Abstract
Objective: The latest nonnucleoside reverse transcriptase inhibitor (NNRTI) rilpivirine (RPV) is indicated for human immunodeficiency virus type-1 (HIV-1) patients initiating antiretroviral treatment, but the extent of genotypic RPV resistance in treatment-naive patients outside clinical trials is poorly defined.
Study Design: This retrospective observational study of clinical data from Belgium and Portugal evaluates genotypic information from HIV-1 drug-naive patients obtained for the purpose of drug resistance testing. Rilpivirine resistance-associated mutations (RPV-RAMs) were defined based on clinical trials, phenotypic studies, and expert-based resistance algorithms. Viral susceptibility to RPV alone and to the single-tablet regimen was estimated using expert-based resistance algorithms.
Results: In 4,631 HIV-1 treatment-naive patients infected with diverse HIV-1 subtypes, major RPV-RAMs were detected in 4.6%, while complete viral susceptibility to RPV was estimated in 95% of patients. Subtype C- and F1-infected patients displayed the highest levels of reduced viral susceptibility at baseline, respectively 13.2% and 9.3%, mainly due to subtype- and geographic-dependent occurrence of RPV-RAMs E138A and A98G as natural polymorphisms. Strikingly, a founder effect in Portugal resulted in a 138A prevalence of 13.2% in local subtype C-infected treatment-naive patients. The presence of transmitted drug resistance did not impact our estimates.
Conclusion: RPV is the first HIV-1 inhibitor for which, in the absence of transmitted drug resistance, intermediate or high-level genotypic resistance can be detected in treatment-naive patients. The extent of RPV susceptibility in treatment-naive patients differs depending on the HIV-1 subtype and dynamics of local compartmentalized epidemics. The highest prevalence of reduced susceptibility was found to be 15.7% in Portuguese subtype C-infected treatment-naive patients. In this context, even in the absence of transmitted HIV-1 drug resistance (TDR), drug resistance testing at baseline should be considered extremely important before starting treatment with this NNRTI.
Introduction
Rilpivirine (RPV) is the latest nonnucleoside reverse transcriptase (RT) inhibitor (NNRTI) approved for antiretroviral treatment (ART) of human immunodeficiency virus type-1 (HIV-1) infection. RPV is currently indicated for treatment-naive patients with a viral load lower than 100,000 copies/mL,1 and predominantly administered first-line as a single-tablet regimen (STR) with a fixed-dose coformulation containing nucleos(t)ide RT inhibitors (NRTIs) tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC).
While the phase 3 clinical studies ECHO and THRIVE demonstrated noninferior virological efficacy and safety of RPV compared to efavirenz,2,3 these clinical studies precluded HIV-1 patients showing NNRTI mutations at baseline. Although a low prevalence of RPV resistance-associated mutations (RAMs) was described outside these clinical trials, RPV-RAMs have been reported to occur naturally as polymorphisms, indicating an impact of HIV-1 subtype on the RPV activity.4–6 In this study, we evaluate the genotypic resistance profile of RPV in a large database with HIV-1 genetic sequences of treatment-naive patients infected with different subtypes. Viral susceptibility predictions were used to better understand the clinical benefit of RPV for first-line HIV-1 treatment.
Materials and Methods
Clinical data of HIV-1 treatment-naive patients pooled from a large HIV-1 drug resistance database in Portugal (n = 4,541) and from the University Hospitals Leuven in Belgium (n = 777), obtained for the purpose of routine genotypic resistance testing before cART initiation, were retrospectively examined.7,8 The study protocol was conducted in accordance with the Declaration of Helsinki and approved by the ethics committees of UZ Leuven (B322201420270/S56109) and Centro Hospitalar de Lisboa Ocidental (108/CES-2014). For each patient, the first available viral isolate spanning the RT region was collected. HIV-1 subtype was assigned by Rega V39 and COMET10 subtyping tools, and only the most prevalent subtypes concordantly determined by both tools were retained for the final analysis, thereby excluding 687 patients. As a result, the analysis was performed on 4,631 patients (715 from Belgium and 3,916 from Portugal).
Major RPV resistance-associated mutations (RPV-RAMs) were defined based on data from the clinical trials, phenotypic RPV resistance analyses, and package inserts: K101E/P, E138A/G/K/Q/R, V179L, Y181C/I/V, Y188L, H221Y, F227C, M230I/L, and the mutational combination of L100I+K103N.1,11,12 In addition, we defined a list of minor RPV-RAMs that have been observed in in vitro or in vivo selection studies and are included in one or more of clinically widely used genotypic resistance interpretation algorithms ANRS (V24), Rega (V9.1.0), and HIVdb (V7.0.1),1–16 encompassing V90I, A98G, L100I/V, K101H/Q/T, K103R/S, V106A/I, V108I, E138S, V179D/E/F/I/T, Y181F/G/S, Y188F, V189I, G190A/C/E/Q/S/T/V, and M230V.
