Abstract
Background: Cervicovaginal HIV shedding is associated with increased female-to-male and mother-to-child transmission. Genital inflammation may increase shedding through cytokines/chemokines which recruit and activate HIV target cells. We evaluated whether cervical immune mediators present before seroconversion affected HIV shedding and whether mediators differed between shedders and nonshedders.
Methods: We used cervical samples from 187 African women with documented HIV seroconversion in the Hormonal Contraception and HIV study. Samples were from the two visits before seroconversion (T-2 and/or T-1), and/or at seroconversion (T0), and/or the two visits (T + 1 and/or T + 2) after seroconversion. We measured interleukin (IL)-1β, IL-1 Receptor Antagonist (IL-1RA), IL-6, IL-8, RANTES (Regulated on Activation, Normal T-Cell Expressed and Secreted), MIP-3α, vascular endothelial growth factor (VEGF), Intercellular Adhesion Molecule-1 (ICAM-1), secretory leukocyte protease inhibitor (SLPI), and BD-2 and used the Wilcoxon test and generalized linear models to evaluate the association between mediators and shedding.
Results: The only immune mediator that differed at T-1 was RANTES, which was higher among shedders (p ≤ .05). HIV seroconversion was followed by significant decreases in many mediators, but a significant increase in RANTES. The magnitude of the change was significantly different for shedders versus nonshedders with regard to RANTES (increased in both groups, significantly more so in shedders), SLPI (decreased in both groups, significantly more so in shedders), and MIP-3α (decreased in shedders and increased in nonshedders). At T0, shedders had lower levels of SLPI and MIP-3α than nonshedders.
Conclusions: In this study, a specific immune mediator profile was associated with risk of cervical HIV shedding. Higher and increasing levels of RANTES and lower and decreasing levels of SLPI and MIP-3α were associated with increased risk of HIV shedding.
Introduction
HIV replication in the female genital tract and shedding into cervicovaginal secretions increase the risk of female-to-male and vertical HIV transmission.1,2 Female genital tract viral load has been associated with plasma viral load (PVL),3 but there is evidence of HIV-1 replication in the genital tract that may be independent of PVL and reflect a milieu specific to the genital tract.4 Genital tract inflammation, whether from trauma or reproductive tract infections (RTIs), appears to increase the risk of shedding, possibly through a direct effect on viral replication or immune mediators that recruit and activate HIV target cells.5–7 Understanding the mechanisms that affect female genital tract viral load could facilitate interventions to reduce HIV shedding and transmission.
Archived endocervical swab specimens were available to us from the Hormonal Contraception and HIV (HC-HIV) study, a multicenter cohort study of over 6,000 women in Uganda, Zimbabwe and Thailand.8 Women were followed quarterly for 15–24 months or until HIV acquisition to examine the association between HC and HIV. Women who used the injectable contraceptive depo-medroxyprogesterone acetate (DMPA), but not combined oral contraceptives (COCs), were found to be at significantly increased risk of HIV acquisition compared with women not using HC.9
The availability of endocervical swabs from the Uganda and Zimbabwe sites allowed us to evaluate the following 10 immune mediators in the genital tract of HIV-uninfected women, some of whom later acquired HIV and were further sampled after seroconversion: RANTES (Regulated on Activation, Normal T-Cell Expressed and Secreted, or CCL5), secretory leukocyte protease inhibitor (SLPI), vascular endothelial growth factor (VEGF), macrophage inflammatory protein 3α (MIP-3α) intercellular adhesion molecule-1 (ICAM-1), interleukin (IL)-6, IL-8, IL-1β, IL-1 receptor antagonist (IL-1RA), and human beta defensin 2 (HBD2). Our recent cross-sectional analysis used 832 swabs from Ugandan and Zimbabwean participants collected at the visit before HIV seroconversion or at a matched time in the control subjects, and showed that higher RANTES and lower SLPI were associated with subsequent HIV acquisition in these women.10 A further secondary analysis showed that the changes in RANTES and SLPI levels, as well as some of the other cervical immune parameters, were dependent on the presence of abnormal vaginal microbiota and vaginal infections established before HC use.11 The question of whether the cervical immunity biomarkers were associated with HIV shedding remained unanswered and is addressed in this analysis.
A separate analysis of a subset of women in the HC-HIV study who seroconverted (the Hormonal Contraception and HIV Genital Shedding and Disease Progression among Women with Primary HIV Infection Study, or GS study) yielded a viral set point in the genital compartment that was reached later than the plasma set point, but was highly correlated with it.9 Subtype C infection, nonviral sexually transmitted infections (STIs), having a partner who spent nights away from home, and recent unprotected sex were associated with higher cervical viral loads, while use of DMPA and COCs was not. The goal of the analysis reported in this study was to assess whether the 10 selected cervical immune mediators listed above and other factors present before or after HIV acquisition were associated with HIV genital shedding.
Materials and Methods
Collection of swabs
We analyzed 443 endocervical swabs from 187 women in the GS study who underwent measurement of the 10 immune mediators listed above at the visit when seroconversion was first documented (T0) and/or at least one visit from before (T-2 and/or T-1) and/or after (T + 1 or T + 2), and measurement of cervical HIV-1 RNA (with detectable or undetectable results) during at least one of the following visits: T0, T + 1, and/or T + 2. The methods for measuring cervical viral load9 and immune mediators10 are described in detail elsewhere and the latter is described briefly below. The lower limit of detectability for cervical and PVL was 50 HIV-1 RNA copies/ml.
