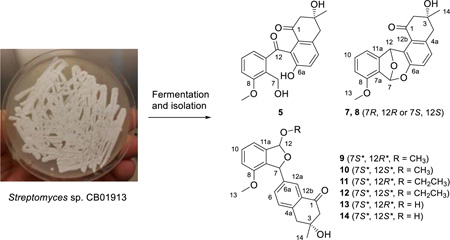
Abstract
Angucyclines and angucyclinones are aromatic polyketides with a tetracyclic benz[a]anthracene skeleton. The benz[a]anthracene scaffold is biosynthesized by type II polyketide synthases that catalyze the decarboxylative condensation of a short acyl-CoA starter and nine extender units. Angucyclines and angucyclinones, the largest group of polycyclic aromatic polyketides, achieve structural diversity via subsequent oxidation, ring cleavage, amino acid incorporation, and glycosylation. We here report the discovery of 14 angucyclinones and two angucyclines (1–16) from Streptomyces sp. CB01913, identifying 12 new compounds featuring various oxidations on rings A and C (1, 2, and 4), different sugar moieties attached to rings A and B (3 and 6), and C-ring cleavage (5 and 10–14) and expansion (8). These new structural features, highlighted by C-ring cleavage and expansion, enrich the structural diversity of angucyclines and angucyclinones. All compounds were tested for cytotoxicity and antibacterial activities, with 1, 5, 15, and 16 showing moderate activities against selected cancer cell lines or bacterial strains.
Graphical abstract
Angucyclines and angucyclinones are aromatic polyketide natural products featuring a tetracyclic benz[a]anthracene skeleton that are distinct from the benz[b]anthracene skeleton found in tetracyclines and anthracyclines.1 Since the isolation of tetrangomycin, the first angucyclinone discovered, from Streptomyces rimosus in 1965,2 the angucycline and angucyclinone family of natural products has grown steadily. To date this family of natural products has been discovered exclusively from actinomycetes with Streptomyces as the major producers.1, 2 Representing the largest family of polycyclic aromatic polyketides known to date, angucyclines and angucyclinones feature a characteristic tetracyclic benz[a]anthracene scaffold that is biosynthesized by type II polyketide synthases (PKSs) via decarboxylative condensations of a short acyl-CoA starter and nine extender units.1 The structural diversity of angucyclines and angucyclinones mainly comes from: (i) oxidations on the angular tetracyclic backbone, including hydroxy substitutions at C-4/5/6a/7/12/12a in the saccharothrixins,3 epoxidation at C-5/6 or C-6a/12a in the panglimycins,4 or carbonyl substitutions at C-7 and C-12 in the hatomarubigins;5 (ii) amino acid incorporations, as in the two point attachment of different amino acids to form yet another ring in jadomycins6 or of tyrosine and tryptophan into urdamycins C and D;7 (iii) ring cleavages including A-ring cleavage in the grincamycins,8 B-ring cleavage in the gilvocarcins,9 or C-ring cleavage in emycins E and F;10 and (iv) glycosylation at various positions, such as the multiple trisaccharide units of d-olivose-4-1-d-olivose-3-1-l-rhodinose attached at the O-8 position in the landomycins11 or deoxysugars attached at the O-3 and C-9 positions in the saquayamycins.12
As a part of the Natural Products Library Initiative (NPLI) at The Scripps Research Institute (TSRI) dedicated to the discovery of natural products from our Actinomycetales strain collection,13–17 we report the discovery of 14 angucyclinones and two angucyclines (1–16) from Streptomyces sp. CB01913, including 12 new compounds with (i) various oxidations on rings A and C (1, 2, and 4), (ii) different glycosylations on rings A and B (3 and 6), and (iii) C-ring cleavage (5 and 10–14) and expansion (8). The natural products isolated in this study show unique characteristics compared with known angucyclines and angucyclinones – (i) 9–14 are the only members with C-ring cleavage between C-12 and C-12a; (ii) 5, 7, and 8 represent rare C-ring cleavage or expansion between C-6a and C-7; (iii) 3, with previously reported TAN-1085,2 represent the only two angucyclines with sugar moieties attached at C-6; and (iv) 1, 4, and 6 contain a hydroxy group, instead of the usual carbonyl, at C-12. These new structural features, especially the C-ring cleavage and expansion represented by 5, 8, and 10–14, enrich the structural diversity of angucycline and angucyclinone family of natural products. All compounds were tested for cytotoxicity and antibacterial activities, with 1, 5, 15, and 16 showing moderate activities against selected cancer cell lines or bacterial strains.
RESULTS AND DISCUSSION
Strain Selection, Taxonomy, and Fermentation Optimization
The NPLI at TSRI aims at constructing a natural products library with unique chemical and structural diversities that complements TSRI’s small molecule collection. The NPLI biases natural products from Actinomycetales that are isolated from unexplored or underexplored ecological niches and unavailable in public strain collections. The current library at TSRI consists of: (i) purified natural products with fully assigned structures, (ii) medium pressure liquid chromatography (MPLC) (on C-18 semipreparative column) fractions, and (iii) extracts. The extracts are generated by fermenting each strain in two media (medium A and medium F, Table S1), and the resultant extracts are subjected to HPLC-photodiode array (PDA)-based chemical profiling, which then guides strain selection for subsequent MPLC fractionation and natural product isolation. Figure S1A depicts HPLC analysis of extracts made from 20 strains in a typical run of fermentation in medium F. Among the 20 strains, S. sp. CB01913 was found to produce the most detectable natural products based on chemical profiling as exemplified by the HPLC chromatograms with UV detection at 254 nm. We recently reported methods to guide strain prioritization for natural product discovery by surveying the biosynthetic gene clusters for the targeted class of natural products.17 While these genomics-based methods are effective in prioritizing strains according to the biosynthetic potential, it is far from certain that the predicted biosynthetic machinery will be functional under the fermentation conditions of investigation for natural product production and isolation. The method reported here, chemical profiling of extracts, complements the genomics-based strain prioritization strategies and allows direct assessment of natural products produced under the conditions of investigation. On the basis of the observed natural product abundance, S. sp. CB01913 was selected as a priority strain for natural product isolation.
S. sp. CB01913 was isolated from a soil sample collected in Weishan county, Yunnan province, China. It grows well on ISP4 or TSB agar medium. CB01913 was classified as a Streptomyces species on the basis of a phylogenetic analysis using the concatenated partial sequences of the four housekeeping genes 16S rRNA, recA, rpoB, and trpB.17 As shown in Figure S2, CB01913 clearly lies in the same clade with other Streptomyces species.
It is well known that the metabolite profile of a given strain is medium dependent.17 Thus, we re-fermented S. sp. CB01913 in four media (medium A, B, C, and F, Table S1). HPLC-PDA analysis showed that S. sp. CB01913 yielded the richest metabolite profile in medium B among the four media examined, as exemplified by the HPLC chromatograms with UV detection at 254 nm (Figure S1B). Medium B was selected for large-scale fermentation of S. sp. CB01913, from which the 16 compounds, including 12 new ones (1–6, 8, and 10–14), were isolated.
