Abstract
Tissue engineering is a rapidly expanding field that aims to establish feasible techniques to fabricate biologically equivalent replacements for diseased and damaged tissues/organs. Emerging from this prospect is the development of in vitro representations of organs for drug toxicity assessment. Due to the ever-increasing interest in ocular drug delivery as a route for administration as well as the rise of new ophthalmic therapeutics, there is a demand for physiologically accurate in vitro models of the eye to assess drug delivery and safety of new ocular medicines. This review summarizes current existing ocular models and highlights the important factors and limitations that need to be considered during their use.
Key words: : cell culture, drug delivery, ophthalmology, tissue engineering, toxicity testing
Introduction
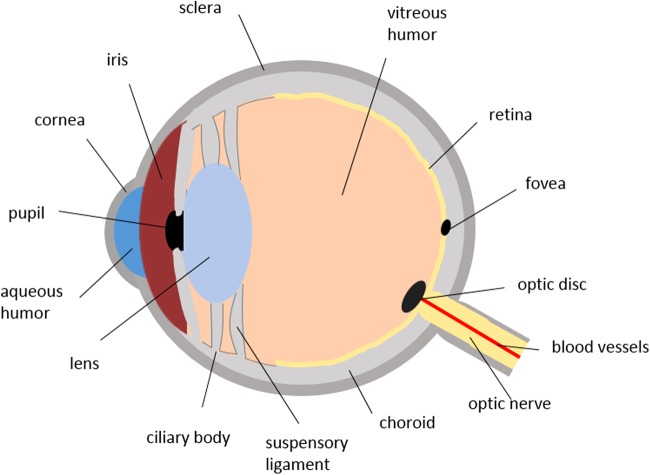
The human eye, a fluid-filled sphere, is a unique and highly protected organ with a complex anatomy and physiology, containing several specialized structures with distinct physiological functions. It is mainly divided into two parts: the anterior and the posterior segments. The anterior segment comprises the cornea, conjunctiva, iris, ciliary body, aqueous humor, and lens, and the posterior segment contains the sclera, choroid, retina, and vitreous humor1 (Fig. 1).
FIG. 1.
Schematic diagram of the human eye.
In the anterior and posterior segments of the eye, ocular barriers play an important part in controlling and regulating the inward and outward transport of solutes, fluids, and also administered drugs. Upon topical administration to the anterior part of the eye, drug molecules are prevented from reaching their ocular site of action as a result of anterior static barriers (i.e., tight junctions of corneal epithelium [CE] and blood–aqueous barriers) and also anterior dynamic barriers (i.e., lacrimal drainage and tear fluid barrier, conjunctival blood and lymphatic clearance). In addition, posterior ocular tissue hinders drug permeation due to expression of efflux pumps on the cell membrane and also by the presence of static (i.e., sclera, retinal pigment epithelium [RPE], and blood capillary endothelial cells) and dynamic barriers (i.e., choroidal blood and lymph circulation).2 Therefore, the administered drug is often required to overcome such ocular barriers before reaching its site of action. For many years, in vivo animal models have been used in ophthalmic studies, mainly to evaluate the level of irritation and toxicity of administered molecules to the ocular cells and tissue. However, in recent years, due to cost, time, and ethical issues associated with animal use,3,4 researchers have been encouraged to develop alternative techniques for ocular investigations. These techniques include ex vivo models of deceased animal tissue and in vitro human and animal cell culture models. The aim of this review is to provide an overview of the established and recently constructed ocular models and the advantages and limitations associated with these models.
Ocular Models
In vivo/ex vivo models
Animal experimentation plays an important role in the research and development of ophthalmic drugs and ocular delivery systems.3 For many years, live animals have been utilized to assess the effect of various ocular products to the eye.5,6 The rabbit is known as the most commonly used animal model with larger animals such as pigs, monkeys, dogs, and cats being less frequently used. In addition, the value of mice and rats are limited in ocular studies due to their small eye size.3
Following permanent eye injuries caused by a cosmetic dye sold in the 1930s,7 the FDA developed the rabbit in vivo Draize test for evaluating acute ocular toxicity.8 Draize test is an international standard assay in which New Zealand white rabbits are mostly used as they are readily obtainable, relatively inexpensive, and have a well-described anatomy with large eyes.6 In this protocol, 0.1 mL of the test substance is applied onto only one eye of the conscious rabbit, whereas the untreated eye serves as a control.8 After 72 h exposure of the test substance on the cornea, conjunctiva, and iris, chemicals can be classified on a subjective scoring, which ranges from nonirritating to severely irritating.5
Despite its gold standard status and being the only validated test for evaluating irritation severity in full range, the Draize test has been criticized for numerous limitations, including its time consuming and subjective nature of assessment, its lack of repeatability and reproducibility,9 high dosage of test materials used,10 variable estimation of results, and overprediction of human response,11 which is mainly related to interspecies differences.
Consequently, the Draize test has been modified both in protocol and data analysis from its original form.12 In 1980, Griffith and colleagues developed the low volume eye irritation test (LVET), as an alternative animal method and following a recommendation from the National Research Council.13 In 1977, the National Research Council committee suggested that the Draize test drawbacks might be more of a volume–response correlation rather than a species–response difference between rabbits and humans. LVET is an alteration to Draize testing in which test substances are only applied to the corneal surface of the rabbit's eye and at a lower volume (0.01 mL vs. 0.1 mL). The rationale in reducing the instilled volume is that it is more representative of the lacrimal fluid volume of both the human and the rabbit eye. Therefore, the LVET was described to cause less stress to tested rabbits and also results could better predict human ocular irritation response.11 However, results obtained following exposure to severe irritants in LVET were considered to be an underestimation of results in comparison with the Draize data.14 Therefore, it is debatable whether to accept LVET as a more accurate test as it lacks the element of exaggeration and overprediction of human responses present in Draize testing.15–18 This, on the other hand, raises concerns over assuring public safety due to its moderate protocol.12,19 As a result, it is still criticized for using animals and it has yet to be accepted as an alternative test by regulatory agencies.
More recently, ocular organotypic models (Table 1) have been used to minimize the use of live animals in experimental studies. These isolated ocular systems retain physiological and biochemical functions of the mammalian enucleated eye or cornea.20,21 Opacity and permeability of the isolated cornea under the effect of a test substance is quantitatively measured using opacitometry and spectrophotometry, respectively. These measurements combined with histological analysis evaluate the extent of damage caused by the test substance, and subsequently drive an eye irritation classification for prediction of potential in vivo ocular irritation of a test substance.5,22
Table 1.
Ex Vivo Organotypic Models Used in Ocular Testing
| Name | Test method indicator | Testing objective | Validation status | Limitations | References |
|---|---|---|---|---|---|
| Bovine corneal opacity and permeability (BCOP) | Increase in corneal thickness, permeability, and opacity | Ocular sensitivity and corrosion | EVCAM statement of scientific validity for identification of severe irritants and ocular corrosives | Not as sensitive in distinguishing between mild irritants with the standard protocol | 22,34 |
| Isolated chicken eye (ICE) | Increase in corneal thickness, permeability, and opacity | Ocular sensitivity and corrosion | EVCAM statement of scientific validity for identification of severe irritants and ocular corrosives | Possible limitation for solids | 22,34 |
| Isolated rabbit eye (IRE) | Increase in corneal thickness, and opacity | Ocular sensitivity and corrosion | Further review is recommended | Possible limitation for solids | 22,34 |
EVCAM, European Center for the Validation of Alternative Methods.
