Abstract
RATIONALE
Delay discounting is a behavioral economic index of impulsivity that reflects a person’s relative preference for small immediate rewards versus larger delayed rewards. Elevated delay discounting is robustly linked to addictive disorders and has been increasingly investigated as a viable endophenotype for genetic influences on addiction.
OBJECTIVE
To examine associations between delay discounting and two a priori loci, rs4680 in COMT and rs1800497 in ANKK1, and three exploratory haplotypes proximal to rs1800497 in a sample of daily smokers.
METHODS
Participants were 713 (60.2% male) daily smokers of European ancestry who completed a delay discounting assessment and provided a DNA sample.
RESULTS
Significant associations were detected between greater discounting of medium magnitude rewards (~$55) and the G allele of rs4680 as well as the T allele of rs1800497. Exploratory haplotype analyses identified two haplotypes (rs1160467/rs1800497; rs6277/rs1079597) significantly associated with delay discounting rates. However, the rs1160467/rs1800497 haplotype associations appeared to be entirely attributable to variation in rs1800497, suggesting that the association of rs1800497 with discounting is best understood at the individual SNP level. Similarly, the rs6277/rs1079597 haplotype findings suggested the association was specific to rs1079597.
CONCLUSIONS
This study provides further evidence that rs4680 and rs1800497 genotype are significantly associated with delay discounting preferences and does so among smokers for the first time. The study also provides evidence of specificity for the rs1800497 association, and identifies a novel locus, rs1079597, as a genetic contributor to higher delay discounting rates.
Keywords: Delay Discounting, Endophenotype, Genetics, Smoking, Nicotine Dependence
INTRODUCTION
The heritability of addictive disorders is estimated to be 50–60% (Goldman et al. 2005), but the results of molecular genetic studies to date have fallen short of expectations in providing an explanation for this magnitude of genetic influences. Early candidate gene association studies have been inconsistently replicated and more recent genome-wide association studies have largely identified only very small associations (Treutlein and Rietschel 2011). Given these challenges, an endophenotype approach has been proposed to shift focus to more tractable phenotypes (Gottesman and Gould 2003; Flint and Munafò 2007). Compared to diagnostic phenotypes that are inherently heterogeneous, endophenotypes are more narrow characteristics that are mechanistically related to a given disorder and theoretically more closely linked to genetic variation. Thus, this strategy seeks to “connect the dots” between genome and syndrome by identifying genetic influences on characteristics that are linked with a disorder. Consistent with this perspective, a recent meta-analysis found that trait, neuropsychological, and neurobiological measures of impulsivity had stronger genetic effects than a diagnostic phenotype (i.e., ADHD symptoms; Jonas and Markon 2014). One promising endophenotype for addictive disorders is delay discounting (Mitchell 2011; MacKillop 2013). Delay discounting is a behavioral economic measure of impulsivity that assesses a person’s preferences for smaller immediate rewards compared to larger delayed rewards. Elevated delay discounting rates (i.e., high preference for smaller-sooner rewards over larger-later rewards) have been robustly associated with addictive disorders, including drug addiction, pathological gambling, and obesity (Bickel et al. 1999; Petry 2001; Weller et al. 2008; MacKillop et al. 2011).
Studies showing evidence of heritability of delay discounting using both animal models and human twin designs are critical for establishing delay discounting as an endophenotype. An increasing number of studies comparing different strains of isogenetic inbred rodents reared in identical environments have identified systematic differences in discounting across strains that are attributable to genetic differences (Isles et al. 2004; Anderson and Woolverton 2005; Madden et al. 2008). Interestingly, one study found significantly greater discounting in Fischer rats compared to Copenhagen and Noble rats, but failed to replicate previous findings of differences between Fischer and Lewis rats (Wilhelm and Mitchell 2009). However, a more recent study suggested that this replication failure may be due to the method of task administration rather than the strain (Stein et al. 2012). In addition, a recent investigation including eight rat strains estimated the specific heritability at 50% (Richards et al. 2013).
Complementing these preclinical findings, four human twin studies have been conducted to date and all four support the heritability of delay discounting. The first study assessed early adolescent twins and found evidence of genetic influences at both age 12 and 14 (30 and 51%, respectively) (Anokhin et al. 2011). The second study comprised 17-year-old twins from the Minnesota Twin Family Enrichment study and found that discounting had a quite similar heritability rate to the previous study (51%) (Sparks et al. 2014). In the same sample, a third study examined the heritability of a different delay discounting phenotype and found similar rates of heritability (47%) (Isen et al. 2014). Finally, Anokhin et al. (in press) analyzed the heritability of two indices of delay discounting, finding 35–46% heritability for age 16 and 55–62%% for age 18. In sum, these studies all identified a robust genetic basis of delay discounting (i.e., ~50%) that seems to be increase in effect in late adolescence, likely paralleling ongoing adolescent brain maturation of prefrontal regions implicated in intertemporal choice (Steinberg 2010; Carter et al. 2010; Peters and Büchel 2011; Luo et al. 2012).