Structural Elucidation
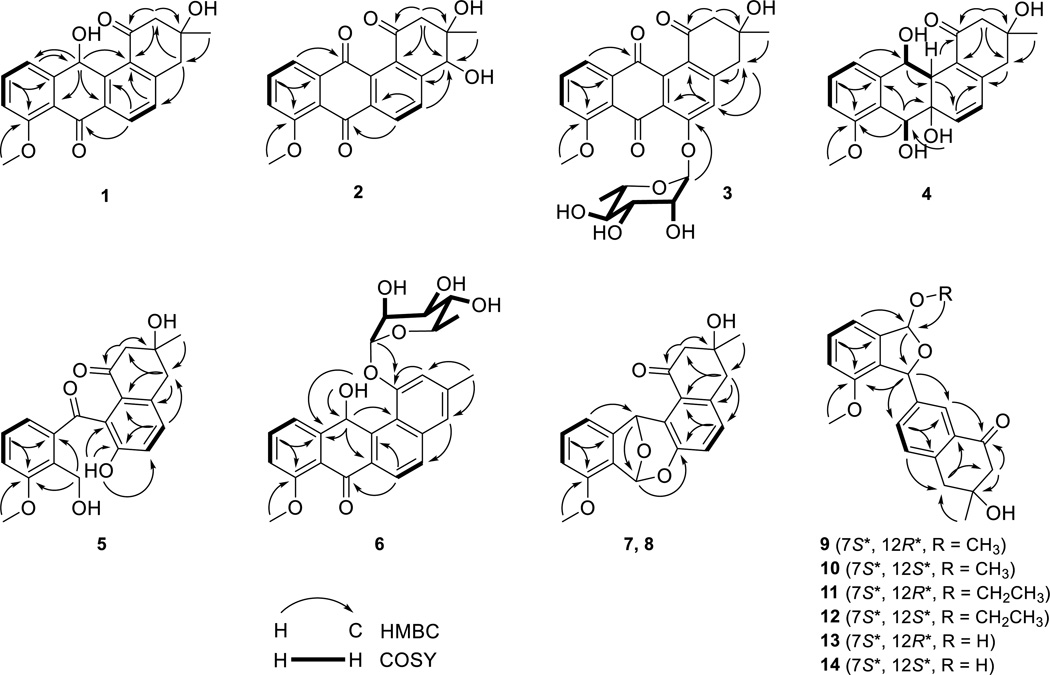
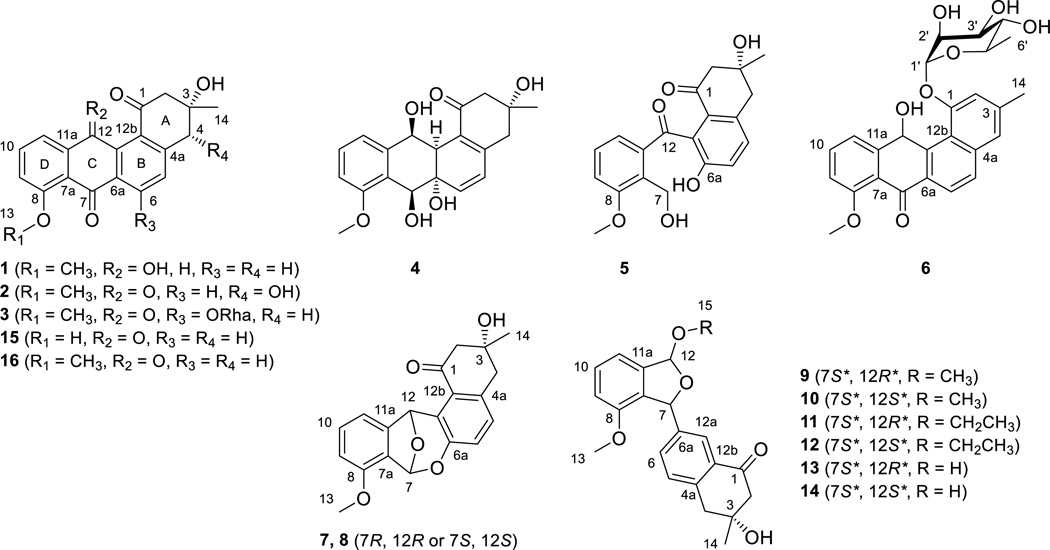
Compound 1 was obtained as a brown powder. High-resolution electro-spray ionization mass spectrometry (HRESIMS) analysis afforded an [M + H]+ ion at m/z 339.1226, giving the molecular formula of 1 as C20H18O5. The 1H, 13C, and COSY NMR spectra of 1 resembled those of 8-O-methyltetrangomycin (16)18a, 18b (Figure 1), except that the resonances at δH 6.39 (s, H-12) (Table 1) and δC 64.3 (C-12) (Table 4), attributed to a methine group in 1, replaced the resonance at δC 184.5 (C-12), attributed to a carbonyl group in 16. These differences indicated one of the carbonyl groups at C-7 or C-12 in 16 was reduced to a hydroxy group in 1. The correlations of H-12 with C-11 and C-12b, H-11 with C-12, and H-6 with C-7 in the HMBC spectrum of 1 identified the hydroxy substitution at C-12 (Figure 1). On that basis, compound 1 was identified as 12-deoxo-12-hydroxy-8-O-methyltetrangomycin through the relative configuration between C-3 and C-12 was not established (Figure 1).
Figure 1.
Key COSY and HMBC correlations supporting structural assignments of 1–14.
Table 1.
1H (700 MHz) NMR Data (δH, J in Hz) for 1–2, 4–5, and 7–8 in DMSO-d6a
| position | 1 | 2 | 4 | 5 | 7 | 8 |
|---|---|---|---|---|---|---|
| 2 | 2.93, d (14.9) | 3.05, d (14.9) | 2.55, d (15.6) | 2.64, br s | 2.94, d (15.1) | 2.67, d (16.6) |
| 2.73, dd (14.9, 2.2) | 2.77, d (14.9) | 2.43, d (15.6) | 2.46, dd (16.2, 1.8) | 2.59, dd (15.1, 2.2) | 2.72, dd (16.6, 1.8) | |
| 4 | 3.22, d (16.9) | 4.72, s | 2.65, d (16.7) | 3.04, d (16.3) | 3.05, d (16.2) | 2.95, d (16.1) |
| 3.11, dd (16.9, 1.8) | 2.58, d (16.7) | 2.91, dd (16.3, 1.5) | 2.83, dd (16.5, 1.9) | 2.87, dd (16.2, 1.3) | ||
| 5 | 7.49, d (8.0) | 8.05, d (8.1) | 6.03, d (10.1) | 7.27, d (8.3) | 7.11, d (8.3) | 7.14, d (8.3) |
| 6 | 8.10, d (8.1) | 8.23, d (8.2) | 6.31, d (10.1) | 7.17, d (8.3) | 6.91, d (8.3) | 6.92, d (8.3) |
| 7 | 4.83, br d (9.7) | 4.85, s | 6.84, s | 6.85, s | ||
| 9 | 7.15, d (8.1) | 7.57, d (8.0) | 7.00, d (7.9) | 7.19, dd (8.2, 1.8) | 6.97, d (8.2) | 6.96, d (8.3) |
| 10 | 7.63, dd (8.2, 7.6) | 7.85, t (8.0) | 7.28, t (8.1) | 7.24, t (7.8) | 7.29, dd (8.2, 7.4) | 7.27, dd (8.2, 7.4) |
| 11 | 7.22, d (7.4) | 7.57, d (8.0) | 6.90, d (7.9) | 6.83, br s | 7.14, d (7.4) | 7.05, d (7.3) |
| 12 | 6.39, s | 4.86, br s | 6.64, s | 6.80, s | ||
| 12a | 2.67, br s | |||||
| 13 | 3.88, s | 3.96, s | 3.81, s | 3.85, s | 3.86, s | 3.86, s |
| 14 | 1.32, s | 1.34, s | 1.20, s | 1.25, s | 1.30, s | 1.23, s |
| 3-OH | 4.82, br s | 4.82, s | 4.78, br s | 4.74, s | 4.85, s | |
| 4-OH | 5.93, br s | |||||
| 6a-OH | 5.15, s | 9.89, s | ||||
| 7-OH | 5.24, br d (9.3) | 4.78, br s | ||||
| 12-OH | 5.48, br s |
Assignments are based on COSY, HMBC, HSQC, and NOESY experiments.
Table 4.
13C (175 MHz) NMR Data (δC, type) for 1–8 in DMSO-d6a
| position | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 200.4, C | 196.2, C | 196.2, C | 197.7, C | 198.1, C | 155.8, C | 200.2, C | 200.0, C |
| 2 | 54.4, CH2 | 52.3, CH2 | 53.4, CH2 | 52.4, CH2 | 52.1, CH2 | 113.6, CH | 53.5, CH2 | 53.3, CH2 |
| 3 | 70.8, C | 74.9, C | 71.6, C | 69.7, C | 70.9, C | 139.0, C | 71.6, C | 69.9, C |
| 4 | 44.7, CH2 | 73.8, CH | 44.1, CH2 | 44.3, CH2 | 42.5, CH2 | 122.0, CH | 43.0, CH2 | 43.2, CH2 |
| 4a | 148.6, C | 151.6, C | 149.5, C | 146.5, C | 134.0, C | 137.8, C | 135.1, C | 135.2, C |
| 5 | 130.5, CH | 132.4, CH | 120.3, CH | 124.7, CH | 131.6, CH | 128.8, CH | 130.3, CH | 130.5, CH |
| 6 | 131.1, CH | 129.5, CH | 157.2, C | 138.7, CH | 122.1, CH | 123.6, CH | 122.3, CH | 122.6, CH |
| 6a | 133.7, C | 135.0, C | 124.0, C | 71.4, C | 153.0, C | 131.9, C | 148.2, C | 148.3, C |
| 7 | 183.2, C | 180.7, C | 180.1, C | 67.7, CH | 54.9, CH2 | 183.7, C | 99.0, CH | 99.1, CH |
| 7a | 120.2, C | 120.4, C | 123.1, C | 127.4, C | 129.9, C | 120.3, C | 123.3, C | 123.2, C |
| 8 | 159.7, C | 135.0, C | 158.7, C | 156.9, C | 158.2, C | 159.5, C | 154.0, C | 154.1, C |
| 9 | 112.5, CH | 118.8, CH | 118.5, CH | 111.0, CH | 115.4, CH | 112.4, CH | 112.0, CH | 112.0, CH |
| 10 | 135.1, CH | 136.3, CH | 135.2, CH | 128.5, CH | 128.6, CH | 134.8, CH | 131.8, CH | 131.9, CH |
| 11 | 122.5, CH | 118.9, CH | 117.7, CH | 119.6, CH | 122.8, CH | 122.6, CH | 112.3, CH | 111.9, CH |
| 11a | 146.4, C | 137.7, C | 136.9, C | 142.6, C | 139.3, C | 146.9, C | 148.7, C | 148.7, C |
| 12 | 64.3, CH | 184.6, C | 186.2, C | 70.1, CH | 199.8, C | 65.2, CH | 77.7, CH | 77.6, CH |
| 12a | 142.0, C | 134.5, C | 138.9, C | 46.6, CH | 128.1, C | 139.6, C | 126.3, C | 126.5, C |
| 12b | 130.1, C | 134.1, C | 127.9, C | 125.8, C | 130.6, C | 120.7, C | 127.6, C | 127.1, C |
| 13 | 56.3, CH3 | 56.9, CH3 | 56.9, CH3 | 55.6, CH3 | 56.5, CH3 | 56.3, CH3 | 56.0, CH3 | 56.0, CH3 |
| 14 | 29.6, CH3 | 26.8, CH3 | 30.6, CH3 | 28.4, CH3 | 29.6, CH3 | 22.0, CH3 | 30.1, CH3 | 29.1, CH3 |
| 1' | 99.4, CH | 100.9, CH | ||||||
| 2' | 70.4, CH | 70.4, CH | ||||||
| 3' | 70.4, CH | 72.0, CH | ||||||
| 4' | 72.1, CH | 72.5, CH | ||||||
| 5' | 70.7, CH | 70.4, CH | ||||||
| 6' | 18.4, CH3 | 18.5, CH3 |
Assignments are based on COSY, HMBC, HSQC, and NOESY experiments.