Burton et al. developed the first ocular organotypic model known as the isolated rabbit eye (IRE) test method.23 The IRE, also known as rabbit enucleated eye test, was originally used to detect irreversible eye damage caused by severe irritants.24 IRE protocols have developed over time and have been widely assessed by regulatory bodies (e.g., The European Commission/British Home Office).5 In 1997, an evaluation of the test concluded that the assay lacks the ability to predict irritation over the full range and can only be useful for evaluating severe irritants.25 To date, IRE is mainly used for nonregulatory optimization studies as it is not characterized as a valid assay for ocular irritancy classification.26 In response to the deficiencies associated with IRE, Prinsen and Koëter developed the isolated chicken eye (ICE) test method.27 Chicken eyes are readily available from slaughter houses with consistent quality and dimensions that make them a practical replacement for IRE. Toxic responses are measured by changes in opacity, fluorescein retention, thickness of tissue upon swelling, and assessment of changes related to the surface of the tissue.28
In addition, in 1992, Gautheron et al. developed the bovine cornea opacity and permeability (BCOP) assay based on methods originally developed by Muir29 and Tchao.30,31 The BCOP assay was internationally accepted in 2009 and its scientific suitability is recognized in identifying substances that can cause serious damage as well as substances categorized as nonirritants.5 Using porcine cornea in BCOP is advantageous as it more accurately resembles human cornea in terms of thickness and structure and has also been frequently used in ocular studies.32 These models have been able to generate promising results with fewer ethical concerns and at reduced costs. However, they all share the mutual drawback that anatomical and physiological differences among interspecies are still associated with these tests. In addition, these models are only limited to evaluating the corneal effects of the substances and not the systemic effects. In 2007, the scientific advisory committee of the European Center for the Validation of Alternative Methods (ECVAM) issued a statement on organotypic ex vivo assays as ocular screening tests to detect possible corrosives and severe irritants. Based on this statement, both BCOP and ICE test methods are scientifically valid to identify severe ocular irritants, whereas validation of the IRE method required additional work to be performed and further review was recommended.33
Tissue viability is prolonged when the culture medium is periodically refreshed to retain sufficient levels of supplements and to eliminate metabolic waste products from the cellular environment. Recently, bioreactors that perfuse medium have enabled organotypic models to retain cell viability over an extended period of time.35 Bioreactors are commonly described as devices in which development of biological and/or biochemical processes are under closely controlled environmental and operating conditions (e.g., temperature, pH, oxygen, pressure, medium flow rate, pressure, nutrient supply, and waste metabolite elimination).36,37 Perfusion culture systems in bioreactors have shown to improve and prolong cell viability survival while maintaining cellular functionality.38 Thuret et al. used an innovative bioreactor for storage of ex vivo cornea, which maintained intraocular pressure and continuously renewed medium. This allowed rapid reduction of stromal swelling and improvement of endothelial cell viability in comparison to the corneal immersion in a sealed flask.39
In Vitro Cell-Based Models
Limitations associated with in vivo models have encouraged development and validation of various alternative in vitro models derived from animal and human primary and immortalized cells. The exploitation of appropriate in vitro models is crucial for the development of new approaches to overcome ocular barriers. In comparison with in vivo and ex vivo models, in vitro cell-based models offer the advantage of being simple, quick to construct, relatively inexpensive, and reproducible, while providing mechanistic understanding of the results.3,10 In addition, in vitro models can be used to evaluate a number of combinations of experimental parameters, which is often not achievable with animal models.40,41 These models are commonly used for basic science, pharmaceutical research and development, toxicology, and permeability studies. The use of both primary and immortalized cell culture models of ocular barriers is described in the literature. However, in vitro models based on primary cells have limited cell division and growth in culture media, do not retain cell characteristics beyond three or four passages, and may also differ from isolate to isolate. Therefore, the emphasis on various areas of ocular investigation has been on the development of in vitro models based on animal and human immortalized cell lines.12
Primary cells can be transformed using chemicals or viruses to establish continuous/immortalized cells. With immortalized cell lines, there is no longer a need for the long process of tissue isolation, which is followed by primary cell harvesting and cell purification. Furthermore, cells in culture are stable over a greater passage number with the ability to rapidly expand in growth medium if a large number of cells are required for experiments. However, immortalized cells may have altered growth characteristics, become tumorigenic, and secrete abnormal levels of proteases and cell surface markers. Moreover, expression of many differentiated or tissue-specific enzymes can be decreased and it is more likely for them to express chromosomal abnormalities.42
Corneal morphology
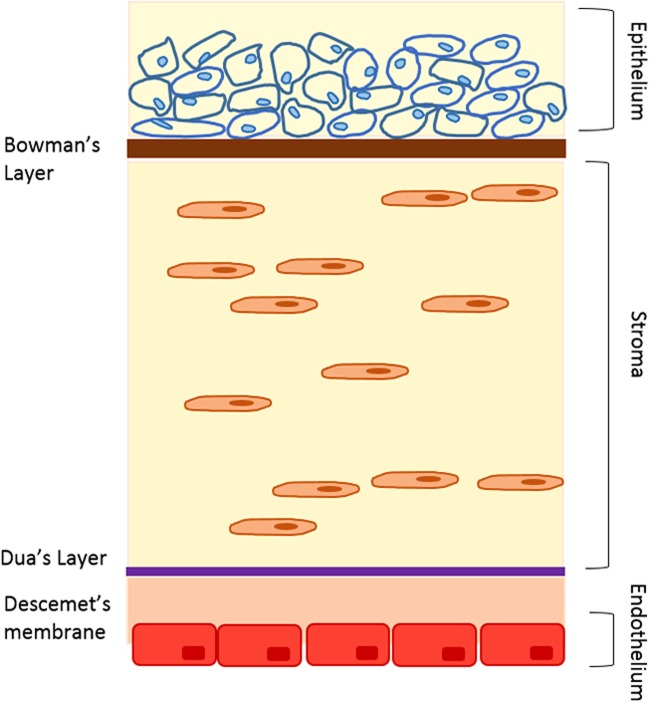
The cornea is the outermost part of the anterior segment of the eye and is always in direct contact with the external environment.43 The transparent and avascular human cornea contains six layers (Fig. 2), and has an average horizontal diameter of about 11.5 mm, a vertical diameter of 10.5 mm, and an average radius of curvature of 7.7 mm curvature that remains constant throughout life.44,45 About 90% of the corneal thickness is made up of 200–250 uniformly spaced collagenous lamellae that intersect and run parallel to the surface to cover the complete extent of the cornea. This layer is called stroma, which offers corneal transparency and physical strength.46 Five more layers make up the remaining 10% of the cornea, which are the epithelium and Bowman's layer at the front of the cornea and Dua's layer, Descemet's membrane, and the endothelium at the back of the cornea.47
FIG. 2.
Schematic presentation of different layers of the cornea.
The cornea is the major route of absorption for topically applied drugs, and CE tight junctions are the most apical intercellular junctions that play an important role in the establishment and maintenance of corneal barrier function.48 The uppermost layers of CE cause over 60% of corneal transepithelial electrical resistance (TEER) and CE is known as the rate-limiting barrier for transcorneal permeation. Therefore, models of the cornea are very useful in studying drug permeation, absorption, and ocular toxicity. To date, cell cultured corneal models range from simple monolayers to stratified epithelium, to cocultures of epithelium and stroma, and to more complex three-dimensional (3D) tissue-engineered corneal equivalents.49
Corneal models
Corneal epithelial models
The CE is the outermost layer of the eye, which acts as a major barrier of the eye. Since the 1960s, many in vitro epithelial models have been established as replacements to Draize test based on primary and immortalized cell lines of human and animal origin.50
Primary models
Most of the primary cell culture models (Table 2) that mimic corneal barrier are constructed from isolated corneal epithelial cells from rabbits.3 For instance, Kawazu et al. established a primary rabbit corneal model which resembled the morphology of an intact cornea and was used for studying permeability of beta adrenergic antagonist and levofloxacin51–53; however, the model's TEER and tight barrier were not comparable to that of cornea in vivo.54,55 In another study by Chang et al., a primary rabbit corneal epithelial membrane was developed with more distinct barrier properties due to air-interface culture conditions.56 Models of human primary epithelial cells have also been investigated as toxicology models.5,57 Furthermore, human primary corneal epithelial (HCE) cell has been used for preparation of tissue sheets to reconstruct the ocular surface in severe ocular surface disorders.58,59 Some authors, such as Ban et al., were successful in developing a stratified epithelium by culturing human corneal limbal cells on human amniotic membrane, which showed tight barrier junctions after 28 days in culture.60 However, the application of HCEs has been limited as alternative in vitro models of corneal barriers due to the low availability of donor corneas.
Table 2.
Summary of the Corneal Epithelial Models
| Species | Application(s) | References |
|---|---|---|
| Primary | ||
| Rabbit | Active transport studies and permeability | 51–53 |
| Rabbit | Permeability studies | 56 |
| Human | Ocular irritation, toxicity, and drug absorption | 79–81 |
| Immortalized | ||
| SIRC | Corneal drug metabolism and transport | 65 |
| EpiOcular™ | Ocular sensitivity and corrosion | 73 |
| SkinEthic™ | Ocular sensitivity and corrosion | 74 |
| Clonetics | Ocular irritation and transepithelial permeability studies | 72 |
SIRC, Statens Seruminstitut rabbit corneal cells.
Immortalized models
Animal
Various models of immortalized animal corneal epithelial cells (Table 2) have been developed from the rabbit,61,62 rat,63 and hamster.64 Models of immortalized rabbit CE cells are more commonly developed and used compared with other animals. In addition, a rabbit corneal cell line known as Statens Seruminstitut rabbit corneal cells is widely used, despite showing a fibroblast phenotype, which in turn decreases its value as a valid model for CE.65 Although these models have helped in reducing the use of animals, the issues related with their nonhuman origin are still present.