A small number of molecular genetic studies have found delay discounting to be associated with single nucleotide polymorphisms (SNPs) in genes putatively associated with dopamine neurotransmission, primarily COMT (rs4680) and ANKK1 (rs1800497) (Boettiger et al. 2007; Eisenberg et al. 2007; Paloyelis et al. 2010; Gianotti et al. 2012; Smith and Boettiger 2012). The relationship between COMT and dopamine neurotransmission is fairly well understood: COMT encodes the enzyme catechol-O-methyl transferase, which plays a critical role in the post-synaptic breakdown of dopamine and has been shown to be a major regulator of dopamine levels in the prefrontal cortex of rodents (Käenmäki et al. 2010). The variability in the rs4680 polymorphism has been strongly associated with levels of enzymatic activity (Chen et al. 2004).
The link between ANKK1 and dopamine neurotransmission is more ambiguous. This is because the SNP rs1800497 was previously believed to be located within the dopamine D2 receptor gene (DRD2), but is actually located in the adjacent ANKK1 gene. Although loci across several genes in this region (NCAM1-TTC12-ANKK1-DRD2) are in high linkage disequilibrium (LD) (i.e., non-random associations among alleles; Mota et al. 2012), this particular locus has been of focus due frequent association with addictive phenotypes and its putative role in the dopaminergic system (Ma et al. 2015). Furthermore, positron emission tomography has revealed that rs1800497 is related to dopamine D2/D3 receptor density (Thompson et al. 1997; Pohjalainen et al. 1998; Jönsson et al. 1999; Savitz et al. 2013). Three current hypotheses exist for the mechanism of influence of rs1800497 on addiction-related phenotypes: a) it is indeed exerting an individual effect related to addictive behavior; b) it is in partial or total LD with a nearby locus in DRD2 that is the actual risk locus; and c) it is primarily influential as part of a larger haplotype (Gelernter et al. 2006). In other words, the specificity of the link between rs1800497 and addiction phenotypes is not clear.
The principal aim of the current study was to extend the understanding of genetic influences on delay discounting in a number of ways. First, no previous studies have examined genetic influences on delay discounting in smokers, a group for whom delay discounting is particularly relevant. Specifically, delay discounting rates have been found to significantly positively correlate with severity of nicotine dependence (Sweitzer et al. 2008; Amlung and MacKillop 2014), with more dependent smokers exhibiting greater impulsivity. Furthermore, more impulsive discounting has been found to predict both the onset of smoking (Audrain-McGovern et al. 2009) and poor smoking cessation treatment response (MacKillop and Kahler 2009; Sheffer et al. 2011), suggesting it plays roles in the etiology and maintenance of smoking. Furthermore, the loci that have been associated with delay discounting have also been associated with nicotine dependence (Huang et al. 2009; Voisey et al. 2012) and smoking cessation treatment response (Swan et al. 2005; Munafò et al. 2009). Based on these overlapping relationships, the current study examined two a priori loci, rs4680 in COMT and rs1800497 in ANKK1, in relation to delay discounting in a relatively large sample of smokers. We hypothesized that the T allele of rs1800497 would be significantly associated with higher discounting rates (Eisenberg et al. 2007) and that the G allele of rs4680 would be significantly associated with greater delay discounting rates (Boettiger et al. 2007; Gianotti et al. 2012). The second goal of the study was exploratory. Given the ambiguity with regard to the specific role of rs1800497, we sought to examine the relationship between delay discounting and haplotypes comprised of loci that are proximal to rs1800497. This permitted us to address the specificity of rs1800497 with delay discounting.
METHODS
Participants and procedure
Participants were adult current smokers (age 18+; ≥ 5 cigarettes per day; ≥ 8th grade education) who were recruited from the general community in Athens, GA, USA and Providence, RI, USA to participate in a parent study examining the relationship between cigarette consumption and price (MacKillop et al. 2012b). To avoid potential population stratification, the current study exclusively examined a subsample of 736 daily smokers of European ancestry. After completing the informed consent, participants completed a variety of assessments, including delay discounting, and provided a DNA sample. This study was approved by the Brown University and University of Georgia Institutional Review Boards.
Assessment
Demographics were assessed by self-report of sex, age, race, gender, and income (assessed in $15,000 intervals, 1 = <15,000; 9 = >$120,000). Of note, although participants were explicitly asked to report pre-tax household income, it is possible that university students in the sample, despite being dependents of their parents, could have reported personal income. Nicotine dependence was assessed using the Fagerstrom Test for Nicotine Dependence (FTND; Heatherton et al. 1991). Alcohol use was assessed using the Alcohol Use Disorders Identification Test (AUDIT; Saunders et al. 1993), but other drug use was not systematically assessed. Delay discounting was assessed using the Monetary-Choice Questionnaire (MCQ) (Kirby et al. 1999), a widely used measure that consists of 27 choices between smaller immediate rewards and larger delayed rewards. The rewards ranged from $11 to $85 and the larger delayed rewards were available at varying intervals of delay from 7 to 186 days. The measure has three sets of nine items grouped into three delayed reward sizes: small ($25–$35, median = $30), medium ($50–$60, median = $55), and large ($75–$85, median = $80); items from the three reward magnitudes are randomly ordered and intermixed.
DNA was collected using a widely-used buccal swab approach (e.g., MacKillop et al. 2007; McGeary et al. 2007). Genotyping comprised 14 putatively dopamine-related SNPs, including the two a priori SNPs (rs1800497 and rs4680). The exploratory SNPs were loci proximal to rs1800497, including chromosome 11 SNPs in the region of chromosome 11 that includes NCAM1, TTC12, DRD2, and ANKK1. The loci were selected from tag SNPs (i.e., atheoretically-selected SNPs that are positionally representative of a region of the genome containing high LD) employed in previous studies exploring variation in this region (Gelernter et al. 2006; Dick et al. 2007). Samples were genotyped using a MassEXTEND Sequenom assay based on the annealing of an oligonucleotide primer adjacent to the SNP of interest. Duplicate runs were conducted for 20% of samples. Primer sequences are available upon request.