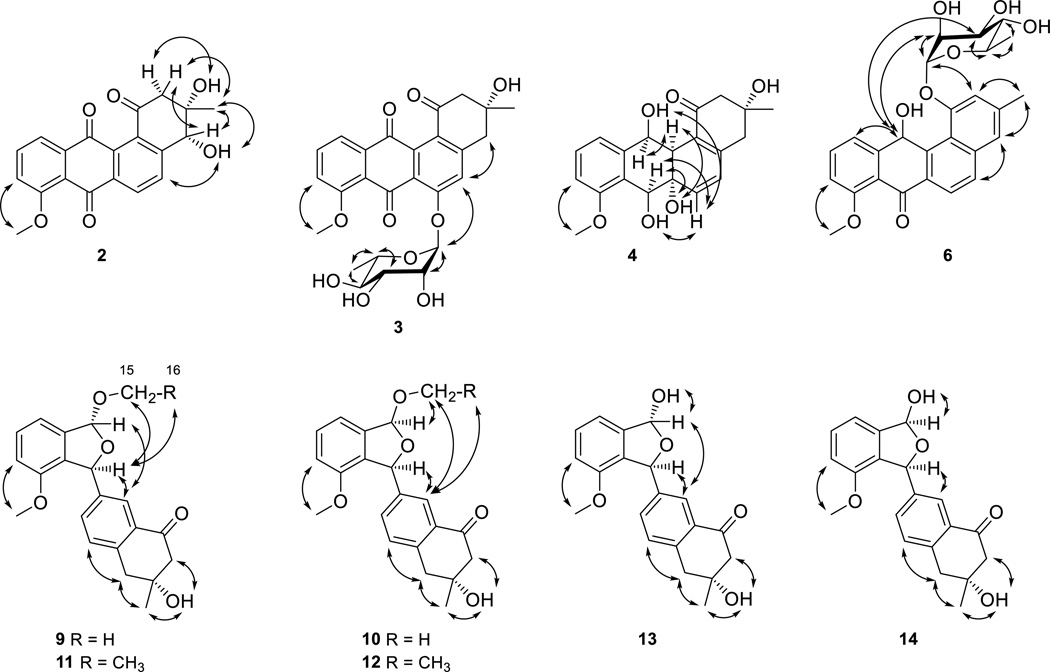
Compound 2 was obtained as a yellow powder. HRESIMS analysis afforded an [M + H]+ ion at m/z 353.1020, giving the molecular formula of 2 as C20H16O6. The chemical shifts and coupling systems in the 1H, 13C, and COSY NMR spectra of 2 resembled those of 16, except that the resonances attributed to the methylene group at C-4 in 16 were replaced by resonances at δH 4.72 (s, H-4) (Table 1) and δC 73.8 (C-4) (Table 4) attributed to one methine group in 2, indicating that the only difference between 2 and 16 was one additional hydroxy group at C-4 in 2. The correlations of H-2 with C-1 and C-4, H-14 with C-4, and H-4 with C-4a in the HMBC spectrum of 2 confirmed the hydroxy substitution at C-4 (Figure 1). Among all natural angucyclines and angucyclinones known to date, the configuration of the methyl and hydroxy groups at C-3, as drawn in Figure 2, is absolutely conserved. Because the absolute R-configuration at C-3 of the co-purified 16 has been confirmed by total synthesis,18c the absolute configuration of C-3 in 2 was assigned as S based on their shared biosynthetic origin. This assignment was further supported by the NOESY spectrum of 2 – the correlations of H-4 with Ha-2, H3-14 with both H-4 and Ha-2, and 3-OH group with Hb-2 unambiguously established that H3-14, H-4, and Ha-2 were on the same face of ring A, with Hb-2 and the 3- and 4-OH groups lying on the opposite face of ring A (Figure 2). Taken together, the absolute configuration of C-4 in 2 was assigned as R, and 2 was identified as 4R-hydroxy-8-O-methyltetrangomycin.
Figure 2.
Key NOESY correlations supporting the relative configurations of 2–4, 6, and 9–14.
Compound 3 was obtained as a green-yellow powder. HRESIMS analysis afforded an [M + H]+ ion at m/z 499.1590, giving the molecular formula of 3 as C26H26O10. Comparison of the 1H and COSY NMR spectra between 3 and 1618a, 18b showed that the AB coupling system of H-5 with H-6 in ring B of 16 was replaced by a methine singlet at δH 7.38 (s, H-5) and resonances attributed to a 6-deoxysugar moiety at δH 5.66 (br s, H-1'), δH 3.98 (dd, J = 3.2, 1.8 Hz, H-2'), δH 3.90 (dd, J = 9.5, 3.2 Hz, H-3'), δH 3.36 (t, J = 9.4 Hz, H-4'), δH 3.55 (m, H-5') and δH 1.13 (d, J = 6.2 Hz, H3-6') were observed in 3 (Table 2). Comparison of chemical shifts, coupling patterns, and correlations of resonances attributed to this sugar moiety in the 1H, 13C, COSY, and HSQC NMR spectra (Figure 1 and Tables 2 and 4) with literature data,19 in combination with the correlations in the NOESY spectrum (Figure 2), identified the sugar as an α-rhamnose. The absolute configuration of the l-rhamnose moiety was assigned on the basis of a common biosynthetic pathway shared with 6 (see structural elucidation of 6). In the HMBC spectrum of 3, the correlations of H-1’ with C-6, H-5 with C-6a, C-12b, and C-4, and H-4 with C-5 identified the attachment of α-l-rhamnose at C-6 (Figure 1). Therefore, compound 3 was identified as 6-O-α-l-rhamnosyl-8-O-methyltetrangomycin.
Table 2.
1H (700 MHz) NMR Data (δH, J in Hz) for 3 and 6 in DMSO-d6a
| position | 3 | 6 | position | 3 | 6 |
|---|---|---|---|---|---|
| 2 | 2.91, d (14.4), 2.69, dd (14.4, 1.6) |
7.31, d (1.3) | 1' | 5.66, br s [d (1.7)]b | 5.60, d (1.7) [d (1.8)]b |
| 4 | 3.14, d (16.7), 3.01, dd (16.7, 1.2) |
7.43, br s | 2' | 3.98, dd (3.2, 1.8) [dd (3.4, 1.8)]b | 4.41, br s [dd (3.4, 1.9)]b |
| 5 | 7.38, s | 7.88, d (8.7) | 3' | 3.90, dd (9.5, 3.2) [dd (9.5, 3.4)]b | 3.97, br s [dd (9.2, 3.5)]b |
| 6 | 8.02, d (8.6) | 4' | 3.36, t (9.4) [t (9.5)]b | 3.42, m [t (9.2)]b | |
| 9 | 7.51, d (8.5) | 7.18, d (8.1) | 5' | 3.55, m | 3.62, m |
| 10 | 7.76, t (8.0) | 7.65, br t (8.2) | 6' | 1.13, d (6.2) | 1.23, d (6.2) |
| 11 | 7.44, d (7.5) | 7.29, d (7.4) | 3-OH | 5.00, br s | |
| 12 | 6.86, d (6.5) | 12-OH | 5.56, d (6.6) | ||
| 13 | 3.93, s | 3.90, s | 2’-OH | 5.00, br s | 5.15, d (4.1) |
| 14 | 1.31, s | 2.46, s | 3’-OH | 5.00, br s | 4.96, br d (5.4) |
| 4’-OH | 5.00, br s | 4.97, br d (6.0) |
Assignments are based on COSY, HMBC, HSQC, and NOESY experiments.
Coupling constants in square brackets are calculated after D2O was added into DMSO-d6.