Human
To overcome this limitation, since early 1990s, considerable efforts have been focused on developing in vitro models derived from human cells.66–69 Recently, a large amount of research is being done in the field of 3D tissue engineering models.70,71 Examples of the in vitro human-derived CE models that are commercially available as a 3D human cornea equivalents include: EpiOcular™ (MatTek), HCE (SkinEthic), and Clonetics (Lonza). EpiOcular is a stratified, squamous epithelial model in which a permeable polycarbonate membrane is used to culture human epidermal keratinocytes from neonatal human foreskin.72,73 Having an air–liquid interface gives these models the advantage of exhibiting closer morphological and growth characteristics to that of in vivo conditions.22 Similar to the EpiOcular model, HCE model of SkinEthic™ is developed from immortalized human CE mucosa cells cultured at the air–liquid interface using a polycarbonate substrate membrane. This model is structurally very comparable to the corneal mucosa of human eye due to the lack of stratum corneum in the air–epithelial tissue.74 Both EpiOcular and SkinEthic are currently used as an eye irritation test in place of the Draize test for product development.69 Finally, Clonetics, a model of human CE cells cultured on permeable membrane supports and provides useful information on assessing corneal penetration of ophthalmic drugs and transepithelial permeability studies.75 In this model, the lifespan of primary human corneal cells is extended by infecting the cells with a recombinant retrovirus.76
In terms of model validation, both EpiOcular and SkinEthic HCE models have undergone prospective validation by European Union Reference Laboratory for alternatives to animal testing (EURL-ECVAM) and Cosmetics Europe to distinguish potential irritants from nonirritants.77,78 The EpiOcular model was found to be acceptable only for the liquids and the protocol for testing the solids required further optimization. It is now accepted for differentiating irritants from nonirritants, however, EpiOcular model is not intended to distinguish between categories of irritation. In addition, to date, the SkinEthic HCE model is also validated for the testing of liquids protocol, however solids protocol is still undertaking additional optimization.78 Since these models are developed based on noncorneal and immortalized cell lines, there are some differences between them and intact native human cornea, which should be taken into account. For instance, primary mechanical or enzymatic detachment from corneal tissue would result in an induced traumatic stimuli and a various range of responses from the cell.9 Consequently, this may affect the integrity of cellular structure and cell differentiation. In addition, the immortalization process followed by culturing conditions may also alter gene expression; hence the use of immortalized cell lines may not always truly correlate with in vivo behavior of corneal cells.5 Additionally, due to the fragile nature of epithelial models, they should be handled with great care to prevent cells from drying or being damaged. In vitro cell-based assays also lack the presence of hormonal, immune, and neural influences, as well as cell–cell interactions. Although this will result in a less complex model, it can also be a disadvantage for an organ as specialized and complex as the eye since the interactions occurring throughout the whole tissue are not taken into account.22
Corneal equivalents
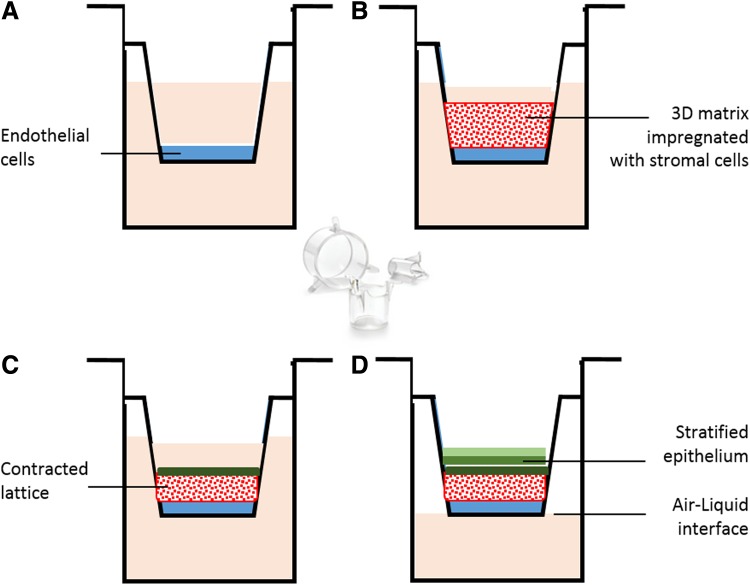
Due to the limitations associated with in vitro corneal epithelial models, several groups have attempted to develop more complex multicellular corneal equivalents. In comparison with models consisting of only corneal epithelial cells, multicellular models better replicate cellular interplay and characteristics of native cornea.5 The first model of a human corneal equivalent from immortalized human corneal cells was developed by Griffith et al.82 This model is composed of a thin layer of endothelial cells cultured on a tissue culture dish with keratocytes and support proteins in the middle covered with a layer of epithelial cells. The stromal matrix was further improved from the original study for understanding recovery mechanisms83 and nerve–target cell interactions.84 Moreover, Reichl et al. (2004) developed a bioengineered human cornea construct for permeation studies containing immortalized endothelium and immortalized stratified epithelium on top derived from HENC and CEPI 17 Cl 4, respectively with native stromal cells (fibroblasts) in a collagen hydrogel matrix (Fig. 3). Three different model drugs were used to test the barrier properties of this cornea equivalent model, such as pilocarpine hydrochloride, befunolol hydrochloride, and hydrocortisone.71 In addition to human corneal equivalents, there are a few models of complete cornea that have been derived from animal cells. The first in vitro model of the whole cornea was developed by Minami et al. derived from isolated bovine endothelial, stromal, and endothelial cells in a collagen gel matrix.85 Cornea-specific keratin was expressed by epithelial cells and the epithelium was a 5–6 layer of superficial cells with microvilli, wing, and basal cells. In addition, Zieske et al. have demonstrated feasibility of developing an in vitro model of all three corneal layers from primary rabbit CE, keratocytes, and a mouse endothelial cell line.86 Consequently, in vitro models of the epithelium, fibroblasts, and endothelium from pig and bovine were constructed for cytotoxicity testing of chemicals and surfactants.87,88 Unfortunately, all multicellular in vitro models do not exhibit the complexity of the native organ89 and factors such as the composition of the aqueous humor, tear fluid, and tear flow90 are not taken into account.
FIG. 3.
Schematic presentation of a corneal equivalent in vitro culture model: (A) Corneal endothelial cells are grown to confluency on a culture insert. (B) A collagen layer containing stromal cells is grown on top of the corneal endothelial cell layer. (C) Epithelial cells are seeded on top of the collagen layer. (D) Exposure to air–liquid interface results in a stratified epithelium.71
Conjunctival Morphology
The conjunctival epithelium is a transparent mucous membrane containing only two to three cell layers and lines the inner eyelid and anterior sclera to the edge of the cornea. It serves as a protective barrier for the ocular surface, generates mucus glycoproteins (mucins) to facilitate eye lubrication and aids adherence of tear films. The conjunctiva is permeable to drugs of different size and polarity91,92 and plays an important part in ocular and systemic absorption of topically administered ophthalmic medications through the noncorneal route.3,93 For a drug to cross the conjunctiva, a significant portion of it is lost to the systemic circulation through the noncorneal route. This intraocular route of drug entry is more applicable to large and hydrophilic molecules that are poorly absorbed through the cornea. The remaining part of the drug can diffuse through sclera, which mainly contains collagen, and unlike conjunctiva, is poorly vascularized. In vitro models of conjunctival epithelium (Table 3) are useful tools in understanding approaches to modulate ocular noncorneal and systemic drug absorption.3
Table 3.
In Vitro Conjunctival Models Derived from Primary Cells
| Species | TEER (Ω cm2) | Application(s) | References |
|---|---|---|---|
| Primary | |||
| Rabbit | ∼1900 | Active transport studies and permeability | 94–99 |
| Rabbit | ∼1100 | Transport studies and metabolism | 100–102 |
| Cow | ∼5600 | Toxicity studies | 103 |
| Immortalized | |||
| Conjunctival (HCjE) cell line | Ocular surface defence mechanism | 107 | |
TEER, transepithelial electrical resistance.
Conjunctival Models
To date, conjunctival epithelial cells have been mostly derived from rabbit primary cells as submerged culture models94–99 and the newer air–liquid interface cell culture systems.100–102 Recently, cell culture models of conjunctiva were developed from primary bovine conjunctival cells103 and immortalized rat cells.104 The next step in establishing a functional in vitro conjunctival model is to develop a system derived from human conjunctival cells. Primary human conjunctival culture models are already used for conjunctival tissue transplantation.105,106 In addition, two immortalized human conjunctival cell lines have also become available and characterized,107,108 but have not yet been used to develop an in vitro model of human conjunctival epithelium.
Retinal Morphology
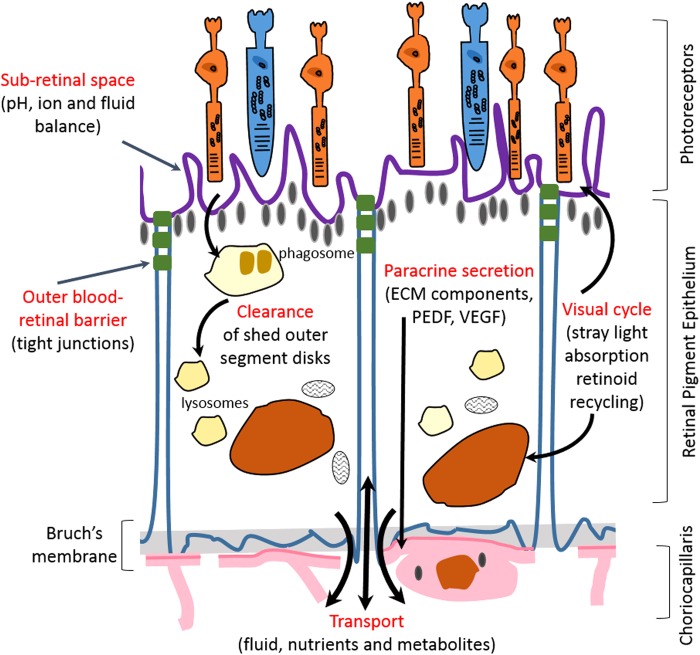
The retina lines the inner surface of the eye, surrounds the vitreous cavity, and has an average thickness of 250 μm. It is designed to capture light and initiate processing of the image by the brain. The retina is protected and held in position by the surrounding sclera and cornea.45 The blood–retinal barrier (BRB) consists of three layers: the outer BRB, known as the RPE, the Bruch's membrane, and the inner BRB, which is the underlying choriocapillaris known as the retinal capillary endothelium109 (Fig. 4). The RPE is a tight monolayer of epithelial cells, which regulates the transport of nutrients and solutes, as well as the diffusion of systemic drugs from the choroid into the retina and vitreous humor.110 In contrast, the inner BRB is located in the inner retinal microvasculature and contains the microvascular endothelium that lines these vessels. Diffusion of many molecules from the blood to the retina is hindered by the tight junctions located between these cells, which are important in maintaining retinal homeostasis.111 Several cell culture models of the inner and outer BRB have been developed as useful tools for investigating cell biology and transport functions of RPE and retinal capillary endothelium (Table 4).
FIG. 4.
Schematic presentation of the functions of the retinal pigment epithelium within the retina.
Table 4.
Cell Culture Models of Blood–Brain Barriers
| Species | Application(s) | References |
|---|---|---|
| Retinal pigment epithelium | ||
| Primary isolated bovine cells | Assessment of barrier function | 116 |
| Primary isolated rat cells | Assessment of tight junctions | 114 |
| Immortalized rat RPE-J cell line | polarity and functions of the retinal pigment epithelium | 131 |
| Immortalized human cells (ARPE-19) | Toxicity, gene delivery, and polarity studies | 136,138,140 |
| Retinal capillary endothelium | ||
| Primary isolated bovine retinal capillary (BRCEC) | Permeability studies | 147 |
| Immortalized rat retinal capillary endothelium | Barrier properties | 146,149 |
Retinal Models
Retinal pigment epithelium
Primary models
Primary cell culture models of RPE derived from frog,112,113 rat,114,115 bovine,116 and chick117 have been widely described in the literature, however, most researchers are interested in models based on human RPE cells to avoid species-related applicability problems. Primary culture models of human RPE118–120 have been used for drug uptake and transport,121–124 protein expression,125 and cytotoxicity studies.126–128 As mentioned previously, there is limited availability of human donor eye cells in comparison with primary animal cells.
