2 to 6. Mitoxantrone 6 mg/m2 was given on day 2 of course 1 and then on day 1 of courses 2 to 6. Rituximab 375 mg/m2 was given on day 1 of course 1 and at a dose of 500 mg/m2 on days 1 of all subsequent courses. Prior to rituximab, patients received oral acetaminophen 650 mg and diphenhydramine 25 to 50 mg. Before chemotherapy, patients received intravenous ondansetron 24 mg. At the discretion of the treating physician, patients received prophylaxis for Pneumocystis jiroveci (carinii) pneumonia with trimethoprim/sulfamethoxazole or equivalent and antiviral prophylaxis with valacyclovir or equivalent for the duration of therapy. Pegfilgrastim was given routinely following the last day of each treatment cycle. Use of other hematopoietic growth factors was permitted throughout therapy as felt necessary for optimal supportive care. Courses were administered every 4 to 6 weeks as permitted by neutrophil and platelet recovery and/or resolution of toxicities. Patients who achieved a stable partial response or who had continued response after their first 3 courses could receive up to a total of 6 courses. Patients with no response or progressive disease after 1 to 3 courses were considered as having failed therapy and were taken off treatment and observed for progression and survival.
Monitoring of patients consisted of CBC with differential every 1 to 2 weeks as long as on therapy and during 6 monthly follow-up visits thereafter as long as on study. Physical examination and serum chemistries including creatinine, transaminases and total bilirubin were repeated before each treatment course. Dose adjustments were made for any ≥ grade 3 treatment-related toxicity (based on National Cancer Institutes Common Toxicity Criteria [http://ctep.cancer.gov/reporting/ctc.html]) during the preceding course. For patients with pretreatment platelet counts >/= 100 × 109/L and/or ANC >/= 1 × 109/L, the platelet count was required to be >/= 60 × 109/L and/or ANC >/= 1 × 109/L prior to continuation. For patients with pretreatment platelet counts < 100 × 109/L or ANC < 1 × 109/L at the start of therapy, blood counts were required to have recovered to within 20% of pretreatment levels prior to starting the next course. Dose level −1 was fludarabine 20 mg/m2 daily for 3 days and cyclophosphamide 200 mg/m2 daily for 3 days without any change in the dose of mitoxantrone. Dose level −2 included a reduction of the mitoxantrone dose to 4.5 mg/m2. There were no dose adjustments for rituximab.
Response Assessment
Patients were evaluated for response according to 1996 NCIWG criteria after their third and sixth course with physical examination, CBC with differential and bone marrow biopsy and aspiration including immunophenotyping and, in some cases, IgVH molecular studies.10 Immunophenotyping was performed based on standard two-color flow cytometry. Molecular monitoring for residual disease was performed using a polymerase chain reaction (PCR)-based ligase assay for patient-specific clonal IgVH as described elsewhere.3
Statistical Analysis
The objective of the study was to determine the clinical, flow cytometry, and molecular response rate in previously untreated patients with symptomatic CLL, age < 70 years, and beta-2-microglobulin levels < 2× ULN when adding mitoxantrone to the FCR combination. A previous phase II clinical trial of FCR resulted in an overall response rate at 6 months of approximately 95%.2 When measuring flow cytometry for CD5/CD19 at 3 and 6 months it was shown that those patients who achieved <1% CD5/CD19-positive cells had better survival than those whose expression was ≥ 1%. It was therefore concluded that obtaining expression of CD5/CD19 by flow cytometry at 3 months was a useful tool for early assessment of survival. The method of Thall, Simon, and Estey was used to perform interim efficacy (CD5/CD19 expression at 3 months and safety monitoring (≥ grade 3 neutropenia).11 Monitoring for efficacy and safety started once the first 10 patients had been enrolled and was then conducted continuously. Historical data were based from on 189 previously untreated patients who were treated with FCR on the previous phase II trial.2 In this study 34.4% of the patients had CD5/CD19 expression < 1% at 3 months and 74.6% had ≥ grade 3 neutropenia. The study aimed to achieve an increase in patients with favorable flow cytometry (CD5/CD19 < 1%) to 50%, an increase of approximately 15%, while not allowing the ≥ grade 3 neutropenia rate to increase. As this study was designed as a pilot study, not more than 30 patients were included.