Statistical Analysis
Participants’ hyperbolic discounting functions (i.e., k values) across all three magnitudes were inferred from the MCQ choices using standard practices (Kirby et al. 1999). The k values were positively skewed (large k = 1.88; medium k = 1.36; small k = .84), as is common, and square root transformations yielded normal distributions (large k = 1.14; medium k = .62; small k = .22). PLINK (Purcell et al. 2007) was utilized to conduct individual locus diagnostics and to compute a priori loci and haplotype associations with discounting. Using an additive model for maximum resolution, the number of minor alleles (i.e., 0, 1, or 2) was examined in relation to the phenotype. However, to assess for dose effects, significant relationships among a priori loci were followed up with recessive model testing (i.e., coding 1 for possession of 2 minor alleles and 0 for possession of 1 or 0 minor alleles). Regression analyses for testing SNP and haplotype associations with delay discounting were conducted using standard significance values (p ≤ .05). Despite directional a priori hypotheses, we report two-tailed significance values to follow convention. To further control for type-1 error inflation, we applied a family-wise false discovery rate (FDR) correction (Benjamini and Hochberg 1995) to a priori loci association tests. Haplotype blocks were identified using Haploview (Barrett et al. 2005) and LD was defined as 95% confidence of non-random association of alleles at two or more loci (Gabriel et al. 2002). Exploratory analyses consisted of assessing the associations between haplotype blocks derived from the chromosome 11 panel and k values that were significantly associated with rs1800497 in a priori analyses. As the association between rs4680 genotype and discounting has been previously observed to differ by age status (18–21 vs. 22+) (Smith and Boettiger 2012), exploratory analyses were conducted to evaluate an interaction effect of age status on the relationship between discounting and rs4680 genotype. In addition, exploratory analyses were also conducted for sex based on evidence of differential COMT functioning in females and possible influences of menstrual cycle phase (Chen et al. 2004; Smith et al. 2014). We first conducted independent samples t-tests to assess if the average number of minor alleles differed based on age status or sex. Subsequently, to assess all possible main effects and interactions of age status and sex, we conducted a 2 × 2 × 3 ANCOVA (age status by sex by rs4680 genotype), controlling for income.
RESULTS
Preliminary Analyses
Twenty-three participants were excluded for missing >20% genotypes. Participant characteristics (final N = 713) were 60.2% male; age M = 30.3, SD = 12.4; years of education M = 13.2, SD = 2.2; AUDIT M = 9.3, SD = 7.52; FTND M = 4.0, SD = 2.6; number of cigarettes per day M = 16.4, SD = 9.6. Of note, where applicable, two additional subjects were excluded from rs4680 analyses (due to failed genotyping) and four from analyses involving sex (due to missing sex data). On the MCQ, participants exhibited high mean consistency across reward sizes (consistency across all reward sizes M = .99, SD = .04), which suggests that the inferred k values were highly consistent with participant performance. Correlations among delay discounting indices, nicotine dependence (FTND), and income are presented in Table 1. Income was significantly associated with discounting and was included as a covariate by entering it into the first level of the linear regression analyses.
Table 1.
Associations among the delay discounting indices, income, and nicotine dependence (N = 713).
| Variable | M (SD) | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| 1 Large k1 | .18 (.14) | — | — | — | — |
| 2 Medium k | .22 (.14) | .84** | — | — | — |
| 3 Small k | .26 (.14) | .75** | .82** | — | — |
| 4 FTND | 4.01 (2.58) | .22** | .21** | .19** | — |
| 5 Income2 | $15,000–$29,999 | −.11** | −.09* | −.10* | −.34** |
Notes. k = behavioral economic index of impulsivity from the Monetary Choice Questionnaire; medians and (ranges) for k values were: large = .16 (.01–.50), medium = .16 (.01–.50), small = .26 (.01–.50); FTND = Fagerstrom Test for Nicotine Dependence
= all k values were square root transformed
median income; correlations coefficients are pearson coefficients
p < .05
p < .01
With regard to genotyping, of an initial panel of 14 putatively dopamine-related loci genotyped, SNPs were examined for excessive numbers of nonviable samples (>20%), insufficient variability (minor allele frequency [MAF] <10%), and Hardy-Weinberg equilibrium (HWE) violation (p < .05). One SNP (rs1799732) was removed for insufficient variability (MAF = 5.4%) and another (rs4938012) was removed for extreme violation of HWE (p = 1.17 × 10−38). Regarding rs4938012, as there were no minor allele homozygotes, in contrast to previously reported frequencies, the deviation from HWE appears to be attributable to a failed assay. Frequencies and HWE for the a priori loci are in Table 2.
Table 2.