Immortalized models
To date, utilizing immortalized rat and human cell lines has led to different in vitro models of RPE being established.67,129–132 For instance, the rat RPE-J cell line with highly differentiated phenotype in vitro was developed by infection of rat primary RPE cells with a SV40 virus.131 In addition, the first spontaneously arising human RPE cell line was developed by Davis et al. from a primary culture of human RPE.129 The cells showed the ability to mimic metabolic and morphological properties of RPE cells in vivo, however, due to the loss of enzymatic activities and cytoskeletal polarization, the cell line is mainly used for cytotoxicity studies133 rather than for the assessment of drug permeation.3 Subsequently, Dunn et al.130 established and characterized the second human immortalized RPE cell line (ARPE-19) in terms of barrier properties and expression of retina-specific markers. ARPE-19 cells retained distinct cell borders and expressed retina-specific markers, RPE65 and CRLABP. In addition, due to the polarized distribution of cell surface markers, the authors suggested this model to be suitable for polarity studies in RPE cells.130 To more accurately reproduce the in vivo RPE phenotype, culture conditions of RPE cells have been altered, such as cocultivation with C6 glioma cells of immortalized astrocytes.134 However, RPE-19 cell lines did not grow in coculture or under conditioned medium mainly due to their heterogeneous nature.4 A third immortalized human RPE cell line was constructed by Bodnar et al., in which RPE cells were transfected by vectors encoding for human telomerase catalytic unit. As a result, the cellular life span was extended while cells retain normal growth characteristics and gene expression patterns.132,135
RPE-19 cell lines have been used by researchers for various in vitro experiments, such as toxicity studies,136,137 gene delivery,138,139 polarity studies of proteins,140 and as a model for retinal disease.137,141 However, the main limitation associated with models of RPE-19 cells is the long culture duration of up to 2 months for the cultured RPE cells to become more growth quiescent.116,142 In addition, due to the limited availability of human donor eyes, there are insufficient primary RPE cell culture models for comparison in the characterization of RPE immortalized cell lines and validating the in vitro culture models in terms of their in vivo/in vitro correlation. In addition, morphological and functional appearance of the RPE cultured cells is easily changed under cell culture conditions, a phenomenon known as deadaptation, and it is yet a challenge to develop a model of RPE that closely mimics many specialized features of the RPE.143
Retinal capillary endothelium
Several pathogenic conditions of the eye such as diabetic retinopathy are related to the breakdown of the inner BRB.144 Therefore, understanding the underlying factors influencing the permeability of the retinal capillary endothelium is essential for discovering new treatment strategies for such diseases. To date, in vitro models of retinal capillary endothelium have been limited to primary isolated bovine retinal capillary endothelial cells (BRCEC)145 and immortalized rat retinal capillary endothelial cell line.146 Gillies et al. studied the permeability of insulin, expression of blood–brain barrier-related enzymes and effect of high glucose levels on the permeability of a BRCEC monolayer using bovine retinal capillary endothelial model.147 This model was further developed by Tretiach et al. as a coculture model of glial cells with primary BRCEC.148 However, this coculture model failed to grow as a monolayer, and also the barrier properties of the Gillies et al. model were not reproduced.145 In addition, Hosoya et al. developed a conditionally immortalized rat retinal capillary endothelial cell line,146 which was further evaluated by Shen et al. in terms of barrier properties.149 Such results concluded that so far, a retinal capillary cell culture model with in vivo barrier properties has not been developed. A better understanding of the in vivo barrier properties of the retinal capillary endothelium, detailed characterization of cell lines, and eventually coculture models will be necessary to establish and scientifically validate a more accurate in vitro model of the inner BRB.3
Ocular Disease Models
To date, a number of animal and cell culture models of ocular diseases have been developed that help to investigate the molecular mechanism of ocular diseases and to screen ophthalmic drug candidates. Age-related macular degeneration (AMD) is a leading cause of blindness in people above the age of 60, which leads to visual impairment and a high rate of depression.109 Due to the unique features of the human eye and complexities associated with the nature of the disease, animal models fail to mimic all aspects of AMD.150 Therefore, cell culture models of RPE cells are useful alternative tools to investigate the physiology and pathology of the disease. In addition, cell culture models are advantageous because they are experimentally controlled systems and so the results are more reproducible than those obtained from animal models.3 A standard primary culture of AMD is human fetal RPE, which closely models the function and metabolic activity of native RPE.151 Other RPE cell models include RPE derived from stem cells and the immortalized ARPE-19 cell line.130
In addition, diseases of the optic nerve are also among the most devastating disorders in ophthalmology, which can result in the degeneration of retinal ganglion cell, visual field loss and, potentially, blindness. To date, the most useful glaucoma experimental animal models are monkeys, rats, and mice in which argon laser photocoagulation, diode laser photocoagulation, or translimbal laser photocoagulation are used to induce intraocular pressure. Although animal models are essential to improve our knowledge and to better understand the mechanism of each disease, developing an animal model for a disease is complex, challenging, and these animal models still differ widely in their applicability to the human disease.152 Therefore, there are a range of cell culture models of glaucoma, which include retinal ganglion cells, mixed retinal cells, transformed retinal cells, and neuronal-like cell lines. Once a culture model is established, multiple mechanisms can be used to simulate injury and study the effectiveness of neuroprotective therapies.153
Vitreous Substitutes
The vitreous body is a gelatinous structure mainly composed of hyaluronic acid and different types of collagen (type II, IX, V/IX, and IV), which fills the space between the lens and the retina. The stability of the vitreous structure is mostly dependant on the presence of bound water to the glycosaminoglycans.154
Since 1960, clinical and bioengineering researchers have attempted to find an ideal vitreous humor substitute that would replicate two aspects of the native in vivo vitreous: on one hand, a substance with the same molecular structure to fill the ocular cavity and to mimic the elasticity and pressure within the eye, and on the other hand, presenting similar chemical and physiological characteristics of this gelatinous substance to allow perfusion of drugs and diffusion of metabolites.155,156 Some of the substances have been known for more than 20 years, however, others have been developed only recently to improve tolerability, stability, and tamponade effect (Table 5).
Table 5.
Vitreous Experimental Substitutes
| Types | Examples | Properties | References |
|---|---|---|---|
| Natural polymers | Hyaluronic acid and collagen | Great biocompatibility; short degradation time; low viscosity | 157,158 |
| Hydrogels | Poly(vinyl alcohol) Poly(1-vinyl-2-pyrrolidone), polyacrylamide | Great biocompatibility, stable transparency, and viscoelastic properties | 159–161 |
| Transplants and Implants | Artificial capsular bodies (foldable capsular vitreous body) | Good mechanical, optical, and biocompatible properties; may cause retinal disorders due to long-term capsule-induced mechanical pressure | 162,163 |
3D In Vitro Models of the Eye
In vitro ocular cell culture models have been widely used in various fields of research; toxicological screening, permeability, studies of drug uptake and transport, cell physiology, and tissue engineering. They provide useful data that compliment findings from in vivo studies and allow significant reduction in the number of animals used. However, there are intrinsic restrictions associated with these models, mainly attributed to the fact that such systems are cell monolayers grown on a two-dimensional (2D) culture scaffold, which do not take into account the response of cells in the 3D curved environment present in the native ocular tissue.164 The in vivo 3D microenvironment can send signals to a cell through cell–cell or cell–extracellular matrix adhesion and mechanical forces.40 Consequently, these signals will activate a cascade of interactive events, which will in turn influence cell proliferation, differentiation, cellular structure morphology, and apoptosis.40 For instance, in drug discovery research, a 2D in vitro cell model that does not present accurate cellular properties and barrier functions may lead to selection of a candidate that cannot reach its target side of action in vivo. In addition, 3D cell culture provides more accurate depiction of cell polarization, since in 2D the cells can only be partially polarized. Moreover, 3D cell cultures have higher stability and longer lifespans than cell cultures in 2D. Also, 3D aggregates can be cultured for a longer period of time, at least up to 4 weeks, in comparison with almost 1 week in a 2D monolayer culture due to cell confluency.40 Therefore, 3D models are more appropriate for demonstrating long-term effects of the drug36 and to create robust and effective cell-based platforms in pharmaceutical research so that cellular responses will be more representative of those under in vivo conditions.165
None of the commercially available in vitro ocular models described in this review have been cultured on curved scaffolds to mimic growth conditions of corneal and retinal cells in vivo. In addition, ocular in vitro models are limited to regional parts of the eye and no model has yet been developed as an in vitro ocular equivalent as an organ. Only recently, a study by Postnikoff et al.50 has taken into account curved cell growth conditions, which focused on the creation of a 3D, stratified, curved epithelium. In this study, human papillomavirus-immortalized HCE cells were cultured on a curved Millicell-HA membrane (mixed cellulose esters; Millipore, Billerica, MA). This culture condition led to a stratified, curved, epithelial model suitable for assessment of cytotoxicity and biocompatibility testing of contact lenses.50 Therefore, the availability of accurate and informative 3D ocular in vitro models is an important challenge for applications in ophthalmic research, toxicity testing, and safety screening.