Clinical implications of observed RPV genotypic resistance were assessed by classifying viral isolates as susceptible, intermediate resistant, or high-level resistant according to Rega and HIVdb, and susceptible or resistant according to ANRS. For HIVdb, five-level scores were simplified to three levels: susceptible and potential low-level resistant were scored as susceptible, low-level and intermediate resistant were scored as intermediate resistant, and high-level resistant as resistant. An estimate of preserved viral susceptibility was obtained by averaging over the three algorithms. The activity of the RPV-containing STR was also assessed by estimating viral susceptibility to NRTIs TDF and FTC.
Evidence of transmitted HIV-1 drug resistance (TDR) was defined by the presence of at least one surveillance drug resistance mutation (SDRM) from the consensus genotypic definition of Bennett et al.,17 including major RVP-RAMs L100I+K103N, Y181C/I/V, Y188L, and M230L, and minor RPV-RAMs L100I, K103S, V106A, V179F, and G190A/E/S.
The origin of E138A in subtype C-infected treatment-naive patients was further investigated by phylogenetic analysis and transmission ratio calculation. Specifically, control sequences were selected using two different approaches. First, a BLAST search was performed to identify the 10 most similar sequences to each subtype C-infected treatment-naive patient included in this study, and their treatment status was retrieved from the original publications. Second, sequence data from treatment-experienced patients infected with subtype C were collected from the Portuguese and Leuven cohorts. HIV-1 subtype was confirmed as described above and duplicate or clonal sequences were removed, resulting in a dataset of 1,121 sequences. SDRM-related positions as well as the E138 codon were excluded from the alignment. For phylogenetic analysis, two separate approaches were used. First, a maximum likelihood tree was built using RaxML, including all 1,121 sequences in the analysis. Second, to evaluate the reproducibility of a monophyletic cluster of sequences containing E138A, 20 maximum likelihood trees with each including 200 sequences randomly selected from this large dataset were constructed using PhyML.18 The transmission ratio of E138A was calculated by dividing its prevalence in treatment-naive patients by the prevalence in treatment-experienced patients and interpreted following Winand et al.19
Data were analyzed using the package R, with a level of significance set at 5% and Benjamini–Hochberg correction for multiple testing.20
Results
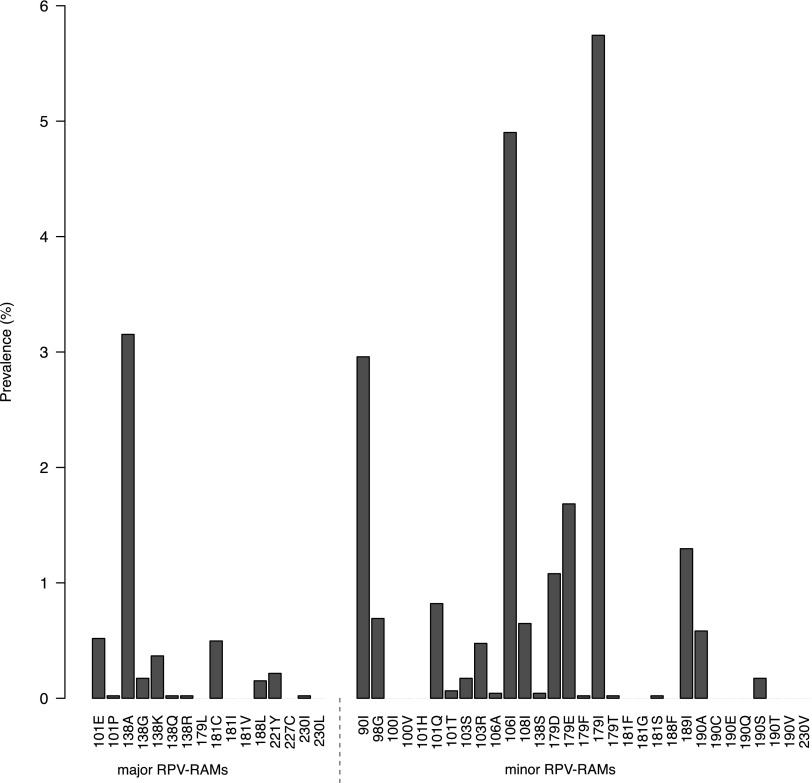