Associations between a priori loci and delay discounting at three magnitudes of reward.
| Genetic Variables | Large k | Medium k | Small k | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Gene | Polymorphism | Mi/Ma | MAF | HWE | β | T | p | β | t | p | β | t | p |
| ANKK1 | rs1800497 | T/C | .22 | 1.00 | .01 | 1.37 | .17 | .02 | 2.48 | .01 | .01 | 1.32 | .19 |
| COMT | rs46801 | G/A | .48 | .71 | .01 | 2.09 | .04 | .02 | 2.67 | .01 | .02 | 2.10 | .04 |
Notes. Alleles are coded higher for larger numbers of minor alleles; income was included as a covariate and k values are square root transformed; Chr = chromosome, Mi = minor allele, Ma = major allele, MAF = minor allele frequency, HWE = Hardy-Weinberg equilibrium
n = 711; nominally significant associations are underlined; statistically significant associations after false discovery rate correction are in boldface.
Individual Locus Analyses
Associations between the three k indices of delay discounting and the a priori loci are presented in Table 2. After FDR correction, the T allele of rs1800497 and the G allele of rs4680 were found to be significantly associated with higher medium k (both R2s = .02), reflecting greater discounting of delayed rewards. Additionally, the relationship between the G allele of rs4680 and large and small k was found to be nominally significant (p < .05) in both cases. A linear regression was conducted to test the association of both loci (rs1800497 and rs4680) simultaneously with medium k. The aforementioned findings held, namely, rs1800497 and rs4680 remained significantly associated with medium k (ps = .01; R2 = .03).
Recessive models were conducted to probe for dose effects of rs1800497 and rs4680 with medium k (i.e., greater differences based on possession of more copies of minor alleles). There was a significant effect of the rs1800497 TT genotype (compared to the other two genotypes; p = .04) on medium k, suggesting dose effects. However, there was no significant effect of the rs4680 GG genotype (as compared to the other two genotypes; p = .43), suggesting a dominance effect, that possession of one or two G alleles puts one at greater risk for increased discounting. These findings are supported by the mean levels of medium k by genotype for each loci (rs1800497: CC M = .21, CT M = .23, TT M = .27; rs4680: AA M = .20, AG M = .24, GG M = .23). Thus, for rs1800497, T allele homozygotes exhibited 29% higher levels of discounting compared to C allele homozygotes, with heterozygotes exhibiting a 10% increase. For rs4680, G allele carriers (homozygous or heterozygous) exhibited 15–20% more impulsive discounting. Of note, the two loci were uncorrelated (r = .05, p = .18) and neither locus was significantly associated with nicotine dependence (rs1800497, p = .39; rs4680, p = .54).
Haplotype Analyses
Characterization of LD blocks is depicted in Figure 1. Linkage disequilibrium analysis identified three haplotype blocks (1: rs2282511/rs877138/rs17115439/rs4938013/rs4938015; 2: rs1160467/rs1800497; 3: rs6277/rs1079597) on chromosome 11. Two loci did not fall into any haplotypes and were not analyzed further. Detailed results are summarized in Table 3. The GT haplotype of Block 2, containing rs1800497, and the CA haplotype of Block 3 were significantly associated (p < .05) with medium logk. For Block 2, only the haplotype containing the T allele of rs1800497 was significantly associated with discounting. For Block 3, only the haplotype containing the A allele of rs1079597 was significantly associated with discounting.
Figure 1.
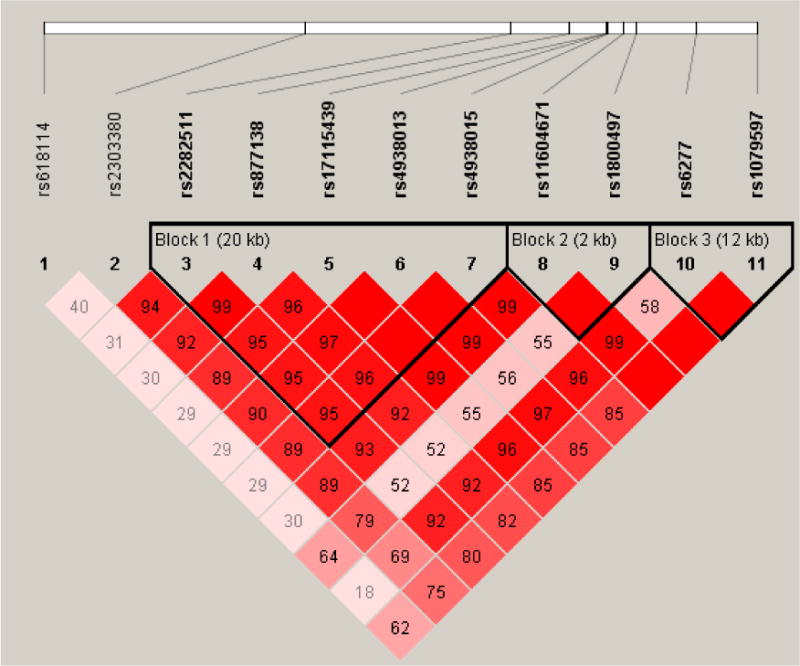
Linkage disequilibrium (LD) map among the proximal chromosome 11 loci. The loci are located in NCAM1 (rs618114), TTC12 (rs2303380, rs2282511), ANKK1 (rs877138, rs17115439, rs4938013, rs4938015, rs11604671, rs1800497), and DRD2 (rs6277, rs1079597).
Table 3.