Conclusion
Every year, around 50–100 million animals, ranging from zebra fish to nonhuman primates, are used worldwide in animal experiments. In 2010, the total number of animals used in the United States was almost 1.37 million, however, these statistics do not include rats and mice as these animals are not covered by the Animal Welfare Act in the United States, but still make up about 90% of research animals. In 2004, over 20,000 rabbits were used in the United Kingdom for the Draize eye irritancy tests and by 2011, over three million animals were generally used for experimentation, which mainly included mice (71%), fish (15%), rats (7%), and birds (4%).166,167 In view of this and to minimize the use of animals, a great amount of research has been dedicated to the development of nonanimal alternatives wherever necessary. Alternatives to animal studies are considered as anything from complete to partial replacement of live animals in biomedical research and experimental studies.168 An apparent 40% decrease in animal use and a simultaneous increase in the use of tissue culture and biotechnology show that scientifically valid nonanimal techniques are implementable.169
The development of alternative ex vivo ocular models has made important contributions to biological research. The BCOP and the ICE test methods have been in development since the early 1990s and are the first ex vivo ocular safety test methods that have been validated by the regulators. In both cases, the animal eyes used in both test methods are slaughterhouse waste, therefore animals were not specifically euthanized to obtain these tissues. William Stokes, director of NICEATM and executive director of ICCVAM, estimated that using these two assays alone could reduce the use of live animals for eye safety testing by 10% or more.170
In addition, the use of in vitro platforms has been greatly attributed to obvious cost and ethical advantages over in vivo models. Finally, the development of 3D in vitro culture models that more closely replicates in vivo and complements 2D cell culture and animal model findings, will help researchers to feel confident that final decisions based on in vivo ocular models are well supported.
Abbreviations Used
- 2D
two-dimensional
- 3D
three-dimensional
- AMD
age-related macular degeneration
- BCOP
bovine cornea opacity and permeability
- BRB
blood–retinal barrier
- BRCEC
bovine retinal capillary endothelial cells
- CE
corneal epithelium
- EURL
European Union Reference Laboratory
- EVCAM
European Center for the Validation of Alternative Methods
- HCE
human corneal epithelial
- IRE
isolated rabbit eye
- LVET
low-volume eye irritation test
- RPE
retinal pigment epithelium
- SIRC
Statens Seruminstitut rabbit corneal cells
- TEER
transepithelial electrical resistance
Acknowledgment
The authors would like to thank the University of Hertfordshire for their support on this work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Patel A, Cholkar K, Agrahari V, et al. Ocular drug delivery systems: an overview. World J Pharmacol 2013;2:47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cholkar K, Patel A, Vadlapudi AD, et al. Novel nanomicellar formulation approaches for anterior and posterior segment ocular drug delivery. Recent Pat Nanomed 2012;2:82–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharmaceut Biopharmaceut 2005;60:207–225 [DOI] [PubMed] [Google Scholar]
- 4.Barar J, Asadi M, Mortazavi-Tabatabaei SA, et al. Ocular drug delivery; impact of in vitro cell culture models. J Ophthalmic Vis Res 2009;4:238–252 [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson SL, Ahearne M, Hopkinson A. An overview of current techniques for ocular toxicity testing. Toxicology 2015;327:32–46 [DOI] [PubMed] [Google Scholar]
- 6.Wilhelmus KR. The Draize eye test. Surv Ophthalmol 2001;45:493–515 [DOI] [PubMed] [Google Scholar]
- 7.Calabrese EJ. Principles of Animal Extrapolation: CRC Press, USA, 1991 [Google Scholar]
- 8.Draize JH, Woodard G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Therapeut 1944;82:377–390 [Google Scholar]
- 9.Davila JC, Rodriguez RJ, Melchert RB, et al. Predictive value of in vitro model systems in toxicology. Annu Rev Pharmacol Toxicol 1998;38:63–96 [DOI] [PubMed] [Google Scholar]
- 10.Curren RD, Harbell JW. Ocular safety: a silent (in vitro) success story. Altern Lab Anim 2002;30:69–74 [DOI] [PubMed] [Google Scholar]
- 11.Jester JV, Li L, Molai A, et al. Extent of initial corneal injury as a basis for alternative eye irritation tests. Toxicol In Vitro 2001;15:115–130 [DOI] [PubMed] [Google Scholar]
- 12.Ubels JL, Clousing DP. In vitro alternatives to the use of animals in ocular toxicology testing. Ocul Surf 2005;3:126–142 [DOI] [PubMed] [Google Scholar]
- 13.Griffith JF, Nixon GA, Bruce RD, et al. Dose-response studies with chemical irritants in the albino rabbit eye as a basis for selecting optimum testing conditions for predicting hazard to the human eye. Toxicol Appl Pharmacol 1980;55:501–513 [DOI] [PubMed] [Google Scholar]
- 14.Gettings S, Lordo R, Demetrulias J, et al. Comparison of low-volume, Draize and in vitro eye irritation test data. I. Hydroalcoholic formulations. Food Chem Toxicol 1996;34:737–749 [DOI] [PubMed] [Google Scholar]
- 15.Ghassemi A, Sauers L, Bruner L, et al. (eds.) Demonstrating the Human Safety of a New Household Cleaning (HSC) Product Using Alternatives to the Draize Eye Irritation Test. Presentation made at the US Society of Toxicology meeting, 1993 [Google Scholar]
- 16.Freeberg F, Hooker D, Griffith J. Correlation of animal eye test data with human experience for household products: an update. Cut Ocular Toxicolo 1986;5:115–123 [Google Scholar]
- 17.Freeberg F, Griffith J, Bruce R, et al. Correlation of animal test methods with human experience for household products. Cut Ocular Toxicol 1984;3:53–64 [Google Scholar]
- 18.Roggeband R, York M, Pericoi M, et al. Eye irritation responses in rabbit and man after single applications of equal volumes of undiluted model liquid detergent products. Food Chem Toxicol 2000;38:727–734 [DOI] [PubMed] [Google Scholar]
- 19.Freeberg F, Nixon G, Reer P, et al. Human and rabbit eye responses to chemical insult. Toxicol Sci 1986;7:626–634 [DOI] [PubMed] [Google Scholar]
- 20.Cooper K, Earl L, Harbell J, et al. Prediction of ocular irritancy of prototype shampoo formulations by the isolated rabbit eye (IRE) test and bovine corneal opacity and permeability (BCOP) assay. Toxicol In Vitro 2001;15:95–103 [DOI] [PubMed] [Google Scholar]
- 21.Gettings S, Lordo R, Hintze K, et al. The CFTA evaluation of alternatives program: an evaluation of in vitro alternatives to the Draize primary eye irritation test. (Phase III) Surfactant-based formulations. Food Chem Toxicol 1996;34:79–117 [DOI] [PubMed] [Google Scholar]
- 22.Barile FA. Validating and troubleshooting ocular invitro toxicology tests. J Pharmacol Toxicol Methods 2010;61:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burton A, York M, Lawrence R. The in vitro assessment of severe eye irritants. Food Cosmet Toxicol 1981;19:471–480 [DOI] [PubMed] [Google Scholar]
- 24.Xiang G, Yang XF, Ying Y, et al. Prediction of ocular irritancy of 26 chemicals and 26 cosmetic products with isolated rabbit eye (IRE) test. Biomed Environ Sci 2012;25:359–366 [DOI] [PubMed] [Google Scholar]
- 25.Chamberlain M, Gad S, Gautheron P, et al. Irag working group 1: organotypic models for the assessment/prediction of ocular irritation. Food Chem Toxicol 1997;35:23–37 [DOI] [PubMed] [Google Scholar]
- 26.Scott L, Eskes C, Hoffmann S, et al. A proposed eye irritation testing strategy to reduce and replace in vivo studies using bottom–up and top–down approaches. Toxicol In Vitro 2010;24:1–9 [DOI] [PubMed] [Google Scholar]
- 27.Prinsen M, Koëter H. Justification of the enucleated eye test with eyes of slaughterhouse animals as an alternative to the Draize eye irritation test with rabbits. Food Chem Toxicol 1993;31:69–76 [DOI] [PubMed] [Google Scholar]
- 28.Test Guideline 438. Isolated Chicken Eye Test Method for Identifying Ocular Corrosives and Severe Irritants. Organization for Economic Cooperation and Development, OECD: Paris, France, 2009. [Google Scholar]
- 29.Muir C. A simple method to assess surfactant-induced bovine corneal opacity in vitro: preliminary findings. Toxicol Lett 1984;22:199–203 [DOI] [PubMed] [Google Scholar]
- 30.Tchao R. Trans-epithelial permeability of fluorescein in vitro as an assay to determine eye irritants. Altern Methods Toxicol 1988;6:271–283 [Google Scholar]
- 31.Gautheron P, Dukik M, Alix D, et al. Bovine corneal opacity and permeability test: an in vitro assay of ocular irritancy. Toxicol Sci 1992;18:442–449 [DOI] [PubMed] [Google Scholar]
- 32.Van den Berghe C, Guillet M, Compan D. Performance of porcine corneal opacity and permeability assay to predict eye irritation for water-soluble cosmetic ingredients. Toxicol In Vitro 2005;19:823–830 [DOI] [PubMed] [Google Scholar]