Compound 4 was obtained as a colorless gum. HRESIMS analysis afforded an [M + Na]+ ion at m/z 381.1306, giving the molecular formula of 4 as C20H22O6. The 1H and COSY NMR spectra of 4 showed similar resonances, attributed to one methyl group and two methylene groups at ring A, one methoxy group, and an ABC coupling system at ring D, with those in 16. However, the resonances for an AB coupling system of aromatic methine groups at ring B in 16 shifted to δH 6.03 (d, J = 10.1 Hz, H-5) and δH 6.31 (d, J = 10.1 Hz, H-6) in 4. In addition, 4 showed two more methine group resonances at δH 4.83 (br d, J = 9.7 Hz, H-7) and δH 4.86 (br s, H-12) that were coupled with the two hydroxy resonances at δH 5.24 (br d, J = 9.3 Hz, 7-OH) and δH 5.48 (br s, 12-OH), one more aliphatic methine group at δH 2.67 (br s, H-12a), and one more hydroxy resonance at δH 5.15 (br d, J = 9.3 Hz, 6a-OH) (Table 1). The 13C NMR spectrum of 4, compared to that of 16, showed that the two carbonyl resonances at C-7 and C-12 and the two aromatic carbon resonances at C-6a and C-12a in 16 were replaced by three aliphatic methine and one aliphatic non-protonated carbon resonances in 4 (Table 4). These differences suggested that the two carbonyl groups in 16 were reduced to two hydroxy groups and the double bond at C-6a/12a in 16 was reduced to a saturated bond, respectively, in 4. These were confirmed by the correlation of 12-OH with H-12a in the COSY spectrum, and the correlations of 6a-OH with C-7, H-7 with C-8, H-12a with C-1 and C-6, H-12 with C-12a, and H-11 with C-12 in the HMBC spectrum of 4, which also established the attachment of the hydroxy groups at C-6a, C-7 and C-12 (Figure 1). Finally, the relative configuration of 4 was solved by a NOESY experiment – correlations of H-6 with H-7 and 7-OH suggested that the C-6a/C-6 bond took an equatorial conformation, the correlations of the axial 6a-OH with both H-7 and H-12a suggested that 6a-OH, H-7 and H-12a were on the same side of ring C, and the correlations of H-12a with H-12, 12-OH with H-6 suggested that H-12 and H-12a were on the same side of ring C (Figure 2). Taken all together, compound 4 was identified as another congener of 16, and the relative configuration at C-6a, C-7, C-12 and C-12a in 4 was assigned as S*, S*, S* and S*, respectively.
Compound 5 was obtained as a green-yellow powder. HRESIMS analysis afforded an [M + Na]+ ion at m/z 379.1161, giving the molecular formula of 5 as C20H20O6. The 1H and 13C NMR spectra of 5 resembled those of angucyclinone C,20 except for one hydroxy resonance occurring at δH 9.89 (s, 6a-OH) in the 1H NMR spectrum of 5 (Table 1). This difference in combination with the molecular formula of 5 indicated that the ether ring in angucyclinone C was oxidatively cleaved in 5. This was confirmed by the correlations of 6a-OH with C-6, C-6a and C-12a and of H-7 with C-8 and C-11a in the HMBC spectrum (Figure 1). Thus compound 5 was identified as the C-ring cleavage product of angucyclinone C.
Compound 6 was obtained as a yellow powder. HRESIMS analysis afforded an [M + H]+ ion at m/z 467.1691, giving the molecular formula of 6 as C26H26O8. The chemical shifts and coupling systems in the 1H and COSY NMR spectra of 6 resembled those of pseudonocardone A21 except the resonances attributed to one 6-deoxysugar moiety instead of the β-glucuronic acid moiety in pseudonocardone A (Table 2). The sugar moiety was identified as α-rhamnose by methods similar to those described for 3 (Tables 2 and 4), and the absolute configuration of l-rhamnose was established upon mild acid hydrolysis followed by optical rotation comparison with literature value.22 The different chemical shifts of H-2' and C-3' between 3 and 6 are due to the different sugar moiety environments. This is supported by the strong correlations between H-12 with H-2' and H-3' in the NOESY spectrum of 6 (Figure 2). The correlations of H-1' with C-1, 12-OH with C-11a and C-12a, H-12 with C-12b, and H-6 with C-7 in the HMBC spectrum of 6 identified the attachment of α-l-rhamnose at C-1 and the hydroxy group at C-12 (Figure 1). The absolute configuration at C-12 was not assigned. Compound 6 was thus identified as 1-O-α-l-rhamnosyl-12-hydroxy-8-O-methyltetrangulol.
Compounds 7 and 8 were obtained as yellow powders. HRESIMS analysis for 7 and 8 afforded the same [M + H]+ ion at m/z 339.1225, giving the molecular formula of 7 and 8 as C20H18O5. Analysis for the 1H, 13C, COSY, HSQC, and HMBC NMR spectra of 7 and 8 (Figure 1) showed that they had the same planar structure as LS1924, a known angucyclinone isolated from Streptomyces sp. LS1924 with its absolute configuration unassigned.23 However, 7 and 8 had distinct chemical shifts for H-11 and H-12 in their 1H NMR spectra, indicating that 7 and 8 are isomers with different configurations at C-7 and C-12 (R, R or S, S) (Table 1). Comparison of the 1H NMR spectra of 7, 8, and LS1924A in acetone-d6 clearly showed that 7 had nearly identical resonances at δH 6.76 (s, H-7), 6.72 (s, H-12), and 7.21 (d, J = 7.2 Hz, H-11) to those of LS1924A, while 8 had distinct resonances at δH 6.84 (s, H-7), 6.76 (s, H-12), and 7.14 (d, J = 7.2 Hz, H-11) (Table S3). These data supported the final assignment of 7 as LS1924A and 8 as a diastereomer of LS1924A (i.e., 7), respectively.
Compounds 9–14 were obtained as white powders. HRESIMS analysis for 9 and 10 afforded the same [M + Na]+ ion at m/z 377.1358, giving the molecular formula of 9 and 10 as C21H22O5. Analysis of the 1H, 13C, COSY, HSQC, and HMBC NMR spectra of 9 showed that it was same as the angucyclinone from Streptomyces sp. M268 (Tables 3 and 5).24 However, close examination of the NMR spectra of 9 indicated that the relative configuration of the angucyclinone from S. sp. M268 was incorrectly assigned. In the NOESY spectrum of 9, the correlations of H-7 with both H-15 and H-12a, H-12 with H-12a, which was similar to that of the angucyclinone from S. sp. M268,24 suggested that H-7 and H-12 adopted “trans” positions on the different sides of the five-membered ether ring (Figure 2). Thus, we assigned the relative configuration of 9, thereby correcting the assignment for the angucyclinone from S. sp. M268, at C-7 and C-12 as S* and R*, respectively.
Table 3.
1H (700 MHz) NMR Data (δH, J in Hz) for 9–14 in DMSO-d6a
| position | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|
| 2 | 2.73, d (16.1) | 2.72, d (16.0) | 2.73, d (16.1) | 2.71, d (16.0) | 2.72, d (16.1) | 2.73, d (16.1) |
| 2.59, dd (16.1, 2.1) | 2.59, dd (16.0, 1.8) | 2.59, dd (16.1, 2.1) | 2.59, dd (16.0, 2.0) | 2.59, dd (16.1, 2.1) | 2.60, dd (16.0, 2.0) | |
| 4 | 3.11, d (16.5) | 3.10, d (16.5) | 3.10, d (16.5) | 3.09, d (16.5) | 3.09, d (16.5) | 3.10, d (16.6) |
| 2.95, dd (16.6, 1.8) | 2.94, br d (16.5) | 2.94, dd (16.7, 1.8) | 2.94, dd (16.6, 1.8) | 2.93, dd (16.5, 2.1) | 2.94, dd (16.3, 2.1) | |
| 5 | 7.28, d (7.9) | 7.27, d (7.8) | 7.28, d (7.9) | 7.27, d (7.8) | 7.25, d (8.3) | 7.27, d (8.7) |
| 6 | 7.45, dd (7.8, 2.0) | 7.51, dd (7.8, 1.7) | 7.45, dd (7.9, 2.0) | 7.57, dd (7.9, 2.0) | 7.44, dd (7.9, 2.0) | 7.62, dd (7.9, 1.9) |
| 7 | 6.33, d (2.2) | 6.18, s | 6.31, d (2.3) | 6.17, s | 6.26, d (2.2) | 6.08, s |
| 9 | 6.98, d (8.1) | 6.98, d (8.1) | 6.97, d (8.1) | 6.96, d (8.1) | 6.92, d (8.1) | 6.94, d (8.1) |
| 10 | 7.41, t (8.1) | 7.39, t (7.7) | 7.40, t (8.0) | 7.37, br t (8.0) | 7.38, t (8.0) | 7.36, t (8.0) |
| 11 | 7.06, d (7.6) | 7.05, d (7.5) | 7.05, d (7.6) | 7.03, d (7.5) | 7.03, d (7.5) | 7.01, d (7.5) |
| 12 | 6.43, d (2.3) | 6.13, s | 6.48, d (2.3) | 6.22, s | 6.58, br d (5.1) | 6.43, d (5.0) |
| 12a | 7.69, d (1.9) | 7.89, d (1.5) | 7.67, d (2.0) | 7.96, d (1.9) | 7.68, d (1.9) | 7.96, d (1.8) |
| 13 | 3.63, s | 3.66, s | 3.62, s | 3.66, s | 3.63, s | 3.64, s |
| 14 | 1.29, s | 1.29, s | 1.29, s | 1.29, s | 1.28, s | 1.29, s |
| 15 | 3.30, s | 3.43, s | 3.61, q (7.5) | 3.82, dq (9.5, 7.1) | ||
| 3.69, dq (9.6, 7.0) | ||||||
| 16 | 1.16, t (7.1) | 1.23, t (7.1) | ||||
| 3-OH | 4.79, s | 4.79, s | 4.78, s | 4.78, s | 4.79, s | 4.79, s |
| 12-OH | 6.88, d (7.4) | 7.23, d (5.5) |
Assignments are based on COSY, HMBC, HSQC, and NOESY experiments.