Descriptive statistics were used to summarize patient characteristics and response data. Duration of response and progression-free survival were estimated according to Kaplan-Meier method. Time intervals were measured from the first day of treatment until progression or relapse.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. A total of 31 patients have been registered of whom 30 patients are evaluable. One patient decided to come off study because of uncertainty whether the health insurance plan would cover the costs of treatment. As per eligibility requirements, all patients had serum beta-2-microglobulin levels less than twice the upper limit of normal, i.e. < 4 mg/L. Only a minority of patients presented with advanced clinical stage as their treatment indication and in most cases treatment was initiated based on a rapid lymphocyte doubling time and/or worsening and significant B-symptoms. Fourteen of 30 patients had > 20% CD38-positive CD5-positive B cells in the marrow. In 17 of 26 patients ZAP-70 immunostaining of marrow lymphoctes was positive, and in 12 of 18 patients IgVH mutational analysis revealed < 2% deviation from germline consistent with unmutated IgVH. A fluorescence in situ hybridization (FISH) panel demonstrated deletions of 17p and 11q in 2 (7%) and 3 (10%) patients, respectively.
Table 1.
Patient Characteristics (N=30)
| Characteristic | Frequency |
|---|---|
| Male, N (%) | 15 (50) |
| Age, yrs | |
| Median | 57 |
| Range | 38-69 |
| WBC, k/μl | |
| Median | 75.1 |
| Range | 5.6-356 |
| Platelet, k/μl | |
| Median | 187 |
| Range | 36-312 |
| Hemoglobin, g/dL | |
| Median | 13.1 |
| Range | 8.5-15.2 |
| Rai stage III and IV, N (%) | 4 (13) |
| Time from diagnosis to treatment, mos | |
| Median | 22 |
| Range | 0-115 |
| Beta-2-microglobulin, mg/L | |
| Median | 2.7 |
| Range | 1.4-3.8 |
| CD38 > 20%, N (%) | 14/30 (47) |
| ZAP-70-positive, N (%)* | 17/26 (65) |
| IgVH unmutated, N (%) | 12/18 (67) |
| FISH abnormalities, N (%) | |
| 17p del | 2/29 (7) |
| 11q del | 3/29 (10) |
| +12 | 7/29 (24) |
| None | 7/29 (24) |
| 13q del (sole) | 10/29 (34) |
Immunohistochemistry
Response
CR was achieved in 25 patients (83%), nPR in 3 patients (10%), and PR in 1 patient (3%) for an OR rate of 96%. Neither of the 2 patients with abnormalities of chromosome 17p achieved a response, whereas the one patient with an 11q23 deletion achieved CR. Response by IgVH mutation status, ZAP-70 staining, and beta-2-microglobulin levels is shown in Table 2. Since the majority of patients achieved a major response (CR/nPR), no significant differences could be observed based on the pretreatment characteristics. No deaths occurred on study. Only 1 patient showed progression while on therapy and was taken off study after 3 cycles to proceed with salvage therapy.
Table 2.