- 33.ECVAM SAC. Statement on the Conclusions of the ICCVAM Retrospective Study on Organotypic In vitro Assays as Screening Tests to Identify Potential Ocular Corrosives and Severe Irritants European Comission 2007. Available from: https://eurl-ecvam.jrc.ec.europa.eu/about-ecvam/scientific-advice-stakeholders-networks/ecvam-scientific-advisory-committee-esac/statements-opinions (accessed November24, 2015)
- 34.Eskes C, Bessou S, Bruner L, et al. Eye irritation. Altern Lab Anim 2005;33:47. [DOI] [PubMed] [Google Scholar]
- 35.Trone M, Campolmi N, Gauthier A, et al. Conception and optimization of a corneal bioreactor. Acta Ophthalmol 2013;91(s252):0 [Google Scholar]
- 36.Antoni D, Burckel H, Josset E, et al. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci 2015;16:5517–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin I, Wendt D, Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol 2004;22:80–86 [DOI] [PubMed] [Google Scholar]
- 38.Maund SL, Nolley R, Peehl DM. Optimization and comprehensive characterization of a faithful tissue culture model of the benign and malignant human prostate. Lab Invest 2014;94:208–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thuret G, He Z, Bernard A, et al. Revisiting corneal storage using an innovative bioreactor. Acta Ophthalmol 2013;91(s252):0 [Google Scholar]
- 40.Elliott NT, Yuan F. A review of three‐dimensional in vitro tissue models for drug discovery and transport studies. J Pharmaceut Sci 2011;100:59–74 [DOI] [PubMed] [Google Scholar]
- 41.Newsam JM, King-Smith D, Jain A, et al. Screening soft materials for their effect on skin barrier function by high throughput experimentation. J Mater Chem 2005;15:3061–3068 [Google Scholar]
- 42.Toropainen E. Corneal Epithelial Cell Culture Model for Pharmaceutical Studies. University of Kuopio, Finland, 2007 [Google Scholar]
- 43.Müller LJ, Marfurt CF, Kruse F, et al. Corneal nerves: structure, contents and function. Exp Eye Res 2003;76:521–542 [DOI] [PubMed] [Google Scholar]
- 44.Cakmak HB, Cagil N, Simavli H, et al. Corneal white-to-white distance and mesopic pupil diameter. Int J Ophthalmol 2012;5:505–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willoughby CE, Ponzin D, Ferrari S, et al. Anatomy and physiology of the human eye: effects of mucopolysaccharidoses disease on structure and function—a review. Clin Exp Ophthalmol 2010;38:2–11 [Google Scholar]
- 46.Rathore K, Nema R. An insight into ophthalmic drug delivery system. Int J Pharm Sci Drug Res 2009;1:1–5 [Google Scholar]
- 47.Dua HS, Faraj LA, Said DG, et al. Human corneal anatomy redefined: a novel pre-descemet's layer (dua's layer). Ophthalmology 2013;120:1778–1785 [DOI] [PubMed] [Google Scholar]
- 48.Uematsu M, Mohamed YH, Onizuka N, et al. A novel in vivo corneal trans-epithelial electrical resistance measurement device. J Pharmacol Toxicol Methods 2015;76:65–71 [DOI] [PubMed] [Google Scholar]
- 49.Huhtala A, Salminen L, Tähti H, et al. Corneal models for the toxicity testing of drugs and drug releasing materials. Topics Multifunct Biomater Devices 2008;1:1–23 [Google Scholar]
- 50.Postnikoff CK, Pintwala R, Williams S, et al. Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PLoS One 2014;16:e96448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawazu K, Shiono H, Tanioka H, et al. Beta adrenergic antagonist permeation across cultured rabbit corneal epithelial cells grown on permeable supports. Curr Eye Res 1998;17:125–131 [DOI] [PubMed] [Google Scholar]
- 52.Kawazu K, Midori Y, Ota A. Cultured rabbit corneal epithelium elicits levofloxacin absorption and secretion. J Pharm Pharmacol 1999;51:791–796 [DOI] [PubMed] [Google Scholar]
- 53.Kawazu K, Yamada K, Nakamura M, et al. Characterization of cyclosporin A transport in cultured rabbit corneal epithelial cells: P-glycoprotein transport activity and binding to cyclophilin. Invest Ophthalmol Vis Sci 1999;40:1738–1744 [PubMed] [Google Scholar]
- 54.Klyce S. Electrical profiles in the corneal epithelium. J Physiol 1972;226:407–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall WS, Klyce D. Cellular and paracellular pathway resistances in the “tight” Cl−-secreting epithelium of rabbit cornea. J Membr Biol 1983;73:275–282 [DOI] [PubMed] [Google Scholar]
- 56.Chang J-E, Basu SK, Lee VH. Air-interface condition promotes the formation of tight corneal epithelial cell layers for drug transport studies. Pharmaceut Res 2000;17:670–676 [DOI] [PubMed] [Google Scholar]
- 57.Tripathi B, Tripathi R. Cytotoxic effects of benzalkonium chloride and chlorobutanol on human corneal epithelial cells in vitro. Lens Eye Toxic Res 1988;6:395–403 [PubMed] [Google Scholar]
- 58.Han B, Schwab IR, Madsen TK, et al. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea 2002;21:505–510 [DOI] [PubMed] [Google Scholar]
- 59.Ramaesh K, Dhillon B. Ex vivo expansion of corneal limbal epithelial/stem cells for corneal surface reconstruction. Eur J Ophthalmol 2003;13:515–524 [DOI] [PubMed] [Google Scholar]
- 60.Ban Y, Cooper LJ, Fullwood NJ, et al. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res 2003;76:735–743 [DOI] [PubMed] [Google Scholar]
- 61.Araki K, Ohashi Y, Sasabe T, et al. Immortalization of rabbit corneal epithelial cells by a recombinant SV40-adenovirus vector. Invest Ophthalmol Vis Sci 1993;34:2665–2671 [PubMed] [Google Scholar]
- 62.Okamoto S, Ohji M, Kosaku K, et al. Characterization of immortalized rabbit corneal epithelial cells with SV40 large T antigen. Jap J Ophthalmol 1994;39:323–333 [PubMed] [Google Scholar]
- 63.Araki K, Sasabe T, Ohashi Y, et al. [Immortalization of rat corneal epithelial cells by SV40-adenovirus recombinant vector]. Nippon Ganka Gakkai Zasshi 1994;98:327–333 (Article in Japanese). [PubMed] [Google Scholar]
- 64.Halenda RM, Grevan VL, Hook RR, et al. An immortalized hamster corneal epithelial cell line for studies of the pathogenesis of Acanthamoeba keratitis. Curr Eye Res 1998;17:225–230 [DOI] [PubMed] [Google Scholar]
- 65.Niederkorn JY, Meyer DR, Ubelaker JE, et al. Ultrastructural and immunohistological characterization of the SIRC corneal cell line. In Vitro Cell Dev Biol 1990;26:923–930 [DOI] [PubMed] [Google Scholar]
- 66.Kahn C, Young E, Lee IH, et al. Human corneal epithelial primary cultures and cell lines with extended life span: in vitro model for ocular studies. Invest Ophthalmol Vis Sci 1993;34:3429–3441 [PubMed] [Google Scholar]
- 67.Araki-Sasaki K, Ohashi Y, Sasabe T, et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci 1995;36:614–621 [PubMed] [Google Scholar]
- 68.Offord EA, Sharif NA, Mace K, et al. Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci 1999;40:1091–1101 [PubMed] [Google Scholar]
- 69.Mohan RR, Possin DE, Mohan RR, et al. Development of genetically engineered tet HPV16-E6/E7 transduced human corneal epithelial clones having tight regulation of proliferation and normal differentiation. Exp Eye Res 2003;77:395–407 [DOI] [PubMed] [Google Scholar]
- 70.Germain L, Carrier P, Auger FA, et al. Can we produce a human corneal equivalent by tissue engineering? Prog Retin Eye Res 2000;19:497–527 [DOI] [PubMed] [Google Scholar]
- 71.Reichl S, Bednarz J, Müller-Goymann C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br J Ophthalmol 2004;88:560–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung K-M, Lee S-H, Ryu Y-H, et al. A new 3D reconstituted human corneal epithelium model as an alternative method for the eye irritation test. Toxicol In Vitro 2011;25:403–410 [DOI] [PubMed] [Google Scholar]
- 73.Stern M, Klausner M, Alvarado R, et al. Evaluation of the EpiOcular™ tissue model as an alternative to the Draize eye irritation test. Toxicol In Vitro 1998;12:455–461 [DOI] [PubMed] [Google Scholar]
- 74.Van Goethem F, Adriaens E, Alepee N, et al. Prevalidation of a new in vitro reconstituted human cornea model to assess the eye irritating potential of chemicals. Toxicol In Vitro 2006;20:1–17 [DOI] [PubMed] [Google Scholar]
- 75.Kulkarni AA, Chang W, Shen J, et al. Use of Clonetics® human corneal epithelial cell model for evaluating corneal penetration and hydrolysis of ophthalmic drug candidates. Invest Ophthalmol Vis Sci 2011;52:3259 [Google Scholar]
- 76.Ehrhardt C, Kim K-J. Drug Absorption Studies: In Situ, In vitro and In Silico Models. Association of Pharmaceutical Scientists. Springer, Germany, 2008 [Google Scholar]
- 77.Pfannenbecker U, Bessou-Touya S, Faller C, et al. Cosmetics Europe multi-laboratory pre-validation of the EpiOcular™ reconstituted human tissue test method for the prediction of eye irritation. Toxicol In Vitro 2013;27:619–626 [DOI] [PubMed] [Google Scholar]
- 78.Zuang V, Schäffer M, Tuomainen AM, et al. EURL ECVAM progress report on the development, validation and regulatory acceptance of alternative methods (2010–2013). Joint Research Centre, Institute for Health and Consumer Protection: European Commission, 2013 [Google Scholar]