Table 5.
13C (175 MHz) NMR Data (δC, type) for 9–14 in DMSO-d6a
| position | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|
| 1 | 197.8, C | 197.7, C | 197.7, C | 197.7, C | 197.8, C | 197.8, C |
| 2 | 52.3, CH2 | 52.4, CH2 | 52.3, CH2 | 52.4, CH2 | 52.3, CH2 | 52.4, CH2 |
| 3 | 70.9, C | 70.9, C | 70.9, C | 70.9, C | 70.9, C | 71.0, C |
| 4 | 42.9, CH2 | 42.9, CH2 | 42.9, CH2 | 42.9, CH2 | 42.8, CH2 | 42.9, CH2 |
| 4a | 142.2, C | 142.0, C | 142.2, C | 141.9, C | 141.9, C | 141.8, C |
| 5 | 129.9, CH | 129.8, CH | 129.9, CH | 129.5, CH | 129.8, CH | 129.8, CH |
| 6 | 133.1, CH | 133.1, CH | 133.1, CH | 133.1, CH | 133.0, CH | 133.4, CH |
| 6a | 139.4, C | 139.6, C | 139.4, C | 139.9, C | 140.0, C | 140.3, C |
| 7 | 83.8, CH | 83.8, CH | 83.6, CH | 83.9, CH | 82.7, CH | 83.2, CH |
| 7a | 130.1, C | 129.9, C | 130.0, C | 130.1, C | 129.7, C | 129.5, C |
| 8 | 154.4, C | 154.4, C | 154.4, C | 154.3, C | 154.3, C | 154.3, C |
| 9 | 112.2, CH | 112.0, CH | 112.1, CH | 111.8, CH | 111.6, CH | 111.4, CH |
| 10 | 131.0, CH | 130.8, CH | 130.9, CH | 130.7, CH | 130.7, CH | 130.6, CH |
| 11 | 115.5, CH | 115.5, CH | 115.5, CH | 115.5, CH | 115.3, CH | 115.4, CH |
| 11a | 139.7, C | 139.7, C | 140.2, C | 139.8, C | 142.7, C | 142.0, C |
| 12 | 107.2, CH | 107.6, CH | 106.3, CH | 106.4, CH | 101.3, CH | 101.0, CH |
| 12a | 124.8, CH | 125.3, CH | 124.8, CH | 125.5, CH | 124.6, CH | 125.5, CH |
| 12b | 132.0, C | 131.9, C | 132.0, C | 132.0, C | 131.9, C | 131.7, C |
| 13 | 55.9, CH3 | 55.9, CH3 | 55.9, CH3 | 55.9, CH3 | 55.8, CH3 | 55.9, CH3 |
| 14 | 29.9, CH3 | 29.9, CH3 | 29.9, CH3 | 29.9, CH3 | 29.8, CH3 | 29.9, CH3 |
| 15 | 53.8, CH3 | 55.5, CH3 | 62.4, CH2 | 63.7, CH2 | ||
| 16 | 15.8, CH3 | 15.7, CH3 |
Assignments are based on COSY, HMBC, HSQC, and NOESY experiments.
Compound 10 had nearly identical 1H, 13C, COSY, HSQC, and HMBC NMR spectra with 9 except that the proton resonance attributed to H-12 shifted from δH 6.43 in 9 to δH 6.13 in 10 (Figure 1 and Tables 3 and 5), indicating that 10 has only a different configuration at C-12 compared with 9. In the NOESY spectrum of 10, the correlation of H3-15, instead of H-12, with H-12a established H-7 and H-12 adopted the “cis” positions on the same side of the five-membered ether ring (Figure 2). Therefore the relative configuration at C-7 and C-12 in 10 was established as S* and S*, respectively. Compounds 11 and 12 were identified as derivatives of 9 and 10, respectively, with the methoxy group at C-12 replaced by one ethoxy group based on their 1H, 13C, COSY, HSQC, HMBC, and NOESY NMR spectra (Figures 1 and 2 and Tables 3 and 5). Compounds 13 and 14 were also identified as derivatives of 9 and 10, respectively, with the methoxy group at C-12 replaced by one hydroxy group based on their 1H, 13C, COSY, HSQC, HMBC, and NOESY NMR spectra (Figures 1 and 2 and Tables 3 and 5). However, 13 and 14 undergo a rapid equilibrium, via H2O attacking at the hemiacetal group, to afford a mixture with equal amount of 13 and 14, giving rise to two sets of resonances in their NMR spectra. As 13 and 14 rapidly equilibrate in the presence of H2O, we cannot rule out the possibility of both 9/10 and 11/12 pairs as isolation artifacts of 13/14 in the presence methanol and ethanol, respectively.
Compounds 15 and 16 were identified as tetrangomycin25 and 8-O-methyltetrangomycin,18a, 18b respectively, based on the comparison of their NMR and MS data with previously published data.
Bioactivity Assays
Angucyclines and angucyclinones are known for their cytotoxicity and antibacterial activities. They are both reported to possess IC50 values between 1 µM and 100 µM against various cancer cell lines.1, 2, 8, 21 In this study, five cancer cell lines, human glioblastoma multiforme SF295, human glioblastoma SF539, human lung squamous carcinoma H226, human melanoma M14, and human breast adenocarcinoma MDA-MB-468, were chosen to test the cytotoxicities of 1–16, using mitomycin C as the positive control. Compounds 1 was active against the SF295 and H226 cell lines, and compound 15 and 16 were active against the H226, SF539, and M14 cell lines (Table 6), while the rest were inactive (IC50 > 10 µM). The potencies for 15 and 16 against the five cell lines tested in this study were similar to other cancer cell lines reported previously.22, 26, 27
Table 6.
Cytotoxicity and Antibacterial Assays for 1, 5, 15 and 16
| cytotoxicity (IC50, µM) |
antibacterial activity (MIC, µg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| SF295 | H226 | SF539 | M14 | MDA-MB-468 | S. aureus | B. subtilis | M. smegmatis | |
| 1 | 3.1±0.1 | 7.2±0.4 | 12±2 | 31±6 | 52±1 | >100 | >100 | >100 |
| 5 | >100 | >100 | >100 | >100 | >100 | >100 | 85 | 93 |
| 15 | 12±1 | 7.2±0.5 | 3.2±0.2 | 2.4±0.1 | 16±1 | 11 | 8.1 | 9.7 |
| 16 | 17±1 | 4.6±0.3 | 10±1 | 9.7±0.6 | 27±1 | >100 | 25 | >100 |
| mitomycin C | 0.54±0.01 | 0.58±0.02 | (3.8±0.3)×10−2 | 0.47±0.01 | 0.50±0.02 | N | N | N |
| tetracycline | N | N | N | N | N | 6.7 | 13 | 11 |
“N” indicates compounds not tested.
The angucycline and angucyclinone family of natural products selectively inhibit Gram-positive bacteria.2,28–31 Compounds 1–16 were tested against the Gram-positive Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 23857, and Mycobacterium smegmatis ATCC 607 and the Gram-negative Escherichia coli ATCC 25922 and E. coli JM109, using tetracycline as the positive control. As summarized in Table 5, both 15 and 16 showed moderate activity against S. aureus ATCC 25923, B. subtilis ATCC 23857, and M. smegmatis ATCC 607, with the minimum inhibitory concentration (MIC) values ranging from 8.1 to 25 µg/mL, which were similar to the reported MIC values for 15 and 16 against other S. aureus or B. subtilis species.26, 27, 32–34 Compound 5 showed weak activity against B. subtilis ATCC 23857 and M. smegmatis ATCC 607. As previously observed for known angucyclines and angucyclinones,2 1–16 showed no activity against the Gram-negative E. coli ATCC 25922 and JM109 species.
In conclusion, we isolated 14 angucyclinones and two angucyclines (1–16), including 12 new compounds (1–6, 8, and 10–14), from S. sp. CB01913, a strain selected based on chemical profiling and fermentation optimization. The new compounds featured unusual structural properties including various oxidations on rings A and C (1, 2 and 4), different sugar moieties attached to rings A and B (3 and 6), and C-ring cleavage (5 and 10–14) and expansion (8). Cytotoxicity and antibacterial assays showed that 1, 5, 15, and 16 gave moderate inhibitory activities against selected cancer cell lines and bacterial strains, respectively. The new structural features, especially C-ring cleavage and expansion disrupting the conjugated system spanning rings B, C, and D, enrich the structural diversity of angucyclines and angucyclinones, and set the stage to further evaluate the structure-activity-relationships of this family of aromatic polyketide natural products.