Exploratory haplotype associations with delay reward discounting.
| Genetic Variables | Medium k | ||||
|---|---|---|---|---|---|
|
| |||||
| Haplotype blocks | Haplotypes | Frequency | β | t | p |
| rs2282511/rs877138/rs17115439/ | AGTAT | .33 | .01 | 1.73 | .19 |
| rs4938013/rs4938015 | CACCC | .64 | −.01 | .99 | .32 |
| rs1160467/rs1800497 | GT | .22 | .02 | 6.00 | .01 |
| AC | .49 | −.01 | 1.38 | .24 | |
| GC | .30 | −.01 | .86 | .35 | |
| rs6277/rs1079597 | CA | .17 | .02 | 6.44 | .01 |
| CG | .30 | −.01 | .93 | .34 | |
| TG | .53 | −.01 | 1.05 | .31 | |
Notes. Nominal statistically significant associations are in boldface.
Exploratory rs4680 (COMT) Analyses
There was no evidence that the genotype distributions differed by sex (male mean minor alleles = .98, SD = .71; and female mean minor alleles = .95, SD = .73, p = .55) or by age status (18–21 mean minor alleles = 1.01, SD = .72; and 22+ mean minor alleles = .94, SD = .71, p = .18). The 2 (age) × 2 (sex) × 3 (genotype) ANOVA identified a main effect of age status (F (1, 694) = 11.63, p = .02, η2 = .02) and rs4680 genotype (F(2, 700) = 5.52, p = .004, η2 = .02), but no main effect of sex (F (1, 694) = 2.62, p = .11, η2 = .00). Additionally, no interaction effects were identified (sex and rs4680: F (2, 694) = .29, p = .75, η2 = .00; age status and rs4680: F (2, 694) = .58, p = .56, η2 = .00; three-way interaction: F (2, 694) = .20, p = .82, η2 = .00). These results indicate that although subjects who were older (i.e., 22+) tended to discount at higher rates, the association between rs4680 genotype and delay discounting did not systematically differ by age.
DISCUSSION
This study sought to contribute to the understanding of genetic influences on delay discounting in a moderately-sized sample of daily smokers. Our findings replicated the associations between two a priori candidate polymorphisms (rs4680 in COMT, rs1800497 in ANKK1) and delay discounting. Specifically, significant associations were found between medium reward delay discounting rates with the G allele of rs4680 and T allele of rs1800497. Similar patterns were evident at the small and large magnitude rewards, reaching nominal statistical significance for rs4680. The association between discounting and the G allele of rs4680 is relatively consistent with the finding among five previous studies, three of which identified the G/G genotype as the risk genotype (Boettiger et al. 2007; Gianotti et al. 2012; Smith and Boettiger 2012) and two of which did not (Paloyelis et al. 2010; Gray and MacKillop 2014). It is worth noting that these mixed findings may be due to substantial methodological differences present in the literature. In particular, different studies have used considerably different tasks and samples (e.g., healthy young adults, adults with and without alcohol use disorders, adolescents with ADHD, adults frequent gamblers), which may influence the observed findings.
The association between the T allele of rs1800497 and discounting is consistent with one previous study (Eisenberg et al. 2007), while two other studies did not find this association (Kawamura et al. 2013; Gray and MacKillop 2014). Again, considerable methodological heterogeneity is present within these studies, which may contribute to these mixed findings. Given the individual association of rs1800497 with discounting identified here, the current results suggest that this SNP exerts an individual influence on discounting rather than being in LD with a nearby locus or part of a broader haplotype. However, an exhaustive examination of all loci proximal to rs1800497 was not conducted, so this conclusion is not definitive. Although the specific functionality of rs1800497 remains unknown, previous studies have identified an association between this SNP and reduced dopamine D2/D3 receptor density and binding affinity (Thompson et al. 1997; Pohjalainen et al. 1998; Jönsson et al. 1999; Savitz et al. 2013), suggesting rs1800497 exerts its effect via dopamine neurotransmission. In general, the relationships identified with rs4680 and rs1800497 are consistent with the hypothesis that dopaminergic hypoactivity is associated with higher rates of delay discounting in adults (MacKillop 2013). Indeed, a recent investigation found that individuals with the GG genotype of rs4680 who were also administered a medication that decreased overall dopamine levels exhibited significantly higher rates of delay discounting and poorer performance on a working memory task (Kelm and Boettiger 2013).
With regard to the small and large magnitude reward discounting, it is notable that the pattern of relationships was consistent, however, the levels of statistical significance for the associations between the two a priori loci and discounting were reduced to nominal significance or nonsignificance. This suggests that genetic associations may meaningfully differ in magnitude based on size of the rewards used. There are two potentially overlapping explanations for the specificity of the effects to medium reward sizes. First, the medium reward range appears to be most quantitatively appropriate as the frequency histograms revealed that there were more people at the highest or lowest k values for smaller and larger reward sizes, respectively, suggesting possible ceiling and floor effects. However, these differences were relatively small in absolute size. Furthermore, in examining the distributions, all three variables were normally distributed and medium magnitude discounting was not markedly more so.
The second possibility pertains to the underlying neural circuitry subserving discounting decision making. Distinct regions of neural activity are recognized to contribute to behavioral choices (Bickel et al. 2009; MacKillop et al. 2012a) and genes are substantially responsible for the architecture of these neurobiological systems. As such, the medium magnitude reward choices may have been at a “sweet spot” in terms of challenging the underlying neural circuitry for this sample and thus bringing genetic influences into sharper relief. This is necessarily speculative but it suggests that delayed rewards that are too large (e.g., $100,000) or too small (e.g., $1) may obscure significant relationships identified at amounts that are more salient to the individual. Again, some caution accompanies this hypothesis as the absolute differences on the MCQ were not dramatically different across magnitudes. Fundamentally, the influence of reward magnitude on detection of genetic associations needs to be examined systematically in future studies, including possible underlying mechanisms.