Cervical mucus was wiped with a cleaning swab provided in the Roche kit before endocervical swabbing for biomarkers. Swabs were extracted in 1 ml Amplicor lysis medium supplemented with proteinase K to degrade residual mucus and after removal of the swab, each extraction was mixed with 1 ml diluent (all provided by Roche Diagnostics, Indianapolis, IN) and processed for STI diagnosis as per the manufacturer's instructions. Leftovers of the extracted solutions were stored at −80°C until analyzed for immune biomarkers at the Laboratory of Genital Tract Biology, Brigham and Women's Hospital.
Measurement of immune biomarkers
IL-1β, IL-1RA, IL-6, IL-8, RANTES, MIP-3α, VEGF, and soluble ICAM-1 were measured using a custom-designed Meso Scale Discovery multiplex and Sector Imager 2400 (Meso Scale Discovery, Gaithersburg, MD). The multiplex was optimized to detect each biomarker within the linearity range of the cervical swab specimens. SLPI and BD-2 were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN and Phoenix Pharmaceuticals, Burlingame, CA, respectively) and total protein by bicinchoninic acid assays were read using Victor2 (Perkin Elmer, Boston, MA). The lower limit of detection of each assay and sample dilutions are reported elsewhere.10 A quality control sample was split and tested on each assay plate showing interplate variation of <25%. Spiking experiments showed no significant interference of the swab extraction buffer and diluent with the immunoassay detection as described.
Lysing the cervical sample in Amplicor buffer precluded removal of cells by centrifugation. Furthermore, the noninvasive swab collection technique with prior removal of mucus and loose cellular material minimized the contribution of nonsoluble cellular material to the swab elutions, and the impact of any potential random variation in cell numbers was diminished by the large sample size of the study.
Statistical analyses
We evaluated whether demographic characteristics were distributed similarly between countries using Cochran-Mantel-Haenszel or Fisher's exact tests. Factors associated with cervical HIV-1 RNA were evaluated using bivariate (unadjusted analysis) and multivariable (adjusted analysis) models (Table 3). In the adjusted analysis, the estimated odds ratio (OR) for each specific covariate (e.g., age <25 at seroconversion) was obtained in a multivariable model which adjusted for all other variates listed in the first column of Table 3 (e.g., country/subtype, time-varying contraceptive use, current pregnancy, etc.).
Table 3.
Factors Associated with Detectable HIV-1 Endocervical Viral Load for HIV+ Women (Restricted to Women with Cervical HIV-1 RNA)
| Unadjusted analysis | Adjusted analysisa | |||
|---|---|---|---|---|
| Variable | OR (95% CI) | p | OR (95% CI) | p |
| Time-invariant variables | ||||
| Age <25 at seroconversion | 0.94 (0.60–1.49) | .799 | 0.76 (0.44–1.29) | .308 |
| Country/HIV-1 subtype | ||||
| Uganda | ||||
| Subtype A | Reference | Reference | ||
| Subtype C | 1.33 (0.08–22.48) | .842 | 2.16 (0.11–44.29) | .618 |
| Subtype D | 1.53 (0.63–3.71) | .343 | 1.51 (0.60–3.83) | .383 |
| Zimbabwe | ||||
| Subtype C | 1.60 (0.87–2.93) | .132 | 1.56 (0.80–3.05) | .196 |
| Time-varying contraceptive use | ||||
| Majority HC use | ||||
| COC | 1.16 (0.73–1.83) | .529 | 0.78 (0.44–1.37) | .385 |
| DMPA | 1.27 (0.77–2.10) | .351 | 1.15 (0.65–2.03) | .628 |
| NH | Reference | Reference | ||
| Reproductive health and STI history | ||||
| Current pregnancy | 0.58 (0.16–2.08) | .405 | 0.43 (0.11–1.71) | .228 |
| Current breastfeeding | 2.09 (1.12–3.89) | .020 | 2.69 (1.33–5.42) | .006 |
| STI symptom | 1.35 (0.87–2.11) | .179 | 1.34 (0.80–2.25) | .265 |
| Clinical/laboratory data | ||||
| Gonorrhea | 2.38 (1.01–5.61) | .047 | 1.57 (0.56–4.43) | .392 |
| Chlamydia | 2.67 (1.09–6.57) | .032 | 2.14 (0.72–6.38) | .171 |
| Trichomoniasis (by wet prep) | 5.24 (2.17–12.64) | <.001 | 8.17 (2.6–25.55) | <.001 |
| Candida (by wet prep) | 1.53 (0.91–2.58) | .109 | 1.57 (0.90–2.75) | .115 |
| HSV-2 | 0.84 (0.43–1.64) | .606 | 0.69 (0.35–1.36) | .280 |
| BV (by Amsel's criteria) | 1.27 (0.83–1.95) | .265 | 1.10 (0.65–1.85) | .716 |
| GUD | 0.94 (0.29–3.09) | .922 | 0.54 (0.11–2.67) | .450 |
| Sexual risk behavior | ||||
| Participant behavioral risk | 0.75 (0.30–1.89) | .545 | 0.75 (0.29–1.98) | .568 |
| Coital frequency per month | ||||
| 0–14 | Reference | Reference | ||
| 15–29 | 1.40 (0.87–2.27) | .168 | 1.52 (0.85–2.70) | .157 |
| 30+ | 2.63 (0.97–7.07) | .056 | 2.45 (1.04–5.80) | .041 |
| Unprotected sex act in last 3 days | 1.30 (0.88–1.93) | .182 | 1.27 (0.77–2.10) | .358 |
| Partner had nights away from home | 1.15 (0.77–1.71) | .499 | 1.21 (0.76–1.92) | .420 |
Cervical samples from 187 African women with documented HIV seroconversion in the HC-HIV study were used. Samples were from at T0 and 2 visits (T + 1 and T + 2) after seroconversion.