EXPERIMENTAL SECTION
General Experimental Procedures
Optical rotations were measured with an AUTOPOL IV automatic polarimeter (Rudolph Research Analytical). UV spectra were collected with a NanoDrop 2000C spectrophotometer (Thermo Scientific). IR spectra were collected with a Spectrum One FT-IR spectrometer (PerkinElmer). NMR data was collected on a Bruker 700 MHz/54mm Ultra Shield Magnet System. HRESIMS data was collected on a Thermo Finnigan LTQ Orbitrap mass spectrometer. MPLC separation was conducted on a Biotage Isolera One using a KP-C18-HS (30 g) column. Size exclusion chromatography was performed on Sephadex LH-20 (GE Healthcare) columns. HPLC was carried out on a Varian semipreparative HPLC system (Woburn, MA) equipped with a Prostar 330 detector, using a GRACE Apollo C18 column ( 250 mm × 4.6 mm, 5 µm) for analysis and an Alltima C18 column (250 mm × 10.0 mm, 5 µm) for purification. All fermentations were carried out in New Brunswick Scientific Innova 44 incubator shakers or New Brunswick BioFlo/celliGen 115 fermentors. Diaion HP-20 resin was purchased from Sigma-Aldrich.
Strain Isolation and Identification
Strain Streptomyces sp. CB01913 was isolated from a soil sample collected in Weishan county (location: 25°23' N, 100°33' E). The strain was purified with standard diluting plate method,35 using agar plates with medium consists of glycerol 10 g, asparagine 1 g, K2HPO4·H2O 1 g, MgSO4·7H2O 0.5 g, CaCO3 0.3 g, vitamin mixture (thiamine-HCl 0.5 mg, riboflavin 0.5 mg, niacin 0.5 mg, pyridoxine 0.5 mg, calcium pantothenate 0.5 mg, inositol 0.5 mg, 4-aminobenzoic acid 0.5 mg, and biotin 0.25 mg), and agar 15 g, in 1 L H2O, pH 7.7. The strain was preserved as spore suspension (20% glycerol, v/v) at −80 °C.
The strain CB01913 was grown on ISP4 medium at 28 °C for 7 days. The spores were harvested and cultured in TSB at 28 °C for 2 days, after which the genomic DNA was isolated following standard protocols.17 Four housekeeping genes, 16S rRNA, recA (encoding recombinase A), rpoB (encoding RNA polymerase β subunit), and trpB (encoding tryptophan synthase β subunit), were amplified by PCR, sequenced, and deposited into GenBank under accession numbers KT581419, KT581420, KT581421, and KT581422, respectively. One Taq Quick-Load 2× Master Mix (New England BioLabs Inc) was used for PCR amplification (PCR primers and PCR conditions were listed in Table S2), and QIAquick Gel Extraction Kit and QIAprep Spin Miniprep Kit (Qiagen) were used for PCR product recovery. The PCR products were sequenced and the resulting sequences were used for BLAST on the NCBI website to search for the homologous gene candidates, which were then used as the representative Streptomyces spp. in the phylogenetic tree, assigning CB01913 as a Streptomyces species (Figure S2).17
Fermentation and Isolation
S. sp. CB01913 was grown on ISP4 medium (Difco) for sporulation. After one week, the spores were harvested and 1 mL of spore suspension was added into 600 mL of seed medium (Tryptic Soy Broth) (Bacto); incubation continued on a rotary shaker with 250 rpm at 28 °C for 2 days. The resultant seed culture (600 mL) was then inoculated into 8 L production medium [dextrin (Sigma-Aldrich) 40 g, tomato paste (Plant Media) 7.5 g, N-Z-Amine A (Sigma-Aldrich) 2.5 g, primary yeast (Acros Organics) 5 g, in 1.0 L deionized H2O, pH 7.0), in a 14 L fermentor, and the fermentation continued with 250 rpm at 28 °C for 7 days. After fermentation, 400 g of Diaion HP-20 resin were added into the medium and stirred overnight. The resin and the cell mass were harvested by centrifugation, washed by deionized H2O, and extracted with MeOH. The MeOH extract was concentrated in vacuo, and the residue was loaded onto a Biotage SNAP Cartridge KP-C18-HS column (30 g) and separated by MPLC using an increasing gradient of 5% MeOH in H2O to 80% MeOH in H2O as the mobile phase. The flowrate was 20 mL/min and every 35 mL was collected as a fraction. In total, 35 fractions (m1 to m35) were collected. All 35 fractions were analyzed by HPLC. Fraction m20 was further purified by preparative HPLC using CH3CN/H2O (32/68) as the mobile phase, with UV detection at 254 nm, to afford 1 (11.7 mg) and 16 (15.1 mg). Fraction m17 was further purified by preparative HPLC using CH3CN/H2O (27/73) as the mobile phase with UV detection at 210 nm to afford 2 (3.4 mg). Fraction m8 was further purified by Sephadex LH-20 column using MeOH as the mobile phase to give four subfractions s1-s4, of which s2 was further purified by preparative HPLC using CH3CN/H2O (27/73) as the mobile phase with UV detection at 300 nm to afford 4 (0.9 mg). Fraction m14 was further purified by preparative HPLC using CH3CN/H2O (27/73) as the mobile phase with UV detection at 260 nm to afford 3 (5.1 mg) and 5 (8.7 mg). Fraction m27 was further purified by preparative HPLC using CH3CN/H2O (45/55) as the mobile phase with UV detection at 254 nm to afford 6 (7.2 mg), 7 (1.5 mg), 8 (1.6 mg), and 15 (4.5 mg). Fraction m9 was further purified by preparative HPLC using an increasing gradient of 30% CH3CN in H2O to 50% CH3CN in H2O over 20 min, then 50% CH3CN in H2O over 5 min as the mobile phase, with UV detection at 254 nm to afford 9 (7.7 mg), 10 (0.6 mg), 11 (2.6 mg), 12 (3.0 mg), 13 (3.7 mg), and 14 (3.5 mg). All preparative HPLC was carried out at a flowrate of 3 mL/min.
Compound 1
Compound 1: brown powder; [α]D27 – 133 (c 0.34, DMSO); UV (DMSO) λmax (log ε) 254 (4.13), 334 (3.66) nm; IR νmax 3441, 2933, 1667, 1595, 1470, 1271, 1236, 1124, 1072, 1022, 966, 819, 757, 723 cm−1; 1H NMR (700 MHz) data, Table 1; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 339.1226 [M + H]+ (calcd for C20H18O5, 339.1231).
Compound 2
Compound 2: yellow powder; [α]D27 – 76.7 (c 0.18, DMSO); UV (DMSO) λmax (log ε) 266 (4.26), 373 (3.52) nm; IR νmax 3663, 3390, 2972, 2901, 1744, 1700, 1670, 1589, 1472, 1443, 1406, 1394, 1382, 1265, 1230, 1065, 1056, 956, 892, 822, 719 cm−1; 1H NMR (700 MHz) data, Table 1; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 353.1020 [M + H]+ (calcd for C20H16O6, 353.1024).
Compound 3
Compound 3: green-yellow powder; [α]D27 – 43.0 (c 0.25, DMSO); UV (DMSO) λmax (log ε) 261 (4.20), 371 (3.52) nm; IR νmax 3663, 3391, 2971, 2901, 1671, 1591, 1406, 1394, 1382, 1251, 1231, 1065, 1056, 892, 880 cm−1; 1H NMR (700 MHz) data, Table 2; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 499.1590 [M + H]+ (calcd for C26H26O10, 499.1602).
Compound 4
Compound 4: colorless gum; [α]D27 – 4.2 (c 0.07, DMSO); UV (DMSO) λmax (log ε) 284 (3.79), 304 (3.72) nm; IR νmax 3277, 2937, 2838, 1645, 1591, 1480, 1437, 1416, 1310, 1266, 1177, 1112, 1092, 1012, 951, 857, 764 cm−1; 1H NMR (700 MHz) data, Table 1; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 381.1306 [M + Na]+ (calcd for C20H22O6, 381.1313).
Compound 5
Compound 5: green-yellow powder; [α]D27 – 1.12 (c 0.36, DMSO); UV (DMSO) λmax (log ε) 252 (4.20), 328 (3.68) nm; IR νmax 3663, 3440, 3290, 2980, 2902, 1778, 1676, 1607, 1586, 1489, 1454, 1405, 1394, 1298, 1266, 1056, 1020, 951, 900, 824, 780 cm−1; 1H NMR (700 MHz) data, Table 1; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 379.1161 [M + Na]+ (calcd for C20H20O6, 379.1156).
Compound 6
Compound 6: yellow powder; [α]D27 – 122 (c 0.23, DMSO); UV (DMSO) λmax (log ε) 274 (4.31), 334 (3.83) nm; IR νmax 3663, 3403, 2973, 2902, 1650, 1596, 1454, 1406, 1394, 1383, 1268, 1231, 1075, 1065, 1051, 955, 892, 807, 764 cm−1; 1H NMR (700 MHz) data, Table 2; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 467.1691 [M + H]+ (calcd for C26H26O8, 467.1704).
Compound 7
Compound 7: yellow powder; [α]D27 + 250 (c 0.07, DMSO); UV (DMSO) λmax (log ε) 261 (3.72), 326 (3.42) nm; IR νmax 3663, 2993, 2958, 2903, 1735, 1453, 1404, 1393, 1382, 1250, 1230, 1083, 1035, 956, 893, 871 cm−1; 1H NMR (700 MHz) data, Table 1; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 339.1225 [M + H]+ (calcd for C20H18O5, 339.1231).