In haplotype analyses, we assessed the relationship between three haplotypes (one of which included rs1800497) from 11 proximal SNPs and delay discounting. This enabled us to be able to both examine rs1800497 at the individual level as well as at the haplotype level. Analyses of the haplotype block containing rs1800497 (i.e., Block 2: rs1160467/rs1800497) identified an association with discounting of medium rewards. Only the haplotype with the T allele of rs1800497 was associated with discounting of medium rewards, suggesting that the variance is best understood as being attributable to the T allele of rs1800497, rather than at the haplotype level. Analyses of the other two haplotype blocks revealed a significant association between Block 3 (rs6277/rs1079597) and discounting of medium rewards, whereby it was only the haplotype with the A allele of rs1079597 that was significantly associated with discounting of medium rewards, suggesting specificity of the underlying SNP. This is the first time this SNP has been linked to delay discounting to our knowledge. It is worth noting, however, that the haplotype analyses do not survive more stringent type 1 error rate correction. As such, they should be interpreted cautiously and primarily as providing an initial basis for future investigation.
A second exploratory strategy included examining possible differences based on age and sex. Part of the rationale for these analyses was the previous observation that COMT may play different roles across the lifespan (Smith and Boettiger 2012). Specifically, in that study, individuals older than 21 exhibited the pattern of associations observed here, whereas individuals < 21 exhibited the reverse, putatively due to developmental differences in COMT activity. In the current study, we did not observe an interaction of age status on the relationship between rs4680 genotype and discounting of medium rewards. However, several differences between the study samples should be noted. In particular, this sample comprised daily smokers who also drank more heavily and were less educated than the previous study, and Smith and Boettiger (2012) used a higher resolution discounting paradigm. Despite evidence of differential COMT functioning based on sex (Chen et al. 2004), we observed no differences between males and females in the relationship between rs4680 genotype and discounting of medium rewards. However, a recent study suggests that rather than merely sex moderating relationships, the specific stage of the ovarian cycle (and associated estradiol levels) may moderate the relationship between rs4680 genotype and discounting (Smith et al. 2014), but hormonal data was not collected in this study and this could not be assessed.
Several aspects of the study’s methodology merit discussion. First, although the focus on smokers was intentional and novel, it is also possible it influenced the findings. For example, there is some evidence of a reciprocal relationship between smoking and impulsivity (Yi et al. 2008). This could mean that investigating genetic associations in an affected population, in this case, smokers, could reduce the magnitude of genetic main effects observed. In future studies, a case-control design (i.e., smokers vs. non-smokers) would provide a comprehensive approach for investigating delay discounting as a nicotine dependence endophenotype. In particular, case-control designs would permit investigating genetic associations with discounting within smokers, within controls, and whether loci associated with discounting are significantly more prevalent in smokers, more directly implicating discounting as a genetically-influenced risk mechanism.
In addition, a number of limitations are worth noting. For example, time since last cigarette was not assessed, meaning both acute exposure to nicotine or potential withdrawal could not be incorporated in the analyses. Similarly, participants’ other substance use and, for females, menstrual cycle phase were not systematically assessed. With regard to the task, the study used a measure that did not provide participants with an actual outcome from the task and generated inferred k values rather than iteratively assessing the points of indifference for a higher resolution characterization of discounting functions. The issue of hypothetical vs. actual rewards is diminished somewhat however, as several studies have suggested that there are limited systematic differences in response based on whether the task is incentivized (Johnson and Bickel 2002; Madden et al. 2003; Madden et al. 2004), including an fMRI study revealing parallel neural activations for hypothetical and incentivized reward (Bickel et al. 2009).
Despite these considerations, the current findings extend the small existing literature on genetic influences on delay discounting in the largest sample to date. The current study further implicates rs4680 and rs1800497 in relation to delay discounting, providing some evidence of specificity for the latter. However, given the somewhat variable findings to date and the widely varying study characteristics, there is a clear need for large studies to more definitively clarify the genetic basis of delay discounting. Furthermore, although genetic variation relating to dopamine neurotransmission has been a dominant focus, studies elucidating of the role of variation in other systems related to reward processing (e.g., GABA, glutamate, endogenous opioids), as well as atheoretical genome-wide association studies, will be needed to comprehensively examine the contribution of genetic factors to delay discounting.
Acknowledgments
The authors are very grateful to the research staff and doctoral students who contributed to data collection, including Lauren Wier, MPH; Lauren Few, PhD; Cara Murphy, MS; John Acker, MS and Monika Stojek, PhD. The study was funded by grants from the Robert Wood Johnson Foundation (JM) and the National Institutes of Health (K23 AA016936; JM). Dr. MacKillop is the holder of the Peter Boris Chair in Addictions Research, which partially supported his role. The funders had no input into the study design, data collection, analysis or manuscript preparation.
Footnotes
CONTRIBUTIONS
The study was designed by JM and JEM. Data collection was overseen by JM. Data processing and analysis was conducted by JCG and JM. The findings were interpreted by JCG, JEM, JM, LCB, CES, and WKB. The initial manuscript was drafted by JM and JCG, with subsequent input from the other authors.