The adjusted analysis includes all covariates listed in the first column of the table in a multivariable model. The estimated OR for each specific covariate (e.g., age <25 at seroconversion) was obtained from the model that adjusted for the other covariates.
Bold represents the values that were significant at p ≤ 0.05.
GUD, genital ulcer disease; OR, odds ratio.
Immune mediator concentrations were first standardized by total protein levels such that standardized mediator levels among specimens were comparable (Table 4). Box-Cox power transformation was used to transform these standardized mediator levels to transformed standardized mediator measures that were more normally distributed, allowing application of parametric statistical approaches.12 The mean change in each mediator level was then calculated for women with detectable cervical HIV-1 RNA and for those without, as well as the difference in mean change between the two groups. These values were then transformed back to their standardized values, expressed as pg per mg total protein (Table 4). Boxplots were used to describe medians and interquartile ranges of transformed mediator levels and compare them between detectable and undetectable cervical HIV-1 RNA results using the Wilcoxon–Mann–Whitney test by each visit (Fig. 1). We used generalized linear models to evaluate the impact of immune mediator levels and other covariates on HIV genital shedding at each visit and across visits. p-Values <.05 were considered statistically significant. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
Table 4.
Average Change of Immune Mediators from the Visit Before Seroconversion (T1) to the Seroconversion Visit (T0) Among Women with Detectable and Undetectable Cervical HIV-1 RNA
| Average change in women with detectable cervical HIV-1 RNA (N = 102) | Average change in women without detectable cervical HIV-1 RNA (N = 55) | Difference in average change between women with detectable cervical HIV-1 RNA vs. undetectable from T-1 to T0 (N = 157) | ||||
|---|---|---|---|---|---|---|
| Immune mediators | T-1 minus T0 | p | T-1 minus T0 | p | Detectable–undetectable | p |
| RANTES | 45.9 | <.001 | 27.8 | .001 | 17.0 | .018 |
| SLPI | −51,626.8 | .001 | −25,647.9 | .268 | −62,761.1 | .009 |
| MIP-3alpha | −46.6 | .136 | 19.5 | .654 | −96.7 | .009 |
| IL-1RA | −127.0 | .012 | −79.8 | .248 | −86.5 | .095 |
| IL-6 | −2.1 | .219 | −4.6 | .050 | −0.7 | .712 |
| IL-8 | −310.6 | <.001 | −306.4 | .002 | −36.5 | .737 |
| VEGF | −262.4 | .004 | −194.8 | .013 | −40.4 | .675 |
| BD-2 | −525.0 | .172 | −895.1 | .041 | 51.2 | .913 |
| IL-1β | −1.1 | .063 | −0.6 | .385 | −0.3 | .582 |
| Ratio (IL-1RA:IL-1β) | 24.9 | .175 | 19.1 | .470 | −3.9 | .786 |
| ICAM-1 | 78.3 | .125 | 95.6 | .132 | 5.3 | .912 |
Changes in mediator levels are shown measured in pg/mg total protein levels.12
Cervical samples from 157 African women with documented HIV seroconversion in the HC-HIV study were used. Samples were from at T0 and/or two visits (T + 1 and T + 2) after seroconversion. Immune mediator concentrations were first standardized by total protein levels such that standardized mediator levels among specimens were comparable. Box–Cox power transformation was used to transform these standardized mediator levels to transformed standardized mediator measures that were more normally distributed, allowing application of parametric statistical approaches. The mean change in each mediator level was then calculated for women with detectable cervical HIV-1 RNA and for those without, as well as the difference in mean change between the two groups. These values were then transformed back to their standardized values, expressed as pg per mg total protein (Table 4).
Bold represents the values that were significant at p ≤ 0.05.
ICAM-1, Intercellular Adhesion Molecule-1; IL, interleukin; IL-1RA, IL-1 Receptor Antagonist; RANTES, Regulated on Activation, Normal T-Cell Expressed and Secreted; SLPI, secretory leukocyte protease inhibitor; VEGF, vascular endothelial growth factor.