- 79.Kruszewski F, Walker T, DiPasquale L. Evaluation of a human corneal epithelial cell line as an in vitro model for assessing ocular irritation. Toxicol Sci 1997;36:130–140 [PubMed] [Google Scholar]
- 80.Ward S, Walker T, Dimitrijevich S. Evaluation of chemically induced toxicity using an in vitro model of human corneal epithelium. Toxicol In Vitro 1997;11:121–139 [DOI] [PubMed] [Google Scholar]
- 81.Toropainen E, Ranta V-P, Talvitie A, et al. Culture model of human corneal epithelium for prediction of ocular drug absorption. Invest Ophthalmol Vis Sci 2001;42:2942–2948 [PubMed] [Google Scholar]
- 82.Griffith M, Osborne R, Munger R, et al. Functional human corneal equivalents constructed from cell lines. Science 1999;286:2169–2172 [DOI] [PubMed] [Google Scholar]
- 83.Doillon C, Watsky M, Hakim M, et al. A collagen-based scaffold for a tissue engineered human cornea: physical and physiological properties. Int J Artif Organs 2003;26:764–773 [DOI] [PubMed] [Google Scholar]
- 84.Suuronen EJ, McLaughlin CR, Stys PK, et al. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol Sci 2004;82:525–533 [DOI] [PubMed] [Google Scholar]
- 85.Minami Y, Sugihara H, Oono S. Reconstruction of cornea in three-dimensional collagen gel matrix culture. Invest Ophthalmol Vis Sci 1993;34:2316–2324 [PubMed] [Google Scholar]
- 86.Zieske JD, Mason VS, Wasson ME, et al. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Exp Cell Res 1994;214:621–633 [DOI] [PubMed] [Google Scholar]
- 87.Schneider AI, Maier-Reif K, Graeve T. Constructing an in vitro cornea from cultures of the three specific corneal cell types. In Vitro Cell Dev Biol Anim 1999;35:515–526 [DOI] [PubMed] [Google Scholar]
- 88.Schneider A, Maier-Reif K, Graeve T. The use of an in vitro cornea for predicting ocular toxicity. In Vitro Toxicol 1997;10:309–318 [Google Scholar]
- 89.Becker RA, Borgert CJ, Webb S, et al. Report of an ISRTP workshop: progress and barriers to incorporating alternative toxicological methods in the US. Regul Toxicol Pharmacol 2006;46:18–22 [DOI] [PubMed] [Google Scholar]
- 90.Tegtmeyer S, Papantoniou I, Müller-Goymann CC. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur J Pharmaceut Biopharmaceut 2001;51:119–125 [DOI] [PubMed] [Google Scholar]
- 91.Wang W, Sasaki H, Chien D-S, et al. Lipophilicity influence on conjunctival drug penetration in the pigmented rabbit: a comparison with corneal penetration. Curr Eye Res 1991;10:571–579 [DOI] [PubMed] [Google Scholar]
- 92.Hayakawa E, Chien D-S, Inagaki K, et al. Conjunctival penetration of insulin and peptide drugs in the albino rabbit. Pharmaceut Res 1992;9:769–775 [DOI] [PubMed] [Google Scholar]
- 93.Ahmed I, Patton T. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci 1985;26:584–587 [PubMed] [Google Scholar]
- 94.Saha P, Kim K-J, Lee VH. A primary culture model of rabbit conjunctival epithelial cells exhibiting tight barrier properties. Curr Eye Res 1996;15:1163–1169 [DOI] [PubMed] [Google Scholar]
- 95.Saha P, Uchiyama T, Kim K-J, et al. Permeability characteristics of primary cultured rabbit conjunctival epithelial cells to low molecular weight drugs. Curr Eye Res 1996;15:1170–1174 [DOI] [PubMed] [Google Scholar]
- 96.Saha P, Yang JJ, Lee V. Existence of a p-glycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci 1998;39:1221–1226 [PubMed] [Google Scholar]
- 97.Basu SK, Haworth IS, Bolger MB, et al. Proton-driven dipeptide uptake in primary cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci 1998;39:2365–2373 [PubMed] [Google Scholar]
- 98.Yang JJ, Kim K-J, Lee VH. Role of P-glycoprotein in restricting propranolol transport in cultured rabbit conjunctival epithelial cell layers. Pharmaceut Res 2000;17:533–538 [DOI] [PubMed] [Google Scholar]
- 99.Scholz M, Lin J-EC, Lee VH, et al. Pilocarpine permeability across ocular tissues and cell cultures: influence of formulation parameters. J Ocul Pharmacol Therapeut 2002;18:455–468 [DOI] [PubMed] [Google Scholar]
- 100.Yang JJ, Ueda H, Kim K-J, et al. Meeting future challenges in topical ocular drug delivery: development of an air-interfaced primary culture of rabbit conjunctival epithelial cells on a permeable support for drug transport studies. J Control Release 2000;65:1–11 [DOI] [PubMed] [Google Scholar]
- 101.Gukasyan HJ, Lee VH, Kim K-J, et al. Net glutathione secretion across primary cultured rabbit conjunctival epithelial cell layers. Invest Ophthalmol Vis Sci 2002;43:1154–1161 [PubMed] [Google Scholar]
- 102.Gukasyan HJ, Kannan R, Lee VH, et al. Regulation of L-cystine transport and intracellular GSH level by a nitric oxide donor in primary cultured rabbit conjunctival epithelial cell layers. Invest Ophthalmol vis Sci 2003;44:1202–1210 [DOI] [PubMed] [Google Scholar]
- 103.Civiale C, Paladino G, Marino C, et al. Multilayer primary epithelial cell culture from bovine conjunctiva as a model for in vitro toxicity tests. Ophthalmic Res 2002;35:126–136 [DOI] [PubMed] [Google Scholar]
- 104.Reichl S, Müller-Goymann CC. The use of a porcine organotypic cornea construct for permeation studies from formulations containing befunolol hydrochloride. Int J Pharmaceut 2003;250:191–201 [DOI] [PubMed] [Google Scholar]
- 105.Scuderi N, Alfano C, Paolini G, et al. Transplantation of autologous cultivated conjunctival epithelium for the restoration of defects in the ocular surface. Scand J Plast Reconstrt Surg Hand Surg 2002;36:340–348 [DOI] [PubMed] [Google Scholar]
- 106.Sangwan VS, Vemuganti GK, Singh S, et al. Successful reconstruction of damaged ocular outer surface in humans using limbal and conjuctival stem cell culture methods. Biosci Rep 2003;23:169–174 [DOI] [PubMed] [Google Scholar]
- 107.Gipson IK, Spurr-Michaud S, Argueso P, et al. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci 2003;44:2496–2506 [DOI] [PubMed] [Google Scholar]
- 108.Diebold Y, Calonge M, de Salamanca AE, et al. Characterization of a spontaneously immortalized cell line (IOBA-NHC) from normal human conjunctiva. Invest Ophthalmol Vis Sci 2003;44:4263–4274 [DOI] [PubMed] [Google Scholar]
- 109.Forest DL, Johnson LV, Clegg DO. Cellular models and therapies for age-related macular degeneration. Dis Models Mech 2015;8:421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mannermaa E, Reinisalo M, Ranta V-P, et al. Filter-cultured ARPE-19 cells as outer blood–retinal barrier model. Eur J Pharmaceut Sci 2010;40:289–296 [DOI] [PubMed] [Google Scholar]
- 111.Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol 2012;763:70–84 [PubMed] [Google Scholar]
- 112.Defoe DM, Ahmad A, Chen W, et al. Membrane polarity of the Na+-K+ pump in primary cultures of Xenopus retinal pigment epithelium. Exp Eye Res 1994;59:587–596 [DOI] [PubMed] [Google Scholar]
- 113.Defoe DM, Easterling KC. Reattachment of retinas to cultured pigment epithelial monolayers from Xenopus laevis. Invest Ophthalmol Vis Sci 1994;35:2466–2476 [PubMed] [Google Scholar]
- 114.Chang C-W, Ye L, Defoe DM, et al. Serum inhibits tight junction formation in cultured pigment epithelial cells. Invest Ophthalmol Vis Sci 1997;38:1082–1093 [PubMed] [Google Scholar]
- 115.Zech J-C, Pouvreau I, Cotinet A, et al. Effect of cytokines and nitric oxide on tight junctions in cultured rat retinal pigment epithelium. Invest Ophthalmol Vis Sci 1998;39:1600–1608 [PubMed] [Google Scholar]
- 116.Hartnett ME, Lappas A, Darland D, et al. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res 2003;77:593–599 [DOI] [PubMed] [Google Scholar]
- 117.Peng S, Rahner C, Rizzolo LJ. Apical and basal regulation of the permeability of the retinal pigment epithelium. Invest Ophthalmol Vis Sci 2003;44:808–817 [DOI] [PubMed] [Google Scholar]
- 118.Holtkamp G, Van Rossem M, De Vos A, et al. Polarized secretion of IL-6 and IL-8 by human retinal pigment epithelial cells. Clin Exp Immunol 1998;112:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol Vis 2001;7:14–19 [PubMed] [Google Scholar]
- 120.Rajasekaran SA, Hu J, Gopal J, et al. Na, K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol 2003;284:C1497–C1507 [DOI] [PubMed] [Google Scholar]
- 121.Lu SC, Sun W-M, Nagineni CN, et al. Bidirectional glutathione transport by cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1995;36:2523–2530 [PubMed] [Google Scholar]
- 122.Huang W, Prasad PD, Kekuda R, et al. Characterization of N 5-methyltetrahydrofolate uptake in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 1997;38:1578–1587 [PubMed] [Google Scholar]
- 123.Hamann S, La Cour M, Lui GM, et al. Transport of protons and lactate in cultured human fetal retinal pigment epithelial cells. Pflügers Arch 2000;440:84–92 [DOI] [PubMed] [Google Scholar]
- 124.Kennedy BG, Mangini NJ. P-glycoprotein expression in human retinal pigment epithelium. Mol Vis 2002;8:30. [PubMed] [Google Scholar]