Genotypic information from 4,631 treatment-naive patients revealed one or more major RPV-RAMs in 4.6% (n = 213) of patients, predominantly as a single mutation (4.1%, n = 189), but also within a combination of two (0.5%, n = 22) or three major mutations (0.04%, n = 2). Furthermore, at least one minor RPV-RAM was present in 18.4% (n = 851) of patients, with one (16.4%, n = 761), two (1.4%, n = 64), and three, or more minor mutations (0.6%, n = 26). In total, 21.5% (n = 997) of the patients presented at least one major and/or minor RPV mutation. Figure 1 shows the prevalence of individual RPV-RAMs. The most prevalent major RPV-RAM was E138A (3.2%, n = 146), occurring in 69% of all patients with ≥1 major RPV-RAM, followed by K101E (0.5%, n = 24). Minor RPV-RAMs V179I (5.7%, n = 266), V106I (4.9%, n = 227), and V90I (3.0%, n = 137) occurred most. Major signature NRTI mutations against TDF and FTC M184V (0.8%, n = 35), M184I (0.07%, n = 3), K65R (0.07%, n = 3), and K70E (0.04%, n = 2) were rarely observed.
FIG. 1.
Prevalence of individual major (left) and minor (right) rilpivirine resistance-associated mutations (RPV-RAMs).
When averaged over the three interpretation systems, viral susceptibility to the STR and RPV was predicted as preserved in 94.0% ± 1.0% and 95.0% ± 0.7% of the patients, respectively, indicating that loss in viral susceptibility to the STR was largely attributable to RPV resistance.
An impact of HIV-1 natural diversity was evaluated by comparing the distribution of RPV-RAMs and the predicted activity across HIV-1 subtypes and CRFs (Table 1). Patients were predominantly infected with HIV-1 subtype B (n = 2,110, 45.6%) and subtype G (n = 1,345, 29.0%), followed by CRF 02_AG (n = 451, 9.7%), subtype C (n = 345, 7.5%), A1 (n = 194, 4.2%), F1 (n = 132, 2.9%), and CRF 01_AE (n = 54, 1.2%). The prevalence of ≥1 major or ≥1 minor RPV-RAMs was significantly different across subtypes (chi-square test, p < .001). The highest prevalence of major RPV-RAMs was observed for subtypes C (12.5%) and F1 (6.8%), largely explained by the subtype-specific occurrence of E138A with, respectively, 11.3% and 6.1% (Table 1). A variable prevalence of minor RPV-RAMs was observed, with minor RPV-RAMs V90I, A98G, V106I, and V179D/E/I/T differing significantly across subtypes, when corrected for multiple testing. A discrepancy in the proportion of patients scored susceptible to RPV was also observed (Table 1). Viral susceptibility was particularly reduced in patients infected with subtypes C or F1, respectively, only 86.8% ± 0.8% and 90.7% ± 4.4% of patients scored susceptible to RPV.
Table 1.
Frequency of Rilpivirine Mutations Across Subtypes
| n | % | A1 | B | C | F1 | G | 01_AE | 02_AG | |
|---|---|---|---|---|---|---|---|---|---|
| Patients | |||||||||
| All | 4,631 | 100 | 4.2 | 45.6 | 7.5 | 2.9 | 29.0 | 1.2 | 9.7 |
| Portugal | 3,916 | 100 | 3.5 | 43.6 | 6.8 | 2.9 | 33.9 | 0.1 | 9.3 |
| Belgium | 715 | 100 | 7.8 | 56.8 | 11.2 | 2.7 | 2.2 | 7.1 | 12.2 |
| ≥1 major | |||||||||
| All | 213 | 4.6 | 3.1 | 4.4 | 12.5 | 6.8 | 3.1 | 1.9 | 4.2 |
| Portugal | 187 | 4.8 | 3.6 | 4.4 | 14.7 | 8.0 | 3.2 | 0.0 | 4.7 |
| Belgium | 26 | 3.6 | 1.8 | 4.4 | 5.0 | 0.0 | 0.0 | 2.3 | 2.0 |
| E138A | |||||||||
| All | 146 | 3.2 | 3.1 | 2.8 | 11.3 | 6.1 | 1.8 | 1.8 | 1.8 |
| Portugal | 124 | 3.2 | 3.6 | 2.6 | 13.2 | 7.1 | 1.8 | 0.0 | 2.2 |
| Belgium | 22 | 3.1 | 1.8 | 3.9 | 5.0 | 0.0 | 0.0 | 2.0 | 0.0 |
| ≥1 minor | |||||||||
| All | 851 | 18.4 | 62.4 | 20.1 | 8.4 | 29.5 | 10.6 | 33.3 | 1.8 |