Compound 8
Compound 8: yellow powder; [α]D27 – 310 (c 0.08, DMSO); UV (DMSO) λmax (log ε) 261 (3.78), 329 (3.46) nm; IR νmax 3675, 3375, 2972, 2902, 1677, 1614, 1598, 1485, 1469, 1436, 1407, 1380, 1276, 1242, 1076, 1047, 960, 924, 895, 820, 774 cm−1; 1H NMR (700 MHz) data, Table 1; 13C NMR (175 MHz) data, Table 4; HRESIMS m/z 339.1225 [M + H]+ (calcd for C20H18O5, 339.1231).
Compound 9
Compound 9: white powder; [α]D27 – 63.6 (c 0.35, DMSO); UV (DMSO) λmax (log ε) 252 (3.95), 297 (3.26) nm; IR νmax 3663, 2971, 2901, 1678, 1611, 1453, 1405, 1393, 1382, 1251, 1231, 1065, 1056, 957, 892, 880, 871 cm−1; 1H NMR (700 MHz) data, Table 3; 13C NMR (175 MHz) data, Table 5; HRESIMS m/z 377.1358 [M + Na]+ (calcd for C21H22O5, 377.1364).
Compound 10
Compound 10: white powder; [α]D27 + 49 (c 0.01, DMSO); UV (DMSO) λmax (log ε) 252 (3.93), 297 (3.21) nm; IR νmax 3663, 2981, 2902, 1682, 1608, 1453, 1406, 1394, 1383, 1251, 1231, 1067, 1057, 893 cm−1; 1H NMR (700 MHz) data, Table 3; 13C NMR (175 MHz) data, Table 5; HRESIMS m/z 377.1358 [M + Na]+ (calcd for C21H22O5, 377.1364).
Compound 11
Compound 11: white powder; [α]D27 – 56.5 (c 0.18, DMSO); UV (DMSO) λmax (log ε) 254 (4.00), 297 (3.33) nm; IR νmax 3661, 3414, 2974, 2901, 1683, 1611, 1486, 1439, 1406, 1377, 1319, 1270, 1230, 1190, 1076, 1068, 1053, 1004, 961, 923, 822, 777 cm−1; 1H NMR (700 MHz) data, Table 3; 13C NMR (175 MHz) data, Table 5; HRESIMS m/z 391.1513 [M + Na]+ (calcd for C22H24O5, 391.1520).
Compound 12
Compound 12: white powder; [α]D27 + 46.7 (c 0.18, DMSO); UV (DMSO) λmax (log ε) 254 (4.13), 297 (3.32) nm; IR νmax 3663, 3398, 2971, 2895, 1683, 1610, 1487, 1439, 1405, 1376, 1349, 1321, 1269, 1231, 1103, 1076, 1052, 1002, 955, 823, 780 cm−1; 1H NMR (700 MHz) data, Table 3; 13C NMR (175 MHz) data, Table 5; HRESIMS m/z 391.1512 [M + Na]+ (calcd for C22H24O5, 391.1520).
Compounds 13+14 (equal amount)
Compounds 13+14 (equal amount): white powder; [α]D27 – 9.1 (c 0.34, DMSO); UV (DMSO) λmax (log ε) 254 (3.97), 297 (3.29) nm; IR νmax 3340, 2965, 1681, 1610, 1486, 1433, 1270, 1231, 1191, 1144, 1104, 1051, 1002, 945, 923, 824, 777 cm−1; 1H NMR (700 MHz) data, Table 3; 13C NMR (175 MHz) data, Table 5; HRESIMS m/z 363.1203 [M + Na]+ (calcd for C20H20O5, 363.1207).
Compound 15
Compound 15: yellow powder; [α]D27 – 119 (c 0.32, DMSO) {[α]D20 – 85.6 (c 0.49, CHCl3)25b}; UV (DMSO) λmax (log ε) 266 (4.32), 396 (3.58) nm; IR νmax 3671, 2973, 2902, 1704, 1637, 1591, 1455, 1405, 1394, 1382, 1264, 1159, 1075, 1049, 892, 722 cm−1.
Compound 16
Compound 16: yellow powder; [α]D27 – 132 (c 0.34, DMSO) {[α]D20 – 140 (c 0.04, MeOH)18b}; UV (DMSO) λmax (log ε) 266 (4.40), 375 (3.64) nm; IR νmax 3484, 2971, 1702, 1670, 1592, 1471, 1303, 1269, 1119, 1071, 1022, 958, 820, 722 cm−1.
Determination of Sugar Absolute Configuration
To determine the absolute configuration of the α-l-rhamnose, 6 (3.0 mg) was first dissolved in 500 µL of acetone, followed by the addition of 4 mL of 2% H2SO4. The resulting solution was stirred at 90 °C for 4 h, and complete hydrolysis was confirmed by HPLC and TCL analysis. After cooling down to room temperature, the hydrolysis mixture was first extracted by 6 mL of CHCl3, and the aqueous layer was then neutralized to pH 7.0 with NaHCO3 and concentrated in vacuum to afford a white solid. The white solid was dissolved in 1 mL of H2O and subjected to the optical rotation dispersion (ORD) determination. The observed ORD value of [α]D27 +7.5 (c 0.10, H2O) agreed with the reported ORD value of [α]D25 +10.2 (c 0.20, H2O),22 thereby establishing the sugar in 6 as l-rhamnose.
Biological Assays
The S. aureus ATCC 25923, B. subtilis ATCC 23857, E. coli ATCC 25922, and M. smegmatis ATCC 607 strains, as well as the human breast adenocarcinoma MDA-MB-468 were purchased from American Type Culture Collection (ATCC). E. coli JM109 was purchased from Promega corporation. The human glioblastoma multiforme SF295, human glioblastoma SF539 and human lung squamous carcinoma H226 were purchased from the Cell Based Screening Core of TSRI Florida. The human melanoma M14 was kindly provided by Dr. Min Guo from the Department of Cancer Biology, TSRI Florida. The cytotoxicity assay was carried out using the standard protocol from the Promega website (https://www.promega.com/resources/protocols/technical-bulletins/0/celltiter-96-aqueous-one-solution-cell-proliferation-assay-system-protocol/), with the CellTiter 96 Aqueous One Solution Proliferation Assay (MTS) Kit (Promega). Cells were plated in 96-well plates at 5,000 cells/well in RPMI 1640 medium (ThermFisher) and allowed to adhere overnight at 37 °C in a humidified atmosphere of 5% CO2. Medium was then removed and replaced by fresh RPMI 1640 medium containing different concentrations of different compounds. The cells were treated for 72 h before the assay was developed. All assay values were measured in triplicate. The IC50 values were determined using GraphPad/Prism software.
The antibacterial activities were first assessed by inhibition zones using the agar diffusion method.36 Each compound (25 µL), prepared to a concentration of 2 mM in H2O, was dropped onto the filter paper on the tryptic soy agar plate. The plate was incubated at 37 °C for 12 h, after which the inhibition zones, if any, were determined, using tetracycline as the positive control. The minimum inhibitory concentration (MIC) values for compounds that showed significant inhibition zones were further determined in the 96-well plate with Müller-Hinton (MH) broth (supplemented with 20 mg Ca2+ and 10 mg Mg2+ per liter).37, 38 The tested strains were grown in MH broth to early stationary phase, diluted by MH broth to an OD625 = 0.005, then pipeted into the 96-well plate with 100 µL of broth in each well. The tested compounds with different concentrations were added into different wells, using the wells containing no compounds or containing tetracycline as negative and positive controls, respectively. The final DMSO concentration in each well was 1%, which did not affect the growth of any of the tested strains. The MIC values were determined after incubation at 37 °C for 18 h, using a plate reader at OD625.
Supplementary Material
Chart 1.
Acknowledgments
This work is supported in part by the Natural Products Library Initiative at The Scripps Research Institute, NIH grant GM086184 (to B.S.), the Chinese Ministry of Education 111 Project B08034 (to Y.D.), and National High Technology Joint Research Program of China grant 2011ZX09401-001 (to Y.D.).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Table S1 summarizes the media A, B, C, and F used for the fermentation of S. sp. CB01913. Table S2 provides the primers and PCR conditions of the four housekeeping genes for the phylogenetic analysis. Table S3 compares 1H NMR data of 7 and 8 with LS1924A23 in acetone-d6. Figure S1 shows the HPLC analysis for the fermentation of S. sp. CB01913. Figure S2 shows the phylogenetic analysis for S. sp. CB01913. Figures S3–S86 show the NMR, HRESIMS, and IR spectra of compounds 1–14. The Supporting Information is available free of charge on the ACS Publication website at DOI:.
Notes. The authors declare no competing financial interest.