References
- Amlung M, MacKillop J. Clarifying the relationship between impulsive delay discounting and nicotine dependence. Psychol Addict Behav. 2014;28:761–768. doi: 10.1037/a0036726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KG, Woolverton WL. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacol Biochem Behav. 2005;80:387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet. 2011;41:175–183. doi: 10.1007/s10519-010-9384-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, Grant JD, Mulligan RC, Heath AC. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol Psychiatry. 2015;77:887–894. doi: 10.1016/j.biopsych.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audrain-McGovern J, Rodriguez D, Epstein LH, et al. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug Alcohol Depend. 2009 doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. doi: 10.2307/2346101. [DOI] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–454. doi: 10.1007/PL00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009;29:8839–8846. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, et al. Immediate reward bias in humans: frontoparietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RM, Meyer JR, Huettel SA. Functional neuroimaging of intertemporal choice models: A review. J Neurosci Psychol Econ. 2010;3:27–45. doi: 10.1037/a0018046. [DOI] [Google Scholar]
- Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Wang JC, Plunkett J, et al. Family-based association analyses of alcohol dependence phenotypes across DRD2 and neighboring gene ANKK1. Alcohol Clin Exp Res. 2007;31:1645–1653. doi: 10.1111/j.1530-0277.2007.00470.x. doi: ACER470 [pii] 10.1111/j.1530-0277.2007.00470.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg DT, Mackillop J, Modi M, et al. Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav Brain Funct. 2007;3:2. doi: 10.1186/1744-9081-3-2. doi: 1744-9081-3-2 [pii] 10.1186/1744-9081-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. doi: S0033291706008750 [pii] 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science (80-) 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Yu Y, Weiss R, et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15:3498–3507. doi: 10.1093/hmg/ddl426. doi: ddl426 [pii] 10.1093/hmg/ddl426. [DOI] [PubMed] [Google Scholar]
- Gianotti LRR, Figner B, Ebstein RP, Knoch D. Why Some People Discount More than Others: Baseline Activation in the Dorsal PFC Mediates the Link between COMT Genotype and Impatient Choice. Front Neurosci. 2012 doi: 10.3389/fnins.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. doi: nrg1635 [pii] 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gray JC, MacKillop J. Genetic basis of delay discounting in frequent gamblers: Examination of a priori candidates and exploration of a panel of dopamine-related loci. Brain Behav. 2014;4:812–821. doi: 10.1002/brb3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Huang W, Payne TJ, Ma JZ, et al. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34:319–30. doi: 10.1038/npp.2008.37. [DOI] [PubMed] [Google Scholar]
- Isen JD, Sparks JC, Iacono WG. Predictive validity of delay discounting behavior in adolescence: A longitudinal twin study. Exp Clin Psychopharmacol. 2014;22:434–443. doi: 10.1037/a0037340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS. Common genetic effects on variation in impulsivity and activity in mice. J Neurosci. 2004;24:6733–6740. doi: 10.1523/JNEUROSCI.1650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas KG, Markon KE. A meta-analytic evaluation of the endophenotype hypothesis: Effects of measurement paradigm in the psychiatric genetics of impulsivity. J Abnorm Psychol. 2014;123:660–675. doi: 10.1037/a0037094. [DOI] [PubMed] [Google Scholar]
- Jönsson EG, Nöthen MM, Grünhage F, et al. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- Käenmäki M, Tammimäki A, Myöhänen T, et al. Quantitative role of COMT in dopamine clearance in the prefrontal cortex of freely moving mice. J Neurochem. 2010;114:1745–55. doi: 10.1111/j.1471-4159.2010.06889.x. [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Takahashi T, Liu X, et al. DNA polymorphism in the FKBP5 gene affects impulsivity in intertemporal choice. Asia Pac Psychiatry. 2013;5:31–8. doi: 10.1111/appy.12009. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Boettiger CA. Effects of acute dopamine precusor depletion on immediate reward selection bias and working memory depend on catechol-O-methyltransferase genotype. J Cogn Neurosci. 2013;25:2061–71. doi: 10.1162/jocn_a_00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037/0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Luo S, Ainslie G, Pollini D, et al. Moderators of the association between brain activation and farsighted choice. Neuroimage. 2012;59:1469–1477. doi: 10.1016/j.neuroimage.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Ma Y, Yuan W, Jiang X, et al. Updated findings of the association and functional studies of DRD2/ANKK1 variants with addictions. Mol Neurobiol. 2015;15:281–299. doi: 10.1007/s12035-014-8826-2. [DOI] [PubMed] [Google Scholar]