| Portugal | 686 | 17.5 | 63.0 | 20.2 | 10.5 | 25.6 | 10.3 | 66.7 | 15.9 |
| Belgium | 165 | 23.0 | 60.7 | 19.4 | 1.3 | 52.6 | 37.5 | 31.3 | 21.8 |
| V90I | |||||||||
| All | 137 | 3.0 | 1.5 | 4.1 | 0.3 | 1.5 | 0.8 | 0.0 | 7.3 |
| Portugal | 101 | 2.6 | 1.5 | 3.8 | 0.4 | 1.8 | 0.7 | 0.0 | 6.0 |
| Belgium | 36 | 5.0 | 1.8 | 5.4 | 0.0 | 0.0 | 12.5 | 0.0 | 12.6 |
| A98G | |||||||||
| All | 32 | 0.7 | 0.5 | 0.6 | 1.7 | 7.6 | 0.1 | 0.0 | 0.4 |
| Portugal | 22 | 0.6 | 0.7 | 0.7 | 2.3 | 0.0 | 0.1 | 0.0 | 0.5 |
| Belgium | 10 | 1.4 | 0.0 | 0.0 | 0.0 | 52.6 | 0.0 | 0.0 | 0.0 |
| V106I | |||||||||
| All | 227 | 4.9 | 1.0 | 7.6 | 0.0 | 5.3 | 3.3 | 7.4 | 1.8 |
| Portugal | 207 | 5.3 | 0.0 | 8.6 | 0.0 | 6.2 | 3.4 | 0.0 | 1.9 |
| Belgium | 20 | 2.8 | 0.0 | 3.7 | 0.0 | 0.0 | 0.0 | 7.8 | 1.1 |
| V179I | |||||||||
| All | 266 | 5.7 | 59.3 | 4.0 | 3.2 | 13.6 | 0.7 | 18.5 | 4.0 |
| Portugal | 192 | 4.9 | 60.1 | 3.5 | 4.2 | 15.9 | 0.7 | 33.4 | 3.0 |
| Belgium | 74 | 10.4 | 57.1 | 6.4 | 0.0 | 0.0 | 0.0 | 17.6 | 8.0 |
| V179D | |||||||||
| All | 50 | 1.1 | 1.0 | 1.9 | 0.6 | 2.3 | 0.1 | 3.7 | 0.0 |
| Portugal | 39 | 1.0 | 0.7 | 1.9 | 0.8 | 2.7 | 0.0 | 0.0 | 0.0 |
| Belgium | 11 | 1.5 | 1.8 | 1.7 | 0.0 | 0.0 | 6.3 | 3.9 | 0.0 |
| V179E | |||||||||
| All | 78 | 1.7 | 0.5 | 1.0 | 0.3 | 0 | 3.6 | 1.9 | 1.1 |
| Portugal | 75 | 1.9 | 0.7 | 1.1 | 0.4 | 0.0 | 3.7 | 0.0 | 1.4 |
| Belgium | 3 | 0.4 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 |
| V179T | |||||||||
| All | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.8 | 0.0 | 0.0 | 0.0 |
| Portugal | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 0.0 | 0.0 | 0.0 |
| Belgium | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Active RPV | |||||||||
| Mean ± SD | |||||||||
| All | — | 95.0 ± 0.7 | 96.6 ± 0.6 | 95.1 ± 0.7 | 86.8 ± 0.8 | 90.7 ± 4.4 | 96.8 ± 0.2 | 98.1 ± 0.0 | 95.4 ± 0.3 |
| Portugal | — | 94.8 ± 0.5 | 96.1 ± 0.4 | 95.1 ± 0.8 | 84.3 ± 1.1 | 92.0 ± 0.0 | 96.7 ± 0.2 | 100 ± 0.0 | 94.9 ± 0.3 |
| Belgium | — | 95.7 ± 1.0 | 97.6 ± 1.0 | 95.4 ± 0.3 | 95.0 ± 0.0 | 82.5 ± 30.1 | 100 ± 0.0 | 98.0 ± 0.0 | 97.7 ± 0.0 |
| ANRS | |||||||||
| All | 4,416 | 95.4 | 96.9 | 95.6 | 87.2 | 93.2 | 96.9 | 98.1 | 95.6 |
| Portugal | 3,727 | 95.2 | 96.4 | 95.6 | 84.9 | 92.0 | 96.8 | 100 | 95.1 |
| Belgium | 689 | 96.3 | 98.2 | 95.6 | 95.0 | 100 | 100 | 98.0 | 97.7 |
| HIVdb | |||||||||
| All | 4,366 | 94.3 | 95.8 | 94.3 | 85.8 | 85.6 | 96.6 | 98.1 | 95.1 |
| Portugal | 3,690 | 94.2 | 95.7 | 94.1 | 83.0 | 92.0 | 96.6 | 100 | 94.5 |
| Belgium | 676 | 94.5 | 96.4 | 95.1 | 95.1 | 47.4 | 100 | 98.0 | 97.7 |
| Rega | |||||||||
| All | 4,413 | 95.3 | 96.9 | 95.5 | 87.2 | 93.2 | 96.9 | 98.1 | 95.6 |
| Portugal | 3,724 | 95.1 | 96.4 | 95.4 | 84.9 | 92.0 | 96.8 | 100 | 95.1 |
| Belgium | 689 | 96.4 | 98.2 | 95.6 | 95.0 | 100 | 100 | 98.0 | 97.7 |
The distribution of HIV-1 subtypes among the 4,631 treatment-naive patients is indicated, with for each subtype, the proportion (%) of patients displaying ≥1 major RPV-RAMs, ≥1 minor RPV-RAMs, single RPV-RAMs, and predicted activity to RPV.