Response
| NCIWG Response (%) | |||||
|---|---|---|---|---|---|
| N | CR | nPR | PR | OR | |
| Overall | 30 | 25 (83) | 3 (10) | 1 (3) | 29 (96) |
| Immunoglobulin status | 26 | ||||
| IgVH unmutated | 12 | 12 (100) | - | - | 12 (100) |
| IgVH mutated | 6 | 6 (100) | - | - | 6 (100) |
| Not available | 12 | 7 (58) | 3 (25) | 1 (8) | 11 (91) |
| ZAP-70 staining | 26 | ||||
| ZAP-70 positive | 17 | 12 (71) | 3 (18) | 1 (6) | 16 (94) |
| ZAP-70 negative | 9 | 9 (100) | - | - | 9 (100) |
| Not available | 4 | 4 (100) | - | - | 4 (100) |
| Beta-2-microglobulin levels | 30 | ||||
| < 2 mg/L | 4 | 4 (100) | - | - | 4 (100) |
| ≥ 2 to < 3 mg/L | 16 | 12 (75) | 3 (19) | 1 (6) | 16 (100) |
| ≥ 3 mg/L to < 4 mg/L | 10 | 9 (90) | - | - | 9(90) |
Assessment of minimal residual disease (MRD) following completion of therapy was done in marrow samples by two-color immunostaining and flow cytometry analysis for CD5- and CD19-positive lymphocytes or PCR to detect the IgVH gene in the malignant clone, or both. The data are summarized in Table 3. A decrease of CD5/CD19-positive lymphocytes to < 1% following therapy was achieved in 17 of 28 patients (61%) of whom 16 occurred in patients (67%) who were also in CR by NCIWG criteria. Of 24 patients for whom PCR data are available, 13 patients in CR were PCR-negative (62%), consistent with achievement of a molecular remission.
Table 3.
Flow Cytometry and PCR Response
| Clinical Response | N | Flow (<1%) or PCR (negative) Response | % |
|---|---|---|---|
| CD5/CD19 (Flow) | |||
| CR | 24 | 16 | 67 |
| nPR | 3 | 0 | 0 |
| PR | 1 | 1 | 100 |
| Total | 28 | 17 | 61 |
| PCR | |||
| CR | 21 | 13 | 62 |
| nPR | 2 | 0 | 0 |
| Total | 24* | 13 | 54 |
includes 1 non-responder (PCR-positive)
To put the results into perspective, we compared them to a historical group of 163 patients with symptomatic CLL who were treated in the past with the FCR frontline program, which did not contain mitoxantrone. The patients of the comparison group had the same entry characteristics as those in the FCM-R trial, i.e. age < 70 years and beta-2-microglobulin levels < 2× ULN (Table 4). CR (85% in FCR group versus 83% in FCM-R group) and OR (92% versus 93%) rates were virtually identical. Furthermore, no significant differences were observed in the number of patients achieving < 1% CD5/CD19 expression in the marrow by two-color flow cytometry (76% versus 67%, p=.322), or molecular remissions by PCR (45% versus 62%, p= .139) (Table 5).
Table 4.
FCR and FCM-R Patient Groups
| Characteristic | FCR | FCM-R |
|---|---|---|
| N | 163 | 30 |
| Median age, yrs (range) | 55 (17-69) | 57 (38-69) |
| Rai stage III and IV, N (%) | 41 (25) | 4 (13) |
| Median WBC, k/μl (range) | 75.8 (5.4-620) | 75.1 (5.6-356) |
| Median beta-2-microglobulin, mg/L (range) | 2.9 (1.6-3.9) | 2.7 (1.4-3.8) |
| CD38 > 20% (%) | 77 | 47 |
| IgVH unmutated (%) | 61 | 67 |
| ZAP-70 positive (%)* | 46 | 65 |
| FISH (%) | ||
| 17p del | 6 | 8 |
| 11q del | 29 | 4 |
| 13q del | 19 | 4 |
Immunohistochemistry
Table 5.