REFERENCES
- 1.Kharel MK, Pahari P, Shepherd MD, Tibrewal N, Nybo SE, Shaaban KA, Rohr J. Nat. Prod. Rep. 2012;29:264–325. doi: 10.1039/c1np00068c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohr J, Thiericke R. Nat. Prod. Rep. 1992;9:103–137. doi: 10.1039/np9920900103. [DOI] [PubMed] [Google Scholar]
- 3.Kalinovskaya NI, Kalinovsky AI, Romanenko LA, Pushilin MA, Dmitrenok PS, Kuznetsova TA. Nat. Prod. Commun. 2008;3:1611–1616. [Google Scholar]
- 4.Fotso S, Mahmud T, Zabriskie TM, Santosa DA, Sulastri Proteau PJ. J. Nat. Prod. 2008;71:61–65. doi: 10.1021/np0704102. [DOI] [PubMed] [Google Scholar]
- 5.Izawa M, Kimata S, Maeda A, Kawasaki T, Hayakawa Y. J. Antibiot. 2014;67:159–162. doi: 10.1038/ja.2013.96. [DOI] [PubMed] [Google Scholar]
- 6.Rix U, Zheng J, Remsing RLL, Greenwell L, Yang K, Rohr J. J. Am. Chem. Soc. 2004;126:4496–4497. doi: 10.1021/ja031724o. [DOI] [PubMed] [Google Scholar]
- 7.Drautz H, Zahner H, Rohr J, Zeeck A. J. Antibiot. 1986;39:1657–1669. doi: 10.7164/antibiotics.39.1657. [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Yang T, Ren X, Liu J, Song Y, Sun A, Ma J, Wang B, Zhang Y, Huang C, Zhang C, Ju J. J. Nat. Prod. 2012;75:202–208. doi: 10.1021/np2008335. [DOI] [PubMed] [Google Scholar]
- 9.Balitz DM, O' Herron FA, Bush J, Vyas DM, Nettleton DE, Grulich RE, Bradner WT, Doyle TW, Arnold E, Clardy J. J. Antibiot. 1981;34:1544–1555. doi: 10.7164/antibiotics.34.1544. [DOI] [PubMed] [Google Scholar]
- 10.Gerlitz M, Udvarnoki G, Rohr J. Angew. Chem. Int. Ed. Engl. 1995;34:1617–1621. [Google Scholar]
- 11.Henkel T, Rohr J, Beale JM, Schwenen L. J. Antibiot. 1990;43:492–503. doi: 10.7164/antibiotics.43.492. [DOI] [PubMed] [Google Scholar]
- 12.Uchida T, Imoto M, Watanabe Y, Miura K, Dobashi T, Matsuda N, Sawa T, Naganawa H, Hamada M, Takeuchi T. J. Antibiot. 1985;38:1171–1181. doi: 10.7164/antibiotics.38.1171. [DOI] [PubMed] [Google Scholar]
- 13.Yu Z, Vodanovic-Jankovic S, Ledeboer N, Huang SX, Rajski SR, Kron M, Shen B. Org. Lett. 2011;13:2034–2037. doi: 10.1021/ol200420u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao LX, Huang SX, Tang SK, Jiang CL, Duan Y, Beutler JA, Henrich CJ, McMahon JB, Schmid T, Blees JS, Colburn NH, Rajski SR, Shen B. J. Nat. Prod. 2011;74:1990–1995. doi: 10.1021/np200603g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Z, Rateb ME, Smanski MJ, Peterson RM, Shen B. J. Antibiot. 2013;66:291–294. doi: 10.1038/ja.2013.1. [DOI] [PubMed] [Google Scholar]
- 16.Rateb ME, Yu Z, Yan Y, Yang D, Huang T, Vodanovic-Jankovic S, Kron MA, Shen B. J. Antibiot. 2014;67:127–132. doi: 10.1038/ja.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Xie P, Ma M, Rateb ME, Shaaban KA, Yu Z, Huang SX, Zhao LX, Zhu X, Yan Y, Peterson RM, Lohman JR, Yang D, Yin M, Rudolf JD, Jiang Y, Duan Y, Shen B. J. Nat. Prod. 2014;77:337–387. doi: 10.1021/np401063s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hindra, Huang T, Yang D, Rudolf JD, Xie P, Xie G, Teng Q, Lohman JR, Zhu X, Huang Y, Zhao L-X, Jiang Y, Duan Y, Shen B. J. Nat. Prod. 2014;77:2296–2303. doi: 10.1021/np5006168. 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Maruna M, Sturdikova M, Liptaj T, Godany A, Muckova M, Certik M, Pronayova N, Proksa B. J. Basic Microbiol. 2010;50:135–142. doi: 10.1002/jobm.200900227. [DOI] [PubMed] [Google Scholar]; (b) Gilpin ML, Balchin J, Box SJ, Tyler JW. J. Antibiot. 2006;42:627–628. doi: 10.7164/antibiotics.42.627. [DOI] [PubMed] [Google Scholar]; (c) Kesenheimer C, Groth U. Org. Lett. 2006;8:2507–2510. doi: 10.1021/ol060667b. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima S, Kojiri K, Suda H, Okanishi M. J. Antibiot. 1991;44:1061–1064. doi: 10.7164/antibiotics.44.1061. [DOI] [PubMed] [Google Scholar]
- 20.Fotso S, Mahmud T, Zabriskie M, Santosa DA, Proteau PJ. J. Antibiot. 2008;61:449–456. doi: 10.1038/ja.2008.61. [DOI] [PubMed] [Google Scholar]
- 21.Carr G, Derbyshire ER, Caldera E, Currie CR, Clardy J. J. Nat. Prod. 2012;75:1806–1809. doi: 10.1021/np300380t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo ZK, Liu SB, Jiao RH, Wang T, Tan RX, Ge HM. Bioorg. Med. Chem. Lett. 2012;22:7490–7493. doi: 10.1016/j.bmcl.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Song F, Guo H, Abdel-Mageed WM, Bai H, Dai H, Liu X, Wang J, Zhang L. J. Antibiot. 2012;65:433–435. doi: 10.1038/ja.2012.39. [DOI] [PubMed] [Google Scholar]
- 24.Xie ZP, Zhang HY, Li FC, Liu B, Yang SX, Wang HP, Pu Y, Chen Y, Qin S. Chin. Chem. Lett. 2012;23:941–944. [Google Scholar]
- 25.(a) Shaaban KA, Stamatkin C, Damodaran C, Rohr J. J. Antibiot. 2007;2007;64:141–150. doi: 10.1038/ja.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kaliappan K, Ravikumar V. J. Org. Chem. 72:6116–6126. doi: 10.1021/jo070709p. [DOI] [PubMed] [Google Scholar]
- 26.Kalyon B, Tan GYA, Pinto JM, Foo CY, Wiese J, Imhoff JF, Sussmuth RD, Sabaratnam V, Fiedler HP. J. Antibiot. 2013;66:609–616. doi: 10.1038/ja.2013.53. [DOI] [PubMed] [Google Scholar]
- 27.Guo ZK, Wang T, Guo Y, Song YC, Tan RX, Ge HM. Planta Med. 2011;77:2057–2060. doi: 10.1055/s-0031-1280097. [DOI] [PubMed] [Google Scholar]
- 28.Igarashi M, Watanabe T, Hashida T, Umekita M, Hatano M, Yanagida Y, Kino H, Kimura T, Kinoshita N, Inoue K, Sawa R, Nishimura Y, Utsumi R, Nomoto A. J. Antibiot. 2013;66:459–464. doi: 10.1038/ja.2013.33. [DOI] [PubMed] [Google Scholar]
- 29.Jakeman DL, Bandi S, Graham CL, Reid TR, Wentzell JR, Douglas SE. Antimicrob. Agents Chemother. 2009;53:1245–1247. doi: 10.1128/AAC.00801-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theriault RJ, Rasmussen RR, Kohl WL, Prokop JF, Hutch TB, Barlow GJ. J. Antibiot. 1986;39:1509–1514. doi: 10.7164/antibiotics.39.1509. [DOI] [PubMed] [Google Scholar]
- 31.Okazaki T, Kitahara T, Okami Y. J. Antibiot. 1975;28:176–184. doi: 10.7164/antibiotics.28.176. [DOI] [PubMed] [Google Scholar]
- 32.Carr G, Derbyshire ER, Caldera E, Currie CR, Clardy J. J. Nat. Prod. 2012;75:1806–1809. doi: 10.1021/np300380t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilpin ML, Balchin J, Box SJ, Tyler JW. J. Antibiot. 2012;42:627–628. doi: 10.7164/antibiotics.42.627. [DOI] [PubMed] [Google Scholar]
- 34.Sakai K, Koyama N, Fukuda T, Mori Y, Onaka H, Tomoda H. Biol. Pharm. Bull. 2012;35:48–53. doi: 10.1248/bpb.35.48. [DOI] [PubMed] [Google Scholar]
- 35.Zhang DF, Pan HQ, He J, Zhang XM, Zhang YG, Klenk HP, Hu JC, Li WJ. Int. J. Syst. Evol. Microbiol. 2013;63:4447–4455. doi: 10.1099/ijs.0.052704-0. [DOI] [PubMed] [Google Scholar]
- 36.Thornsberry C, Barry AL, Jones RN, Baker CN, Badal RE. J. Clin. Microbiol. 1982;15:769–776. doi: 10.1128/jcm.15.5.769-776.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rateb ME, Houssen WE, Harrison WT, Deng H, Okoro CK, Asenjo JA, Andrews BA, Bull AT, Goodfellow M, Ebel R, Jaspars M. J. Nat. Prod. 2011;74:1965–1971. doi: 10.1021/np200470u. [DOI] [PubMed] [Google Scholar]
- 38.Wiegand I, Hilpert K, Hancock REW. Nat. Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.