- 125.Ishida BY, Bailey KR, Duncan KG, et al. Regulated expression of apolipoprotein E by human retinal pigment epithelial cells. J Lipid Res 2004;45:263–271 [DOI] [PubMed] [Google Scholar]
- 126.Ho T-C, Del Priore LV, Hornbeck R. Effect of mitomycin-C on human retinal pigment epithelium in culture. Curr Eye Res 1997;16:572–576 [DOI] [PubMed] [Google Scholar]
- 127.Kon CH, Occleston NL, Foss A, et al. Effects of single, short term exposures of human retinal pigment epithelial cells to thiotepa or 5-fluorouracil: implications for the treatment of proliferative vitreoretinopathy. Br J Ophthalmol 1998;82:554–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoffmann S, Masood R, Zhang Y, et al. Selective killing of RPE with a vascular endothelial growth factor chimeric toxin. Invest Ophthalmol Vis Sci 2000;41:2389–2393 [PubMed] [Google Scholar]
- 129.Davis AA, Bernstein PS, Bok D, et al. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci 1995;36:955–964 [PubMed] [Google Scholar]
- 130.Dunn K, Aotaki-Keen A, Putkey F, et al. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res 1996;62:155–170 [DOI] [PubMed] [Google Scholar]
- 131.Nabi IR, Mathews AP, Cohen-Gould L, et al. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci 1993;104:37–49 [DOI] [PubMed] [Google Scholar]
- 132.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science 1998;279:349–352 [DOI] [PubMed] [Google Scholar]
- 133.Mannerström M, Zorn-Kruppa M, Diehl H, et al. Evaluation of the cytotoxicity of selected systemic and intravitreally dosed drugs in the cultures of human retinal pigment epithelial cell line and of pig primary retinal pigment epithelial cells. Toxicol In Vitro 2002;16:193–200 [DOI] [PubMed] [Google Scholar]
- 134.Constable PA, Lawrenson JG. Glial cell factors and the outer blood retinal barrier. Ophthalmic Physiol Opt 2009;29:557–564 [DOI] [PubMed] [Google Scholar]
- 135.Jiang X-R, Jimenez G, Chang E, et al. Telomerase expression in human somatic cells does not induce changes associated with a transformed phenotype. Nat Genet 1999;21:111–114 [DOI] [PubMed] [Google Scholar]
- 136.Yeung CK, Chan KP, Chiang SW, et al. The toxic and stress responses of cultured human retinal pigment epithelium (ARPE19) and human glial cells (SVG) in the presence of triamcinolone. Invest Ophthalmol Vis Sci 2003;44:5293–5300 [DOI] [PubMed] [Google Scholar]
- 137.Yeung CK, Chan KP, Chan CK, et al. Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: comparison with dexamethasone and hydrocortisone. Jpn J Ophthalmol 2004;48:236–242 [DOI] [PubMed] [Google Scholar]
- 138.Haeseleer F, Imanishi Y, Saperstein DA, et al. Gene transfer mediated by recombinant baculovirus into mouse eye. Invest Ophthalmol Vis Sci 2001;42:3294–3300 [PMC free article] [PubMed] [Google Scholar]
- 139.Aukunuru JV, Ayalasomayajula SP, Kompella UB. Nanoparticle formulation enhances the delivery and activity of a vascular endothelial growth factor antisense oligonucleotide in human retinal pigment epithelial cells. J Pharm Pharmacol 2003;55:1199–1206 [DOI] [PubMed] [Google Scholar]
- 140.Philp NJ, Wang D, Yoon H, et al. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 2003;44:1716–1721 [DOI] [PubMed] [Google Scholar]
- 141.Bailey TA, Kanuga N, Romero IA, et al. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2004;45:675–684 [DOI] [PubMed] [Google Scholar]
- 142.Kaida M, Cao F, Skumatz CM, et al. Time at confluence for human RPE cells: effects on the adherens junction and in vitro wound closure. Invest Ophthalmol Vis Sci 2000;41:3215–3224 [PubMed] [Google Scholar]
- 143.Engelmann K, Valtink M. RPE cell cultivation. Graefes Arch Clin Exp Ophthalmol 2004;242:65–67 [DOI] [PubMed] [Google Scholar]
- 144.Klaassen I, Van Noorden CJ, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res 2013;34:19–48 [DOI] [PubMed] [Google Scholar]
- 145.Gillies MC, Su T, Naidoo D. Electrical resistance and macromolecular permeability of retinal capillary endothelial cells in vitro. Curr Eye Res 1995;14:435–442 [DOI] [PubMed] [Google Scholar]
- 146.Hosoya K-I, Tomi M, Ohtsuki S, et al. Conditionally immortalized retinal capillary endothelial cell lines (TR-iBRB) expressing differentiated endothelial cell functions derived from a transgenic rat. Exp Eye Res 2001;72:163–172 [DOI] [PubMed] [Google Scholar]
- 147.Gillies MC, Su T, Stayt J, et al. Effect of high glucose on permeability of retinal capillary endothelium in vitro. Invest Ophthalmol Vis Sci 1997;38:635–642 [PubMed] [Google Scholar]
- 148.Tretiach M, Van Driel D, Gillies MC. Transendothelial electrical resistanceof bovine retinal capillary endothelial cells is influenced by cell growth patterns: an ultrastructural study. Clin Exp Ophthalmol 2003;31:348–353 [DOI] [PubMed] [Google Scholar]
- 149.Shen J, Cross ST, Tang-Liu DD, et al. Evaluation of an immortalized retinal endothelial cell line as an in vitro model for drug transport studies across the blood-retinal barrier. Pharmaceut Res 2003;20:1357–1363 [DOI] [PubMed] [Google Scholar]
- 150.Zeiss C. Review paper: animals as models of age-related macular degeneration an imperfect measure of the truth. Vet Pathol Online 2010;47:396–413 [DOI] [PubMed] [Google Scholar]
- 151.Ablonczy Z, Dahrouj M, Tang PH, et al. Human retinal pigment epithelium cells as functional models for the RPE in vivo. Invest Ophthalmol Vis Sci 2011;52:8614–8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Levkovitch-Verbin H. Animal models of optic nerve diseases. Eye (Lond 2004;18:1066–1074 [DOI] [PubMed] [Google Scholar]
- 153.Levin LA. Animal and culture models of glaucoma for studying neuroprotection. Eur J Ophthalmol. 2001;11 Suppl 2:S23–S29 [DOI] [PubMed] [Google Scholar]
- 154.Foster WJ. Vitreous Substitutes. Expert Rev Opthalmol 2008;3:211–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Edwards GK, Locke JC. The collection, storage and selection of human vitreous for use in retinal detachment surgery. Am J Ophthalmol 1960;50:108–110 [DOI] [PubMed] [Google Scholar]
- 156.Chirila TV, Tahija S, Hong Y, et al. Synthetic polymers as materials for artificial vitreous body: review and recent advances. J Biomater Appl 1994;9:121–137 [DOI] [PubMed] [Google Scholar]
- 157.Baino F. Towards an ideal biomaterial for vitreous replacement: historical overview and future trends. Acta Biomater 2011;7:921–935 [DOI] [PubMed] [Google Scholar]
- 158.Kleinberg TT, Tzekov RT, Stein L, et al. Vitreous substitutes: a comprehensive review. Surv Ophthalmol 2011;56:300–323 [DOI] [PubMed] [Google Scholar]
- 159.Maruoka S, Matsuura T, Kawasaki K, et al. Biocompatibility of polyvinylalcohol gel as a vitreous substitute. Curr Eye Res 2006;31:599–606 [DOI] [PubMed] [Google Scholar]
- 160.Aliyar HA, Foster WJ, Hamilton PD, et al. Towards the development of an artificial human vitreous. Polym Prep 2004;45:469–470 [Google Scholar]
- 161.Swindle KE, Hamilton PD, Ravi N. Advancements in the development of artificial vitreous humor utilizing polyacrylamide copolymers with disulfide crosslinkers. Polym Prep 2006;47:56–60 [Google Scholar]
- 162.Gao Q, Mou S, Ge J, et al. A new strategy to replace the natural vitreous by a novel capsular artificial vitreous body with pressure-control valve. Eye 2008;22:461–468 [DOI] [PubMed] [Google Scholar]
- 163.Liu Y, Jiang Z, Gao Q, et al. Technical standards of a foldable capsular vitreous body in terms of mechanical, optical, and biocompatible properties. Artif Organs 2010;34:836–845 [DOI] [PubMed] [Google Scholar]
- 164.Bissell M. Biology's new dimension. Nature 2003;424:870–872 [DOI] [PubMed] [Google Scholar]
- 165.Cheng K, Lai Y, Kisaalita WS. Three-dimensional polymer scaffolds for high throughput cell-based assay systems. Biomaterials 2008;29:2802–2812 [DOI] [PubMed] [Google Scholar]
- 166.Liebsch M, Grune B, Seiler A, et al. Alternatives to animal testing: current status and future perspectives. Arch Toxicol 2011;85:841–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Home office GB. Statistics of scientific procedures on living animals 2011. Available from: https://gov.uk/government/uploads/system/uploads/attachment_data/file/115853/spanimals11.pdf (accessed November24, 2015)
- 168.Dewhurst DG, Kojic ZZ. Replacing animal use in physiology and pharmacology teaching in selected universities in Eastern Europe—charting a way forward. Altern Lab Anim 2011;39:15–22 [DOI] [PubMed] [Google Scholar]
- 169.Badyal DK, Desai C. Animal use in pharmacology education and research: the changing scenario. Ind J Pharmacol 2014;46:257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hood E. Alternative test models: ocular safety assays accepted. Environ Health Perspect 2008;116:A381. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Cite this article as: Shafaie S, Hutter V, Cook MT, Brown MB, Chau DYS (2016) In vitro cell models for ophthalmic drug development applications, BioResearch Open Access 5:1, 94–108, DOI: 10.1089/biores.2016.0008.