Outcome of FCR-treated Patients compared with FCM-R
| FCR | FCM-R | p-value | |
|---|---|---|---|
| N | 163 | 30 | - |
| CR+nPR (%) | 85 + 7 | 83 + 10 | >.05 |
| CD5/CD19 < 1% in CR (%) | 76 | 67 | .322 |
| PCR-negative in CR (%) | 45 | 62 | .139 |
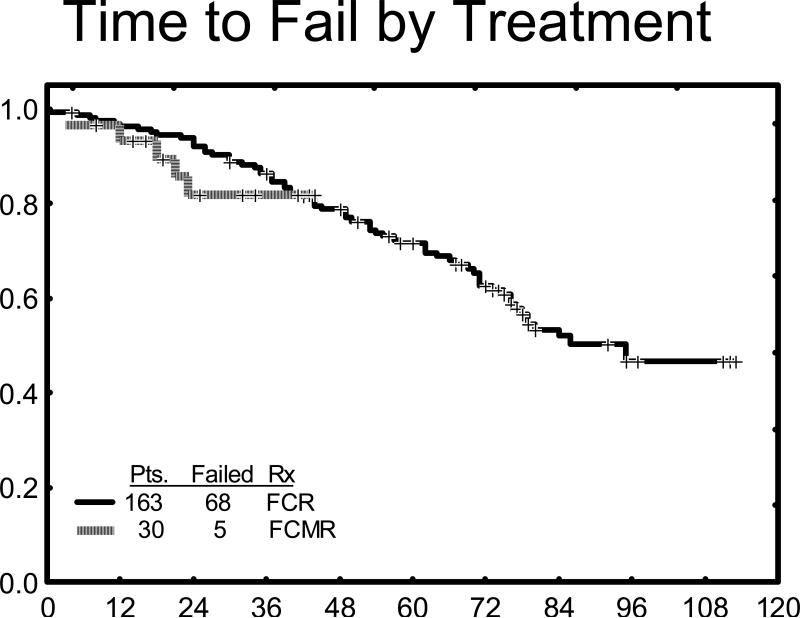
Time to treatment failure (TTF) was determined from time of treatment start until time of progression or relapse. TTF of all patients is shown in Figure 1. With a median follow up of 38.5 months (range 17 to 45+ months), median TTF has not been reached yet as only 5 patients failed therapy. When compared with TTF to the historical FCR only group, the curves are virtually superimposable indicating no difference between these two treatment regimens with the current follow up for FCM-R. No differences yet are discernible in TTF between PCR-negative versus PCR-positive complete responders and those patients with < 1% CD5/CD19-positive marrow cells.
Figure 1.
Time to treatment failure FCM-R (green) versus FCR (yellow)
Toxicity
All six intended courses of treatment were administered to 23 patients (77%). Only 2 patients (7%) received 3 or less courses; one of the patients was taken off therapy with FCM-R following the first course because of disease progression. No deaths on therapy occurred. The most common reason for treatment discontinuation before 6 courses were completed was myelosuppression. Grade 3 and 4 neutropenia was observed in 20 patients (67%) despite routine use of pegfilgrastim with each treatment cycle. Less frequently observed were grade ≥ 3 anemia [4 patients (13%)] and thrombocytopenia [2 (7%)]. No major infections, defined as sepsis, pneumonia, or infection requiring hospitalization, occurred in any of the patients while on study. Fever of unknown origin was seen in 12 patients (40%) and minor infections (upper respiratory tract infections, bronchitis, cellulitis, etc.) were demonstrated in 9 patients (30%). Rates of myelosuppression and infections were similar to the historical FCR experience without significant differences in any of the categories.
One patient (38 year old female achieving a PCR-negative CR and having completed all six cycles of therapy) developed secondary AML after a latency period of around 3 years. No secondary malignancies have been recorded in any of the other patients with the current follow up. No patients developed cardiac toxicity.
Discussion
Recent results from large randomized studies have shown that FCR does achieve higher response and better progression-free survival compared with FC supporting FCR as the new standard for frontline treatment of patients with CLL.4,5 On the other hand, overall survival was not significantly different between treatment arms and even though with longer follow up a survival advantage could become evident, further attempts are being made to identify more effective chemoimmunotherapy combinations.