Single mutations were only listed when their prevalence significantly differed across subtypes. Estimated drug activity is shown for each individual genotypic resistance interpretation algorithm (ANRS V24, Rega V9.1.0 and HIVdb V7.0.1) and the mean of estimated proportion of each resistance interpretation algorithm together with the SD.
HIV-1, human immunodeficiency virus type-1; RPV-RAMs, rilpivirine resistance-associated mutations; SD, standard deviation.
RPV susceptibility in subtype C-infected patients differed according to geographic origin of sampling, with 84.3% ± 1.1% of patients from Portugal who scored susceptible compared to 95.0% ± 0.0% of patients from Belgium (Table 1), which was largely explained by a prevalence of RPV-RAM E138A of, respectively, 13.2% and 5.0% (p = .044). Subtype F1-infected patients displayed a lower proportion that was estimated susceptible (90.7%) together with high between-algorithm variability (4.4%). A higher prevalence of E138A was also observed in subtype F1-infected patients from Portugal (7.1%), explaining that low proportion of patients scored susceptible (92.0% ± 0.0%). Subtype F1-infected patients from Belgium however lacked E138A, but they still displayed a lower average susceptibility score (82.5% ± 30.1%) mainly attributable to the minor RVP-RAM A98G (52.6%). This minor RPV-RAM is however only scored by the HIVdb algorithm, but not by ANRS or Rega, with only 47.4% of patients estimated susceptible by HIVdb.
Evidence of transmitted drug resistance was observed in 401 patients (8.7%), with RPV-RAMs Y181C, Y188L, K103S, V106A, V179F, and G190A/S detected as SDRMs. When excluding these patients, one or more major RPV-RAMs could still be detected in 3.4% of the remaining 4,230 study patients and at least one minor RPV-RAM in 16.6%, while preserved susceptibility to RPV remained in 96.0% ± 1.0% of patients. The frequency of major RPV-RAM E138A in this population was 3.1% compared to 4.2% in the patient population with SDRMs, although strongly varying according to geography and subtype (Table 2). Strikingly, among 116 subtype C-infected treatment-experienced patients from Portugal, the RPV-RAM E138A could be detected in 11 patients (9.5%), compared to 35 treatment-naive patients (13.2%), resulting into a transmission ratio of 1.39.
Table 2.
Frequency of E138A and Transmitted Drug Resistance
| Belgium (n = 715) | Portugal (n = 3,916) | |||||
|---|---|---|---|---|---|---|
| E138A (%) | E138A (%) | |||||
| Subtype | TDR (%) | WT | TDR | TDR (%) | WT | TDR |
| All | 9.9 | 3.3 | 1.4 | 8.4 | 3.0 | 4.9 |
| A1 | 1.8 | 1.8 | 0 | 3.6 | 3.8 | 0.0 |
| B | 14.5 | 4.3 | 1.7 | 10.9 | 2.8 | 0.6 |
| C | 3.8 | 5.2 | 0.0 | 6.0 | 11.7 | 37.5 |
| F1 | 5.3 | 0.0 | 0.0 | 5.3 | 7.5 | 0.0 |
| G | 6.3 | 0.0 | 0.0 | 6.9 | 1.3 | 8.8 |
| AE | 3.9 | 2.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| AG | 4.6 | 0.0 | 0.0 | 7.1 | 2.1 | 3.9 |
The frequency of TDR and of RPV-RAM E138A in patients with (TDR) or without SDRMs (WT), according to subtype and geographical origin of the patient (Belgium–Leuven Cohort, Portugal, Portuguese Cohort).