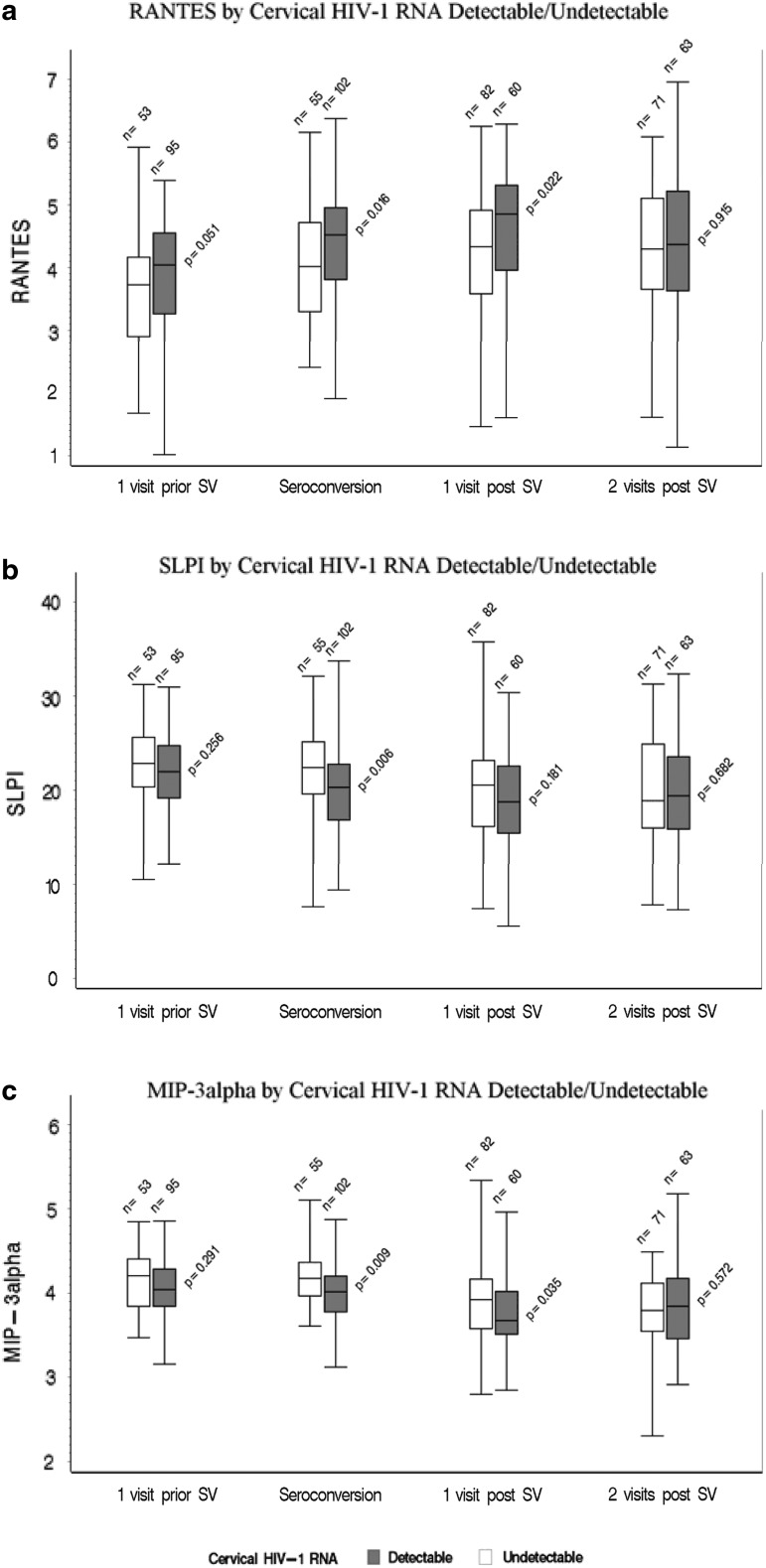
FIG. 1.
Immune mediators in cervical samples from 157 African women with documented HIV seroconversion in the Hormonal Contraception-HIV study. (HC-HIV) Study (a: RANTES; b: SLPI; c: MIP-3α). Samples were from the visit before the seroconversion visit (SV), at the SV, and two visits post SV. Results are shown for women with detectable versus undetectable cervical HIV-1 RNA. The values on the Y-axes are the power-transformed values of pg of immune mediators per mg total protein (see Statistical Analysis section). The only mediator that differed at one visit before SV between women who did and did not shed at SV was RANTES (Regulated on Activation, Normal T-Cell Expressed and Secreted) and the difference was of borderline significance (p = .051). This difference became significant at the SV and persisted at one visit after SV, but not two visits after SV. RANTES was the only marker that was significantly higher in shedders than nonshedders at any visit. At the SV, shedders had lower levels of secretory leukocyte protease inhibitor (SLPI) and MIP-3α than nonshedders, although at one visit before SV, shedders and nonshedders had similar levels.
Results
Demographics
About two-thirds of women used HC, about 40% had bacterial vaginosis, and most (87%) had herpes simplex virus type 2 (HSV-2) (Table 1). None were on antiretroviral therapy. All women infected with subtypes A and D were in Uganda and all, but one woman, with Subtype C were in Zimbabwe (p < .001). The most common Nugent score among Zimbabwean women indicated BV, while the most common score in Uganda indicated normal flora (p = .028). A significantly smaller proportion of Ugandan women had candidiasis (p = .003). Otherwise the populations by country were similar.
Table 1.