Mitoxantrone was shown in vitro to trigger apoptosis in CLL cells and to act synergistically in combination with alkylators and nucleoside analogs such as fludarabine.7 Adding mitoxantrone to the FC combination in 60 patients with relapsed and refractory CLL, Bosch et al. reported CR in 30 patients and, remarkably, molecular remission in 10 of these 30 patients.8 Median response duration was 19 months. A landmark analysis for survival at 4 months supported the value of molecular remissions as all the patients with MRD-negative responses were alive at the end of the analysis unlike for patients with MRD-positive CR or those with not achieving CR at all. These promising leads then led to attempts to explore FCM plus rituximab. A randomized study in 147 patients with relapsed/refractory follicular and mantle cell lymphomas showed significantly higher response rates, longer progression-free, and overall survival with FCM-R compared with FCM.12
In our current study, we added mitoxantrone to FCR. The question of interest to us was not to compare chemoimmunotherapy to chemotherapy, but to evaluate whether addition of mitoxantrone to chemoimmunotherapy provided any substantial benefit over FCR by itself. The study was designed as a pilot trial with not more than 30 patients. Primary endpoints were assessment of clinical and flow cytometry (2-color based on CD5/CD19) response. The latter proved a useful marker for frontline FCR patients to predict progression-free survival early on therapy. We included patients younger than 70 years and those with favorable beta-2-microglobulin levels, i.e. values less then twice the upper level of normal. While the study was accruing patients, there were different treatment programs for patients above 70 years of age and those with unfavorably high beta-2-microglobulin levels. This selectivity should be kept in mind when comparing it to other similar studies.
We report a high CR rate of 83% with on OR of 96%. Sixty-seven percent of patients in CR achieved levels of <1% CD5/CD19-expressing marrow cells by flow cytometry and 62% of CR patients also turned PCR-negative. We could not establish any relationship between response and pretreatment biological or clinical characteristics, probably due to the high number of CR patients in a rather small contingent of patients. With the median follow up time of 38.5 months, only 5 patients failed therapy and therefore the median TTF has not been reached. In the FCR study, both flow cytometry negative and PCR negative responses correlated with longer remission duration. This has not been reproduced in the FCM-R study yet and given a median TTF of 6+ years with FCR, longer follow up will be required for this study as well. Major toxicity was severe neutropenia, which occurred in two thirds of patients despite routine use of pegfilgrastim. Although the frequency of severe neutropenia was similar between FCM-R and FCR, the point could be made that FCM-R was more myelosuppressive since every patients received hematopoietic growth factor support whereas this was not the case with the FCR-treated patients. Other than that, the addition of mitoxantrone did not appear more toxic. Nor was it more effective, whether looking at clinical, flow cytometry, or molecular responses. Bosch et al. recently presented updated data on FCM-R for untreated symptomatic patients with CLL.13 Dose and schedule of the treatment agents were almost identical except for a lower cyclosphosphamide dose in the Bosch study. Similar to our study, the response rate was high (82% CR, 93% OR, and 46% MRD-negative CR rate). When compared with FCM, FCM-R produced higher response rates. Variables that were correlated with a lower CR rate included deletions of chromosome 17 and increased levels of beta-2-microglobulin. We were not able to associate these markers with response, because of the low number of patients with 17p deletion and the upper limit we set for beta-2-microglobulin. Somewhat striking was the low number of severe neutropenia in the study by Bosch et al. (only 13% of the patients) which was well below the chemoimmunotherapy experience in general.
In our experience, FCM-R is highly active. This outcome is in agreement with the study by Bosch et al. Compared to FCR, however, the addition of mitoxantrone does not seem to add more benefit. Obviously, our study had fewer patients so that an influence of mitoxantrone on particular subgroups of patients (similar to what has been shown with cyclophosphamide in reference to 11q deletions) has not become obvious. In the absence of further evidence, our emphasis remains on FCR without the addition of mitoxantrone or other chemotherapy agents. Further improvement may be difficult to achieve with intensification of chemotherapy and may depend more on maintenance strategies and subgroup-specific combination therapies.