SDRM, surveillance drug resistance mutation; TDR, transmitted HIV-1 drug resistance; WT, wild-type.
To investigate whether a founder effect contributed to the propagation of this polymorphism, a set of 1,121 subtype C sequences was compiled, which included the most similar subtype C sequences, determined as described in the Materials and Methods section. Phylogenetic analysis of the 1,121 subtype C-infected patient population revealed that a large number of Portuguese treatment-naive patients with E138A formed a monophyletic cluster (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid). Since this cluster was not supported by a large bootstrap support, we used subsampling to evaluate the reproducibility of this cluster. Twenty separate phylogenetic analyses, each containing a subsample of 200 patients of the initial dataset, demonstrated a consistent monophyletic grouping of this E138A cluster among all 20 ML trees (100%), with an aLRT support for this cluster above 0.8 in 16 trees (80%) (Data not shown).
Discussion
The latest NNRTI RPV is an attractive first-line option for treatment of HIV-1 infection due to its favorable tolerability and dosing, but its use should be guided by resistance testing at baseline as for other antiviral drugs, especially NNRTIs.1,21 Using a large population of treatment-naive HIV-1 patients, we report reduced RPV susceptibility at baseline estimated in ∼5% of HIV-1 treatment-naive patients. However, variable prevalence of RPV-RAMs was detected when HIV-1 subtypes were evaluated separately, with subtypes C and F1 showing a higher prevalence of reduced baseline susceptibility to RPV.
Specifically, subtype C treatment-naive patients in our datasets displayed an increased prevalence of RPV-RAM E138A, which when present alone confers intermediate or high-level resistance by all three algorithms. This natural polymorphism is known to occur in varying frequencies according to viral subtype and is previously shown to have a higher prevalence in subtype C patients compared to subtype B patients.6 In our study, subtype F1 strains circulating in the Portuguese HIV-1 epidemic also showed an increased prevalence of this polymorphism, while subtype F1 patients in Belgium displayed an increased prevalence of mutation A98G. Importantly, the RPV-RAM A98G is only assigned an intermediate resistance score for RPV by the HIVdb algorithm and is not considered by the ANRS and Rega algorithms.
The findings of our study are in large agreement with a similar study by Lambert-Niclot et al. that observed a prevalence of 4.6% for major RPV-RAMs and 19.9% for any RPV mutation in 1,729 drug-naive patients, of which 4.9% were scored resistant by ANRS.4 They also reported a higher prevalence of RPV genotypic resistance in pooled non-B subtype-infected patients compared to subtype B-infected patients, without a stratification by subtypes, however. Furthermore, trends in RPV-RAMs prevalence across subtypes observed in our study were highly comparable with mutation frequencies according to subtype reported by the Stanford HIV Drug Resistance Database.22
Importantly, our study shows that the prevalence of E138A and A98G is highly determined by geographical compartmentalization of subtype epidemics, with a higher prevalence of E138A in subtype C and F1 patients from Portugal and a higher prevalence of A98G in subtype F1 patients from Belgium. These observations suggest founder effects boosting local transmission of these mutations, an HLA impact for the selection of these mutations in each population, and/or differences in selective pressure caused by different treatment strategies between countries.23
Phylogenetic analysis of subtype C sequences supported the hypothesis that a founder effect in the Portuguese treatment-naive patients explains the high prevalence of E138A in Portugal. Furthermore, a transmission ratio of 1.39 was detected for E138A, indicating that high levels of transmission between treatment-naive patients contribute to its higher prevalence. This finding suggests that the origin of E138A in Portugal is most probably not the treated population and confirms its forward transmission among treatment-naive patients.19 Despite its reported selection by ART, the E138A mutation is not included in the surveillance mutation list of Bennett et al. due to the polymorphic nature of RT position 138.17 A prevalent E138A in treatment-naive patients could have accompanied transmitted DRMs, however, our results strongly suggest that the reduced viral susceptibility to RPV and in particular the increased E138A prevalence in a geographic and subtype-dependent manner resulted from the propagation of a natural polymorphism following a founder event.