Participant Characteristics at HIV Seroconversion Visit for Women with Cervical HIV-1 RNA and Cytokine Data
| Characteristic | Uganda (n = 57), n (%) | Zimbabwe (n = 130), n (%) | Total (n = 187), n (%) | p |
|---|---|---|---|---|
| Age at screening | ||||
| 18–24 | 36 (63.16) | 67 (51.54) | 103 (55.08) | .143 |
| 25+ | 21 (36.84) | 63 (48.46) | 84 (44.92) | |
| HIV-1 subtype | ||||
| A | 37 (64.91) | 0 (0) | 29 (19.79) | <.001 |
| C | 1 (1.75) | 130 (100) | 114 (70.05) | |
| D | 19 (33.33) | 0 (0) | 14 (10.16) | |
| HC group | ||||
| COC | 16 (28.07) | 38 (29.23) | 54 (28.88) | .547 |
| DMPA | 19 (33.33) | 52 (40) | 71 (37.97) | |
| NH | 22 (38.6) | 40 (30.77) | 62 (33.16) | |
| Currently pregnant | 2 (3.51) | 2 (1.54) | 4 (2.14) | .143 |
| Currently breastfeeding | 6 (10.53) | 22 (16.92) | 28 (14.97) | .260 |
| 2+ Sexual partners | 4 (7.02) | 2 (1.54) | 6 (3.21) | .071 |
| No. of unprotected acts per month | ||||
| 15+ | 4 (7.02) | 26 (20) | 30 (16.04) | .206 |
| 8–14 | 22 (38.60) | 21 (16.15) | 43 (22.99) | |
| 1–7 | 19 (33.33) | 31 (23.85) | 50 (26.74) | |
| 0 or no sex acts | 12 (21.05) | 52 (40) | 64 (34.22) | |
| Current BV | ||||
| Nugent score 7–10 | 13 (28.89) | 47 (43.12) | 60 (38.96) | .028 |
| Nugent score 4–6 | 10 (22.22) | 33 (30.28) | 43 (27.92) | |
| Nugent score 0–3 | 22 (48.89) | 29 (26.61) | 51 (33.12) | |
| Current candidiasis | 1 (1.75) | 22 (17.05) | 23 (14.01) | .003 |
| Current chlamydia infection | 1 (3.51) | 12 (9.30) | 14 (7.53) | .233 |
| Current gonorrhea infection | 4 (7.02) | 17 (13.18) | 21 (11.29) | .316 |
| HSV-2 infection | 46 (83.64) | 114 (88.37) | 160 (86.96) | .383 |
| Current trichomonas | 4 (7.02) | 13 (10.08) | 17 (9.14) | .592 |
| Any RTI/STI | 51 (96.23) | 123 (98.40) | 174 (97.75) | .584 |
| STIs signs | 5 (8.77) | 15 (11.54) | 20 (10.7) | .574 |
| STIs symptoms | 23 (40.35) | 35 (26.92) | 58 (31.02) | .068 |
Cervical samples from 187 African women with documented HIV seroconversion in the HC-HIV study were used.
Bold represents the values that were significant at p ≤ 0.05.
BV, bacterial vaginosis; COC, combined oral contraceptives; DMPA, depo-medroxyprogesterone acetate; HC, hormonal contraception; HSV-2, herpes simplex virus type 2; NH, non-hormonal; RTI, reproductive tract infection; STI, sexually transmitted infections.
HIV-1 RNA in cervical swabs
Among these 187 women, 138 (74%) had detectable cervical HIV-1 RNA at least once. These 187 women provided 433 specimens for cervical HIV-1 RNA measurements. Of those, 52% (225/433) had detectable cervical HIV-1 RNA (Table 2). The mean (SD) cervical HIV-1 RNA across specimens with detectable cervical HIV-1 RNA was 2.79 (0.79) log10 HIV-1 copies/swab. The cervical viral set point was 2.64 log10 HIV-1 RNA copies/swab at 140 days from the estimated infection date (T0). Eighty-seven women had specimens for all three visits (T0, T1, and T2); of these, 22 showed continuous shedding through all three visits. A woman with detectable cervical HIV-1 RNA at T0 was 2.2 times more likely to have detectable cervical HIV-1 RNA at a subsequent visit compared with a woman without detectable cervical HIV-1 RNA at T0 (p = .011). A significant direct correlation between cervical and plasma HIV-1 RNA levels was seen during early infection (Spearman's r = 0.47 p < .001).9
Table 2.
Cervical HIV-1 RNA by Visit
| T0 | T + 1 | T + 2 | Total | |
|---|---|---|---|---|
| No. of specimens with detectable cervical HIV-1 RNA/No. of specimens tested (%) | 102/157 (65.0) | 60/142 (42.3) | 63/134 (47) | 225/433 (52) |
| Mean (SD) log10 cervical HIV-1 RNA | 2.94 (0.87) | 2.63 (0.67) | 2.70 (0.73) | 2.79 (0.79) |
Cervical samples from 187 African women with documented HIV seroconversion in the HC-HIV study were used.
Demographics, contraception, sexual behavior, and STI/RTIs associated with shedding
Breastfeeding, having a coital frequency of >30 acts in a typical month, and trichomoniasis were each associated with at least a 2.5-fold greater risk of shedding in the adjusted analysis (Table 3). There was a significant negative association (p = .001) between breastfeeding and number of sexual acts (data not shown). Infection with chlamydia or gonorrhea was associated with about a 2.5-fold increased risk of shedding in the unadjusted analysis (Table 3). No association was seen between shedding and candidiasis, HSV-2, BV, age, country, HC, or pregnancy.
