Abstract
Chronic hepatitis C virus (HCV) infection is associated with lower serum concentration of low-density lipoprotein (LDL-C), the primary cholesterol metabolite targeted pharmaceutically to modulate cardiovascular risk. Chronic infection with human immunodeficiency virus (HIV) and treatment with antiretrovirals (ARVs) are associated with dyslipidemia and increased risk of cardiovascular disease. In subjects coinfected with HIV and HCV, lipid abnormalities associated with either infection alone are often attenuated. Treatment of chronic HCV infection in HIV/HCV-coinfected subjects is now possible with interferon (IFN)-free regimens composed of directly acting antivirals (DAAs). We previously observed a marked increase in serum LDL-C in HCV-monoinfected subjects treated with sofosbuvir and ribavirin (SOF/RBV) that correlated with viral decline in serum, suggesting a direct influence of HCV clearance on serum cholesterol. In the present study, we assessed longitudinal changes in cholesterol in HIV/HCV-coinfected subjects during treatment of HCV genotype-1 (GT1) infection with combination DAA therapy. We report a rapid increase in LDL-C and LDL particle size by week 2 of treatment that was sustained during and after treatment in HIV/HCV-coinfected subjects. No change in serum LDL-C was observed at day 3 of treatment, in spite of a marked reduction in serum HCV viral load, suggesting LDL-C increases do not directly reflect HCV clearance as measured in peripheral blood. After effective DAA therapy for HCV, an increase in LDL should be anticipated in HIV/HCV-coinfected subjects.
Introduction
Chronic hepatitis C virus (HCV) infection is the leading cause of chronic hepatitis, liver cirrhosis, hepatocellular carcinoma, and liver transplantation in the United States, and occurs frequency as a coinfection with HIV.1–13 HCV infection is associated with lipid and lipoprotein metabolism disorders including hepatic steatosis, hypobetalipoproteinemia, and hypocholesterolemia.14 Additional studies have linked chronic HCV infection to increased risk for cardiovascular disease.15,16 HCV modulation of serum cholesterol is in part related to viral intrahepatic modulation of cholesterol biosynthesis, and HCV circulates with host lipoproteins in complexes termed lipoviral particles.14,17–22 While HCV genotype-3 (GT3) is notable for inducing hepatic steatosis and having the most marked reduction of serum low-density lipoprotein (LDL) among the HCV genotypes, infection with HCV GT1, the most common genotype in the United States, is also associated with insulin resistance and altered LDL concentrations.12,23
With the advent of combination antiretroviral (ARV) therapy for HIV infection, mortality due to complications of AIDS has declined, while mortality associated with liver and cardiovascular disease has increased.3,24–26 Chronic HIV infection and treatment with ARVs are both associated with dyslipidemia, and cardiovascular risk is often modulated by the administration of LDL-lowering statin therapy.1,2,4,27,28 Interestingly, the phenotypes of low LDL associated with HCV and hypercholesterolemia associated with HIV are often attenuated in the setting of HIV/HCV coinfection.5–8,10
Treatment of chronic HCV infection is now possible with interferon (IFN)-free regimens composed of directly acting antiviral (DAA) agents that directly inhibit viral proteins.11 Marked advances in IFN-free DAA treatment of HCV infection in HIV/HCV-coinfected subjects have also been reported,29,30 such that HCV disease will be increasingly eradicated in this population.
How lipid profiles are altered after sustained virologic response (SVR) is achieved with DAA therapy for HCV in HIV/HCV-coinfected patients is unknown. We recently observed a rapid and sustained increase in LDL concentration in HCV, GT1-monoinfected subjects treated for 24 weeks with SOF/RBV.31 LDL changes were sustained posttreatment in subjects achieving SVR, while LDL declined to pretreatment levels in subjects who experienced treatment relapse.31 In the present study, we preformed longitudinal lipid analyses in HIV/HCV-coinfected subjects who achieved SVR with combination DAA therapy and compared the findings to HCV-monoinfected subjects treated with similar regimens.29,32
Materials and Methods
Study design
Clinical data and serum samples from 90 chronically infected, treatment-naive HCV GT-1 (n = 50 HIV/HCV-coinfected, n = 40 HCV-monoinfected) subjects were considered for analysis. All 90 subjects were enrolled in phase 2, single-center, prospective, community-based clinical trials that evaluated all-oral DAA treatment at the National Institute of Allergy and Infectious Diseases, National Institutes of Health in Bethesda, MD.
Fifty GT1, HCV treatment-naive HIV/HCV-coinfected subjects were treated on the NIH/NIAID ERADICATE (NCT01878799) protocol with the fixed dose combination of ledipasvir (LDV, 90 mg) combined with SOF (400 mg) once daily for 12 weeks.29 Thirty-seven subjects were virally suppressed with ARVs while 13 subjects were not receiving ARVs.29 Study approved ARVs included emtricitabine/tenofovir combined with efavirenz, raltegravir, or rilpivirine. Subjects not on ARVs had a stable CD4 T-lymphocyte count ≥500 cells/mm3 (n = 2) or a stable CD4 count with an HIV viral load <500 copies/ml at baseline (i.e., elite controllers, n = 11). Forty GT1, treatment-naive, HCV-monoinfected subjects were treated on the NIH/NIAID SYNERGY (NCT01805882) protocol with SOF/LDV combined with the investigational NS3/4A inhibitor GS-9451 (80 mg) or the allosteric NS5B inhibitor GS-9669 (500 mg) once daily for 6 weeks.32 Data were analyzed only from subjects achieving SVR. All subjects provided written or oral informed consent approved by the NIAID/NIH Institutional Review Board, and the study protocols conformed to the ethical guidelines of the Declaration of Helsinki.