- MacKillop J. Integrating behavioral economics and behavioral genetics: delayed reward discounting as an endophenotype for addictive disorders. J Exp Anal Behav. 2013;99:14–31. doi: 10.1002/jeab.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung M, Wier L, et al. The neuroeconomics of nicotine dependence: An fMRI study of delay discounting in nicotine dependent adults. Psychiatry Reserch: Neuroimaging. 2012a;202:20–29. doi: 10.1016/j.pscychresns.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, et al. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–21. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Few L, Murphy J, et al. High-resolution behavioral economic analysis of cigarette demand to inform tax policy. Addiction. 2012b;107:2191–200. doi: 10.1111/j.1360-0443.2012.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Kahler CW. Delayed reward discounting predicts treatment response for heavy drinkers receiving smoking cessation treatment. Drug Alcohol Depend. 2009;104:197–203. doi: 10.1016/j.drugalcdep.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Menges DP, McGeary JE, Lisman SA. Effects of craving and DRD4 VNTR genotype on the relative value of alcohol: an initial human laboratory study. Behav Brain Funct. 2007;3:11. doi: 10.1186/1744-9081-3-11. doi: 1744-9081-3-11 [pii] 10.1186/1744-9081-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Lagorio CH, et al. Delay Discounting of Potentially Real and Hypothetical Rewards: II. Between- and Within-Subject Comparisons. Exp Clin Psychopharmacol. 2004;12:251–261. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Smith NG, Brewer AT, et al. Steady-state assessment of impulsive choice in Lewis and Fischer 344 rats: between-condition delay manipulations. J Exp Anal Behav. 2008;90:333–344. doi: 10.1901/jeab.2008.90-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary JE, Esposito-Smythers C, Spirito A, Monti PM. Associations of the dopamine D4 receptor gene VNTR polymorphism with drug use in adolescent psychiatric inpatients. Pharmacol Biochem Behav. 2007;86:401–406. doi: 10.1016/j.pbb.2006.11.001. doi: S0091-3057(06)00366-2 [pii] 10.1016/j.pbb.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behav Processes. 2011;87:10–17. doi: 10.1016/j.beproc.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota NR, Araujo-Jnr EV, Paixão-Côrtes VR, et al. Linking dopamine neurotransmission and neurogenesis: the evolutionary history of the NTAD (NCAM1-TTC12-ANKK1-DRD2) gene cluster. Genet Mol Biol. 2012;35:912–918. doi: 10.1590/S1415-47572012000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Timpson NJ, David SP, et al. Association of the DRD2 gene Taq1A polymorphism and smoking behavior: a meta-analysis and new data. Nicotine Tob Res. 2009;11:64–76. doi: 10.1093/ntr/ntn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Mehta MA, et al. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–2426. doi: 10.1038/npp.2010.124. doi: npp2010124 [pii] 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15:227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance abuse disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110:482–487. doi: 10.1037/0021-843X.110.3.482. [DOI] [PubMed] [Google Scholar]
- Pohjalainen T, Rinne JO, Någren K, et al. The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol Psychiatry. 1998;3:256–260. doi: 10.1038/sj.mp.4000350. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Lloyd DR, Kuehlewind B, et al. Strong genetic influences on measures of behavioral-regulation among inbred rat strains. Genes Brain Behav. 2013;12:490–502. doi: 10.1111/gbb.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Savitz J, Hodgkinson CA, Martin-Soelch C, et al. DRD2/ANKK1 Taq1A polymorphism (rs1800497) has opposing effects on D2/3 receptor binding in healthy controls and patients with major depressive disorder. Int J Neuropsychopharmacol. 2013;16:2095–2101. doi: 10.1017/S146114571300045X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffer C, Mackillop J, McGeary J, et al. Delay discounting, locus of control, and cognitive impulsiveness independently predict tobacco dependence treatment outcomes in a highly dependent, lower socioeconomic group of smokers. Am J Addict. 2011;21:221–232. doi: 10.1111/j.1521-0391.2012.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Boettiger CA. Age modulates the effect of COMT genotype on delay discounting behavior. Psychopharmacology (Berl) 2012;222:609–617. doi: 10.1007/s00213-012-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CT, Sierra Y, Oppler SH, Boettiger CA. Ovarian cycle effects on immediate reward selection bias in humans: a role for estradiol. J Neurosci. 2014;34:5468–5476. doi: 10.1523/JNEUROSCI.0014-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks JC, Isen JD, Iacono WG. Preference on cash-choice task predicts externalizing outcomes in 17-year-olds. Behav Genet. 2014;44:102–112. doi: 10.1007/s10519-013-9638-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Pinkston JW, Brewer AT, et al. Delay discounting in Lewis and Fischer 344 rats: steady-state and rapid-determination adjusting-amount procedures. J Exp Anal Behav. 2012;97:305–321. doi: 10.1901/jeab.2012.97-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A behavioral scientist looks at the science of adolescent brain development. Brain Cogn. 2010;72:160–164. doi: 10.1016/j.bandc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics J. 2005;5:21–9. doi: 10.1038/sj.tpj.6500281. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Donny EC, Dierker LC, et al. Delay discounting and smoking: association with the Fagerstrom Test for Nicotine Dependence but not cigarettes smoked per day. Nicotine Tob Res. 2008;10:1571–1575. doi: 10.1080/14622200802323274. doi: 904706261 [pii] 10.1080/14622200802323274. [DOI] [PubMed] [Google Scholar]
- Thompson J, Thomas N, Singleton A, et al. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: Reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7:479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Treutlein J, Rietschel M. Genome-wide association studies of alcohol dependence and substance use disorders. Curr Psychiatry Rep. 2011;13:147–155. doi: 10.1007/s11920-011-0176-4. [DOI] [PubMed] [Google Scholar]
- Voisey J, Swagell CD, Hughes IP, et al. A DRD2 and ANKK1 haplotype is associated with nicotine dependence. Psychiatry Res. 2012;196:285–9. doi: 10.1016/j.psychres.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Weller RE, Cook EW, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite. 2008;51:563–9. doi: 10.1016/j.appet.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Johnson MW, Giordano LA, et al. The effects of reduced cigarette smoking on discounting future rewards: An initial evaluation. Psychol Rec. 2008;58:163–174. doi: 10.1007/bf03395609. [DOI] [PMC free article] [PubMed] [Google Scholar]