Immune mediators before HIV seroconversion
We assessed immune mediators before HIV seroconversion (at T-1) to determine whether, among these women who acquired HIV, there was a difference in immune mediators between women who went on to shed and those who did not. Such a difference might indicate a preseroconversion condition that facilitates shedding post seroconversion. From the group of mediators we evaluated, the only one that differed at T-1 between women who did and did not shed at T0 was RANTES and the difference was of borderline significance (p = .051, Fig. 1a).
Immune mediators after HIV seroconversion
HIV seroconversion was followed by significant changes in many of the mediators (comparing T0 with T-1) (Table 4). Except for RANTES, all significant changes were decreases. The magnitude of the change was significantly different for shedders versus nonshedders with regard to RANTES (increased in both groups, but significantly more so in shedders, p = .018), SLPI (decreased in both groups, but significantly more so in shedders, p = .009), and MIP-3α (decreased in shedders and increased in non-shedders with a significant difference between groups, p = .009) (Table 4 and Fig. 1). The magnitude of the change in other markers did not differ significantly between shedders and nonshedders and was in the same direction for both groups.
As stated above, before infection (T-1), women who subsequently shed at T0 had higher levels of RANTES than women who did not go on to shed. This difference became significant at T-0 and persisted at T + 1 (p = .022), but not T + 2 (Fig. 1a). RANTES was the only marker that was significantly higher in shedders than nonshedders at any visit.
At T0, shedders had lower levels of SLPI and MIP-3α than nonshedders, although before infection (at T-1), shedders and non-shedders had similar levels (Fig. 1b, c).
Being in the upper quartile for RANTES or lower quartile for MIP-3α or SLPI between T0 and T + 2 was associated with a significantly increased risk of shedding during the same visit (OR, 95% CI = 1.71, 1.17–2.50, p = .005, data not shown).
About 3 months after seroconversion (at T + 1), shedders had significantly lower levels of IL-1RA, IL-1β, and VEGF than nonshedders (p < .05, data not shown). There were no significant differences in BD-2, IL-6, IL-8, ICAM-1, or the ratio IL-1RA:IL-1β between shedders and nonshedders at this visit.
Discussion
We evaluated cell-free cervical HIV-1 RNA from endocervical swabs in a cohort of highly active antiretroviral therapy naive African women. As expected, our demographics were similar to those of the GS analysis.9 In both studies, shedding was associated with PVL and nonviral RTIs, and not with HC. Unlike the GS analysis, we found no correlation between shedding and subtype C infection, having a partner who spent nights away from home, or recent unprotected sex, possibly from the use of continuous cervical HIV-1 RNA measures in the GS analysis rather than the use of a binary categorization (shedding or not) used in the current analysis.
We did see a correlation between shedding and both breastfeeding and coital frequency. The hypoestrogenism that occurs during breastfeeding may make the vaginal epithelium more susceptible to trauma, inflammation, and HIV replication. Endocervical HIV shedding among cycling women is higher in the late luteal phase, when the ratio estradiol/progesterone is relatively low.13 It is also possible that, among women with high coital frequency, some of the cervical HIV-1 RNA detected was of male partner origin, but this is unlikely given the lack of association between cervical HIV-1 RNA and unprotected sex in the last 3 days. In contrast, it is more likely that the semen exposure associated with high coital frequency modulates the cervicovaginal environment in ways that facilitate recruitment and activation of HIV target cells, resulting in increased viral replication.14
In our earlier cross-sectional analysis, higher levels of RANTES were associated with subsequent HIV seroconversion.10 In this study, higher levels of RANTES before seroconversion were also associated with HIV shedding after seroconversion. Importantly, the difference in RANTES between shedders and nonshedders was apparent starting ∼3 months before seroconversion. RANTES was also the only marker that was significantly higher after seroconversion; levels of it increased after seroconversion in both shedders and nonshedders, but the increase was greater among shedders.
RANTES competes with HIV for binding to CCR5 on CD4+ host cells, and it blocks SHIV transmission in rhesus macaques.15 However, it also attracts CD4+ target T cells, which could increase the likelihood of initial HIV infection and viral shedding postinfection; our results are consistent with the latter. An association between cervical RANTES and HIV shedding has been seen in other studies.16–18 The presence of higher levels of RANTES in women who become HIV infected and those who go on to shed, demonstrated in our studies, suggests that elevations in RANTES precede and may be causally related to increased HIV replication. Consistent with this notion is a study in which RANTES levels in the cervico-vaginal lavage (CVL) of highly exposed, but seronegative, women were lower than in HIV-negative (but not highly exposed) and HIV-positive women.19
In our earlier cross-sectional analysis, lower levels of SLPI were associated with seroconversion.10 In this study, we found that lower levels of SLPI before seroconversion were also associated with HIV shedding after seroconversion. SLPI levels decreased after seroconversion, but the decrease was greater among shedders.