Acknowledgement
The authors of the study “Fludarabine, Cyclophosphamide, Mitoxantrone, plus Rituximab (FCM-R) in Frontline CLL < 70 Years” received research support from OSI Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
SF was involved in conception and design of the study, acquisition, analysis, and interpretation of data; drafting of the article, and revising it for critically important intellectual content; and final approval of the version submitted. WW, SOB, AF, and SL were involved in conception and design of the study, acquisition, analysis, and interpretation of data; and final approval of the version submitted.
References
- 1.Dighiero G, Hamblin TJ. Chronic lymphocytic leukemia. Lancet. 2008;371:1017–1029. doi: 10.1016/S0140-6736(08)60456-0. [DOI] [PubMed] [Google Scholar]
- 2.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 3.Wierda W, O'Brien S, Wen S, et al. Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab for relapsed and refractory chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4070–4078. doi: 10.1200/JCO.2005.12.516. [DOI] [PubMed] [Google Scholar]
- 4.Hallek M, Fingerle-Rowson G, Fink A-M, et al. Immunochemotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) versus fludarabine and cyclophosphamide (FC) improves response rates and progression-free survival (PFS) of previously untreated patients (pts) with advanced chronic lymphocytic leukemia. Blood. 2008;112:125. abstract 325. [Google Scholar]
- 5.Robak T, Moiseev SI, Dmoszynska A, et al. Rituximab, fludarabine, and cyclophosphamide (R-FC) prolongs progression free survival in relapsed or refractory chronic lymphoyctic leukemia (CLL) compared with FC alone : final results from the International Randomized Phase III REACH Trial. Blood. 2008;112:LBA–1. [Google Scholar]
- 6.Boettcher S, Fischer K, Stilgenbauer S, et al. Quantitative MRD assessments predict progression free survival in CLL patients treated with fludarabine and cyclophosphamide with or without rituximab – a prospective analysis in 471 patients from the randomized GCLLSG CLL8 trials. Blood. 2008;112:125. abstract 326. [Google Scholar]
- 7.Bellosillo B, Villamor N, Colomer D, et al. In vitro evaluation of fludarabine in combination with cyclophosphamide and/or mitoxantrone in B-cell chronic lymphocytic leukemia. Blood. 1999;94:2836–2843. [PubMed] [Google Scholar]
- 8.Bosch F, Ferrer A, López-Guillermo A, et al. Fludarabine, cyclophosphamide and mitoxantrone in the treatment of resistant or relapsed chronic lymphocytic leukemia. Br J Haematol. 2002;119:976–984. doi: 10.1046/j.1365-2141.2002.03959.x. [DOI] [PubMed] [Google Scholar]
- 9.Bosch F, Ferrer A, Villamor N, et al. Fludrabine, cyclophosphamide, and mitoxantrone as initial therapy of chronic lymphocytic leukemia : high response rate and disease eradication. Clin Cancer Res. 2008;14:155–161. doi: 10.1158/1078-0432.CCR-07-1371. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia : revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 11.Thall PF, Simon R, Estey EH. New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol. 1996;14:296–303. doi: 10.1200/JCO.1996.14.1.296. [DOI] [PubMed] [Google Scholar]
- 12.Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas : results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. doi: 10.1182/blood-2004-04-1323. [DOI] [PubMed] [Google Scholar]
- 13.Bosch F, Abrisqueta P, Villmor N, et al. Rituximab, fludarabine, cyclophosphamide, and mitoxantrone (R-FCM) is a highly active chemoimmunotherapy regimen for chronic lymphocytic leukemia. Blood. 2008;112:730. doi: 10.1200/JCO.2009.22.0442. abstract 2097. [DOI] [PubMed] [Google Scholar]