Regarding the origin of an increased E138A presence in treatment-naive patients, a distinction between original transmission from treated patients and increased prevalence of a natural polymorphism after a founder effect can have important implications for the treatment of HIV-1 infected patients. If E138A predominantly results from transmission from treated patients, its high prevalence would largely coincide with regions with substantial ART coverage and could be addressed by targeted intervention strategies or optimized treatment policies in addition to resistance testing that is usually recommended before ART initiation in these regions.
This study, however, finds that natural variability and founder effects can boost the natural occurrence of E138A, suggesting that even regions with low ART coverage can display a high prevalence of RPV resistance, with potentially devastating implications when RPV-based first-line treatment is initiated in the absence of routine resistance testing of treatment-naive patients.1 In addition to supporting large-scale analyses of RPV genotypic resistance in treatment-naive patients,22 the significance of our findings should also be further elucidated in localized epidemics characterized by similar and other subtypes.
In this study, we show a high prevalence of reduced susceptibility to RPV at baseline in all subtypes, but with a significant variability both between HIV-1 subtypes and between local epidemics. These findings have an important impact for the use of RPV as first-line treatment. Loss of RPV activity directly affects the success of this low genetic barrier drug combination, but also limits future treatment options due to extensive cross-resistance to other NNRTIs and most likely also to other NRTIs upon failure of this regimen. In the context of such a high prevalence of reduced susceptibility to RPV at baseline, even in patients without TDR, drug resistance testing at baseline should be considered extremely important before starting treatment with this NNRTI.
Furthermore, the fact that the prevalence of RPV-RAMs differed between local subtype epidemics indicates a geographical compartmentalization of such variants that should be further investigated and monitored. Finally, the finding of discrepancies between drug resistance algorithms indicates the need for a better consensus on the impact of RPV-RAMs. In summary, our results indicate that drug resistance to RPV should be more thoroughly investigated. Until then, drug resistance testing at baseline is crucial for patients initiating therapy with an RPV containing regimen.
Supplementary Material
Acknowledgments
This work was supported by the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G.0692.14N) and by the AIDS Reference Laboratory of Leuven that receives support from the Belgian Ministry of Social Affairs through a fund within the Health Insurance System. KT is funded as a Postdoctoral Researcher by the Research Foundation - Flanders (FWO). Guy Baele acknowledges funding from the European Research Council under the European Community's Seventh Framework Programme (FP7/2007–2013) under Grant Agreement no. 278433-PREDEMICS and ERC Grant agreement no. 260864. The computational resources and services used in this work were provided by the Hercules Foundation and the Flemish Government–department EWI-FWO Krediet aan Navorsers (Theys, KAN2012 1.5.249.12.). This work was partly supported by BEST HOPE: Bio-Molecular and Epidemiological Surveillance of HIV Transmitted Drug Resistance, Hepatitis Co-Infections, and Ongoing Transmission Patterns in Europe (project funded through HIVERA: Harmonizing Integrating Vitalizing European Research on HIV/Aids, grant 249697), and by L'Oréal Portugal Medals of Honor for Women in Science 2012 (financed through L'Oréal Portugal, Comissão Nacional da Unesco and Fundação para a Ciência e Tecnologia).