SLPI is a broad-spectrum antimicrobial peptide, which inhibits bacteria, fungi, and viruses, including HIV. It also exerts anti-inflammatory effects by inhibiting endogenous proteases and reducing secretion of proinflammatory mediators, the latter by inhibiting NF-κβ, which in turn inhibits HIV replication.7,20 SLPI may also block HIV-1 infection of monocytes and T cells by preventing internalization of the virus.20 A reduction in any of these activities would increase the likelihood of initial infection and viral replication. It has been suggested that an HIV-mediated increase in TGF-β1 secretion and the associated downregulation of SLPI can result in increased HIV-1 shedding.21
In a study among 36 HIV-infected women, cervical levels of SLPI were significantly associated with low cervical viral loads.21 No difference in SLPI was seen between shedders and nonshedders in two other studies, however.4,17 In one of them, the women had low CD4 cell counts and low anti-HIV activity in the CVL despite the presence of antimicrobials, possibly due to disease progression, unlike women in our study who were acutely infected.22
MIP-3α levels were not associated with seroconversion in our earlier cross-sectional analysis. In this study, MIP-3α decreased after seroconversion in shedders and increased in nonshedders, with a significant difference in the magnitude of change between groups. MIP-3α has an antiviral activity and inhibits HIV-1, most likely by direct interaction with the virus rather than through blocking of receptors.22 In one study of 32 HIV-positive women, infectious virus was found in 3 of 32 CVL specimens, and MIP-3α levels in these women were lower than average.23
The presence of RTIs has been associated with HIV shedding. A 2008 meta-analysis showed an association between HIV shedding and gonorrhea, chlamydia, and candidiasis,24 and such an association has been seen with trichomoniasis.25,26 Our study showed similar results with the exception of no association between HIV-1 shedding and candidiasis. The presence of BV and/or intermediate Nugent scores has been associated with shedding in some studies, but not in ours, possibly due to differences in diagnostic methods, the microbiome, and host factors.27 We did not see an association between shedding and HSV-2 most likely because almost all participants (87%) were HSV-2 positive. The presence of genital infection with Mycoplasma genitalium has been associated with cervical HIV-1 RNA shedding in a subset of women in the GS study28; we plan to evaluate the association of both factors with immune mediators.
Most other studies have found associations between shedding and proinflammatory mediators, which are known to stimulate viral replication.4,16,17,21 We found that IL-1β, which did not change significantly after seroconversion (T0) in either group, ended up significantly higher in women without detectable cervical HIV-1 RNA at T + 1. This differs from findings by others using CVLs,4,16 but it appears to have been balanced by an increase in IL-1RA such that the ratio of IL-1RA:IL-1β was not different between groups at T + 1.6 We did not see a difference between shedders and nonshedders in IL-6 or IL-8, two markers that are typically increased in the presence of inflammation,29 suggesting that the higher levels of RANTES observed in the shedders were not simply a result of inflammatory activation.
Our analysis was limited by the type of archived specimens that were available to us, namely cervical swab elutions stored frozen in the lysis medium. A limitation to the study was the fact that we could not perform assays for prostate-specific antigen on the elutions to determine objectively whether some of the cervical HIV-1 RNA detected was of male partner origin. In addition, we were unable to evaluate the cellular immune response, which may have provided information on the relative effects of chemoattraction of target cells versus receptor blockade by RANTES. Recent work has shown a decrease in the ratio of CD4:CD8 cells in early HIV infection, attributed mostly to an influx of CD8 cells, in conjunction with increased levels of cervicovaginal RANTES.30 We also could not evaluate anti-HIV activity, infectious virus, or anti-HIV antibodies in the cervicovaginal secretions. We also did not look at systemic levels of biomarkers, but recently received further funding to do so and correlate those levels with cervical biomarkers and other parameters.
In conclusion, increased RANTES, not accompanied by increases in other classical proinflammatory mediators such as IL-1 and IL-8, was associated with an increased risk of subsequent HIV seroconversion (in our earlier cross-sectional analysis) and cervical shedding (this analysis). The concordance of these two findings reinforces the biological significance of this association. Importantly, increased RANTES preceded both seroconversion and shedding. Similarly, a decrease in SLPI, an antimicrobial factor frequently found in the cervicovaginal secretions, was associated with subsequent seroconversion and shedding. MIP-3α was decreased in shedders and increased in nonshedders. Continued efforts to understand the biological role and determinants of cervical immune mediators may facilitate the development of measures that protect against HIV acquisition and transmission.
Acknowledgments
This study was funded with U.S. federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health through 1RO1HD077888-01, and through an Interagency Agreement with the United States Agency for International Development (USAID) (GHO A 00 09 00016-00); and from funds from USAID provided to CONRAD (GPO-A-00-08-00005-00) and Brigham and Women's Hospital by subagreement PPC-11-127. The authors thank the following members of the Fichorova Laboratory, who contributed to this study by processing the cervical samples and performing all protein assays (listed in alphabetical order): Bi Yu Li, Bisiayo Fashemi, Hassan Dawood, Hidemi Yamamoto, Huaiping Yuan, Noah Beatty, Olimpia Suciu, Raymond Wong, Ryan Murray, Tai Nguyen, Xenia Chepa-Lotrea, Yoshika Yamamoto, and Yujin Lee.