Sequence Data
We acknowledge the ARHR policy about data availability. We have not provided the accession numbers for the dataset because these sequences are not deposited in GenBank as they comprise a dense sampling of one region. They have been deposited in Euresist (www.euresist.org), a project aiming to collect and make available data to study HIV drug resistance and viral diversity in Europe. Access to the sequences linked to all clinical and demographic data is available through Euresist.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.EDURANT® (rilpivirine) package insert. Available at www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002264/WC500118874.pdf, accessed March12, 2014
- 2.Molina J-M, Cahn P, Grinsztejn B, et al. : For ECHO Study Group: Rilpivirine versus efavirenz with tenofovir and emtricitabine in treatment-naive adults infected with HIV-1 (ECHO): A phase 3 randomised double-blind active-controlled trial. Lancet 2011;378:238–246 [DOI] [PubMed] [Google Scholar]
- 3.Cohen CJ, Andrade-Villanueva J, Clotet B, et al. : For THRIVE Study Group: Rilpivirine versus efavirenz with two background nucleoside or nucleotide reverse transcriptase inhibitors in treatment-naive adults infected with HIV (THRIVE): A phase 3, randomised, non-inferiority trial. Lancet 2011;378:229–237 [DOI] [PubMed] [Google Scholar]
- 4.Lambert-Niclot S, Charpentier C, Storto A, et al. : Prevalence of pre-existing resistance-associated mutations to rilpivirine, emtricitabine and tenofovir in antiretroviral-naive patients infected with B and non-B subtype HIV-1 viruses. J Antimicrob Chemother 2013;68:1237–1242 [DOI] [PubMed] [Google Scholar]
- 5.Sungkanuparph S, Jiamsakul A, Kiertiburanakul S, et al. : TREAT Asia studies to evaluate resistance: Rilpivirine resistance-associated mutations among antiretroviral-naive patients infected with HIV-1 in Asia. J Acquir Immune Defic Syndr 2013;62:e98–e100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sluis-Cremer N, Jordan MR, Huber K, et al. : E138A in HIV-1 reverse transcriptase is more common in subtype C than B: Implications for rilpivirine use in resource-limited settings. Antiviral Res 2014;107:31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theys K, Vercauteren J, Abecasis AB, et al. : The rise and fall of K65R in a Portuguese HIV-1 Drug Resistance database, despite continuously increasing use of tenofovir. Infect Genet Evol 2009;9:683–688 [DOI] [PubMed] [Google Scholar]
- 8.Libin P, Beheydt G, Deforche K, et al. : RegaDB: Community-driven data management and analysis for infectious diseases. Bioinformatics 2013;29:1477–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pineda-Peña AC, Faria NR, Imbrechts S, et al. : Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: Performance evaluation of the new REGA version 3 and seven other tools. Infect Genet Evol 2013;19:337–348 [DOI] [PubMed] [Google Scholar]
- 10.Struck D, Lawyer G, Ternes AM, Schmit JC. and Bercoff DP: COMET: Adaptive context-based modeling for ultrafast HIV-1 subtype identification. Nucleic Acids Res 2014;18:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vingerhoets J, Rimsky L, Van Eygen V, et al. : Pre-existing mutations in the rilpivirine Phase III trials ECHO and THRIVE: Prevalence and impact on virologic response. Antivir Ther 2013;18:253–256 [DOI] [PubMed] [Google Scholar]
- 12.Rimsky L, Vingerhoets J, Van Eygen V, et al. : Genotypic and phenotypic characterization of HIV-1 isolates obtained from patients on rilpivirine therapy experiencing virologic failure in the phase 3 ECHO and THRIVE studies: 48-week analysis. J Acquir Immune Defic Syndr 2012;59:39–46 [DOI] [PubMed] [Google Scholar]
- 13.Azijn H, Tirry I, Vingerhoets J, et al. : TMC278, a next-generation nonnucleoside reverse transcriptase inhibitor (NNRTI), active against wild-type and NNRTI-resistant HIV-1. Antimicrob Agents Chemother 2010;54:718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meynard JL, Vray M, Morand-Joubert L, et al. : Phenotypic or genotypic resistance testing for choosing antiretroviral therapy after treatment failure: A randomized trial. AIDS 2002;16:727–736 [DOI] [PubMed] [Google Scholar]
- 15.Liu TF, Shafer RW: Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006;42:1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vercauteren J, Beheydt G, Prosperi M, et al. : Clinical evaluation of Rega 8: An updated genotypic interpretation system that significantly predicts HIV-therapy response. PLoS One 2013;8:e61436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF: Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther 2008;2:25–36 [PubMed] [Google Scholar]
- 18.Guindon S, Dufayard JF, Lefort V, et al. : New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst Biol 2010;59:307–321 [DOI] [PubMed] [Google Scholar]
- 19.Winand R, Theys K, Eusebio M, et al. : Assessing transmissibility of HIV-1 drug resistance mutations from treated and from drug-naive individuals. AIDS 2015;29:2045–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Development Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2011 [Google Scholar]
- 21.Wittkop L, Günthard HF, de Wolf F, et al. : Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European Multicohort Study. Lancet Infect Dis 2011;5:363–371 [DOI] [PubMed] [Google Scholar]
- 22.Rhee SY, Kantor R, Katzenstein DA, et al. : HIV-1 pol mutation frequency by subtype and treatment experience: Extension of the HIVseq program to seven non- B subtypes. AIDS 2006;20:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gatanaga H, Murakoshi H, Hachiya A, et al. : Naturally selected rilpivirine-resistant HIV-1 variants by host cellular immunity. Clin Infect Dis 2013;7:1051–1055 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.