Presented as a poster at HIV Research for Prevention 2014: AIDS Vaccine, Microbicide and ARV-Based Prevention Science (HIV R4P) in Cape Town, South Africa, October 28–31.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Baeten JM, Kahle E, Lingappa JR, et al. : Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 2011;3:77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mofenson LM, Lambert JS, Stiehm ER, et al. : Risk factors for perinatal transmission of human immunodeficiency virus type 1 in women treated with zidovudine. N Engl J Med 1999;341:385–393 [DOI] [PubMed] [Google Scholar]
- 3.Bere A, Denny L, Naicker P, et al. : HIV-specific T-cell responses detected in the genital tract of chronically HIV-infected women are largely monofunctional. Immunology 2013;139:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell C, Hitti J, Paul K, et al. : Cervicovaginal shedding of HIV type 1 is related to genital tract inflammation independent of changes in vaginal microbiota. AIDS Res Hum Retroviruses 2011;27:35–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fichorova RN: Guiding the vaginal microbicide trials with biomarkers of inflammation. J Acquir Immune Defic Syndr 2004;37(Suppl. 3):S184–S193 [PMC free article] [PubMed] [Google Scholar]
- 6.Fichorova RN, Tucker LD, Anderson DJ: The molecular basis of nonoxynol-9-induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis 2001;184:418–428 [DOI] [PubMed] [Google Scholar]
- 7.Ghosh M: Secreted mucosal antimicrobials in the female reproductive tract that are important to consider for HIV prevention. Am J Reprod Immunol 2014;71:575–588 [DOI] [PubMed] [Google Scholar]
- 8.Morrison CS, Richardson BA, Mmiro F, et al. : Hormonal contraception and the risk of HIV acquisition. AIDS 2007;21:85–95 [DOI] [PubMed] [Google Scholar]
- 9.Morrison CS, Demers K, Kwok C, et al. : Plasma and cervical viral loads among Ugandan and Zimbabwean women during acute and early HIV-1 infection. AIDS 2010;24:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison CS, Fichorova RN, Mauck C, et al. : Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014;66:109–117 [DOI] [PubMed] [Google Scholar]
- 11.Fichorova RN, Chen PL, Morrison CS, et al. : The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio 2015;6:e00221-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Box GEP, Cox DR: An analysis of transformation. J R Stat Soc Ser B Methodol 1964;26:211–246 [Google Scholar]
- 13.Reichelderfer PS, Coombs RW, Wright DJ, et al. : Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. AIDS 2000;14:2101–2107 [DOI] [PubMed] [Google Scholar]
- 14.Doncel GF, Anderson S, Zalenskaya I: Role of semen in modulating the female genital tract microenvironment—Implications for HIV transmission. Am J Reprod Immunol 2014;71:564–574 [DOI] [PubMed] [Google Scholar]
- 15.Lederman MM, Veazey RS, Offord R, et al. : Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science 2004;306:485–487 [DOI] [PubMed] [Google Scholar]
- 16.Herold BC, Keller MJ, Shi Q, et al. : Plasma and mucosal HIV viral loads are associated with genital tract inflammation in HIV-infected women. J Acquir Immune Defic Syndr 2013;63:485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukura LR, Ghosh M, Fahey JV, et al. : Genital tract viral load in HIV type 1-positive women correlates with specific cytokine levels in cervical-vaginal secretions but is not a determinant of infectious virus or anti-HIV activity. AIDS Res Hum Retroviruses 2012;28:1533–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iversen AK, Fugger L, Eugen-Olsen J, et al. : Cervical human immunodeficiency virus type 1 shedding is associated with genital beta-chemokine secretion. J Infect Dis 1998;178:1334–1342 [DOI] [PubMed] [Google Scholar]
- 19.Yao XD, Omange RW, Henrick BM, et al. : Acting locally: Innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol 2014;7:268–279 [DOI] [PubMed] [Google Scholar]
- 20.Aboud L, Ball TB, Tjernlund A, Burgener A: The role of serpin and cystatin antiproteases in mucosal innate immunity and their defense against HIV. Am J Reprod Immunol 2014;71:12–23 [DOI] [PubMed] [Google Scholar]
- 21.Thakar M, Patil R, Shukre S, et al. : Short communication: Genital tumor growth factor-β1 levels in HIV-infected Indian women are associated with reduced levels of innate antimicrobial products and increased HIV shedding. AIDS Res Hum Retroviruses 2014;30:648–653 [DOI] [PubMed] [Google Scholar]
- 22.Ghosh M, Shen Z, Schaefer TM, et al. : CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol 2009;62:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghosh M, Fahey JV, Shen Z, et al. : Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One 2010;5:e11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson LF, Lewis DA: The effect of genital tract infections on HIV-1 shedding in the genital tract: A systematic review and meta-analysis. Sex Transm Dis 2008;35:946–959 [DOI] [PubMed] [Google Scholar]
- 25.Tanton C, Weiss HA, Le Goff J, et al. : Correlates of HIV-1 genital shedding in Tanzanian women. PLoS One 2011;6:e17480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kissinger P, Amedee A, Clark RA, et al. : Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis 2009;36:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitchell C, Marrazzo J: Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol 2014;71:555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napierala Mavedzenge S, Müller EE, Lewis DA, et al. : Mycoplasma genitalium is associated with increased genital HIV type 1 RNA in Zimbabwean women. J Infect Dis 2015;211:1388–1398 [DOI] [PubMed] [Google Scholar]
- 29.Mauck CK, Lai JJ, Weiner DH, et al. : Toward early safety alert endpoints: Exploring biomarkers suggestive of microbicide failure. AIDS Res Hum Retroviruses 2013;29:1475–1486 [DOI] [PubMed] [Google Scholar]
- 30.McKinnon LR, Nyanga B, Kim CJ, et al. : Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J Acquir Immune Defic Syndr 2015;68:6–12 [DOI] [PubMed] [Google Scholar]