Serum cholesterol measurements
Measurements for total serum cholesterol content, cholesterol content of HDL (HDL-C) and LDL (LDL-C), and triglycerides were performed in the NIH Clinical Research Center laboratory on the day of collection. Subjects were not instructed to fast prior to blood draws. Subjects who recently started or had a dose modification of statin therapy during HCV treatment were excluded from analysis. Subjects on stably dosed, long-standing lipid-modulating drugs or metformin therapy for diabetes were included.
The Vantera Clinical Nuclear Magnetic Resonance (NMR) Analyzer was used to identify and quantify lipoprotein particle distribution and generate an NMR LipoProfile using stored patient serum, as previously described.12,33 The NMR Analyzer detects specific lipoproteins by subjecting patient serum to radio frequency energy within a magnetic field, with determination of particle lipid composition by proprietary Vantera Clinical Analyzer software.
In HIV/HCV-coinfected subjects treated for 12 weeks, clinical serum lipids were measured pretreatment and at weeks 2, 4, 12 (end of treatment), and 24 (SVR12). Data are reported from 45 of 50 treated patients, with five patients excluded due to missing data, initiation of lipid modulating therapy during study, or relapse. Serum from a subset of 34 HIV/HCV-coinfected subjects (n = 22 on ARVs, n = 12 untreated) was selected for NMR LipoProfile analysis based on availability. NMR LipoProfile data were measured at baseline, day 3, week 2, and week 12. In HCV-monoinfected subjects treated for 6 weeks, clinical serum lipids were measured pretreatment and at weeks 4, 14 (SVR8), and 30 (SVR24). Three patients did not have week 30 data collected, and subsequent data collected at week 42 were used for analysis. Data are reported from 31 of 40 treated patients, with nine patients excluded for the aforementioned reasons.
Statistical analysis
Baseline demographics were compared using the Mann–Whitney test for continuous outcomes and Fisher's exact t-test for binary outcomes. Clinical serum lipids and NMR data were analyzed by ANOVA relative to pretreatment values with a multiple test correction. Prism 6.0 (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis and data presentation. Subjects with a missing time point for select components of the LipoProfile analyses were not included.
Results
In these two phase 2 clinical trials, 88 out of 90 subjects (98%) achieved SVR with undetectable HCV RNA 12 weeks after treatment, and as such, analysis was restricted to subjects achieving SVR.29,32 A summary of baseline patient demographics is shown in Table 1. One HIV/HCV-coinfected patient treated with SOF/LDV for 12 weeks and one HCV-monoinfected patient treated with SOF/LDV/GS-9669 for 6 weeks relapsed within 4 weeks of completing treatment.
Table 1.
Baseline Demographics and Clinical Characteristics of Patients
| Characteristics | HIV/HCV coinfected(n = 50) | HCV monoinfected(n = 40) | p-value |
|---|---|---|---|
| Median age, (IQR) | 59 (52–63) | 55 (51–60) | 0.14 |
| Male gender, no. (%) | 37 (74) | 29 (73) | 1.00 |
| Race, no. (%) | |||
| Black | 42 (84) | 37 (92) | 0.33 |
| White | 8 (16) | 3 (8) | |
| Median body mass index, IQR | 26 (22–29) | 28 (24–31) | 0.14 |
| Advanced liver disease, no. (%) | 13 (26) | 12 (30) | 0.22 |
| Steatosis, no. (%)a | |||
| None or trace | 31 (74) | 26 (68) | 0.63 |
| ≥1 | 11 (26) | 12 (32) | |
| HCV genotype subtype, no. (%)b | |||
| 1a | 37 (77) | 31 (78) | 1.00 |
| 1b | 11 (23) | 9 (22) | |
| IFNL4 genotype, no. (%) | |||
| TT/TT | 8 (16) | 8 (20) | 0.78 |
| ΔG/TT or ΔG/ΔG | 42 (84) | 32 (80) | |
| Median baseline HCV-RNA (log10 IU/ml) | 6.0 (5.3–6.5) | 6.2 (5.8–6.4) | 0.52 |
| Mean baseline albumin, (g/dl) (SD) | 3.7 (0.4) | 3.8 (0.3) | 0.03 |
| Mean baseline metabolic parameters (SD) | |||
| Total cholesterol | 150 (33) | 152 (45) | 0.63 |
| LDL-C | 76 (31) | 80 (36) | 0.84 |
| Triglycerides | 111 (63) | 104 (91) | 0.93 |
| HDL-C | 46 (26) | 49 (19) | 0.41 |
| Glucose | 95 (34) | 96 (20) | 0.72 |
| HbA1C | 5.6 (0.7) | 5.8 (0.6) | 0.11 |
| Taking metabolic drugs, no. (%) | |||
| Statins or fibrates | 6 (12) | 7 (18) | 0.55 |
| Metformin or insulin | 7 (14) | 6 (15) | 1.00 |
Steatosis was graded on liver biopsy only where available (n = 81/83).
Two Eradicate patients were classified as genotype 1 without a subtype.
Continuous variables are shown as median (with interquartile range, IQR) with analysis by the Mann–Whitney test or mean (with standard deviation, SD) with analysis by unpaired t-test. Categorical variables are expressed as number of patients (no.) with frequencies (%), with analysis by the Fisher's exact t-test. Race was self-reported. Body mass index is the weight in kilograms divided by the height in meters. Advanced liver disease (HAI-fibrosis 3–4) was determined by pretreatment biopsy (n = 83) or Fibrosure (n = 8) IFNL4 genotype was determined using the rs368234815 dinucleotide variant.
HVC, hepatitis C virus.
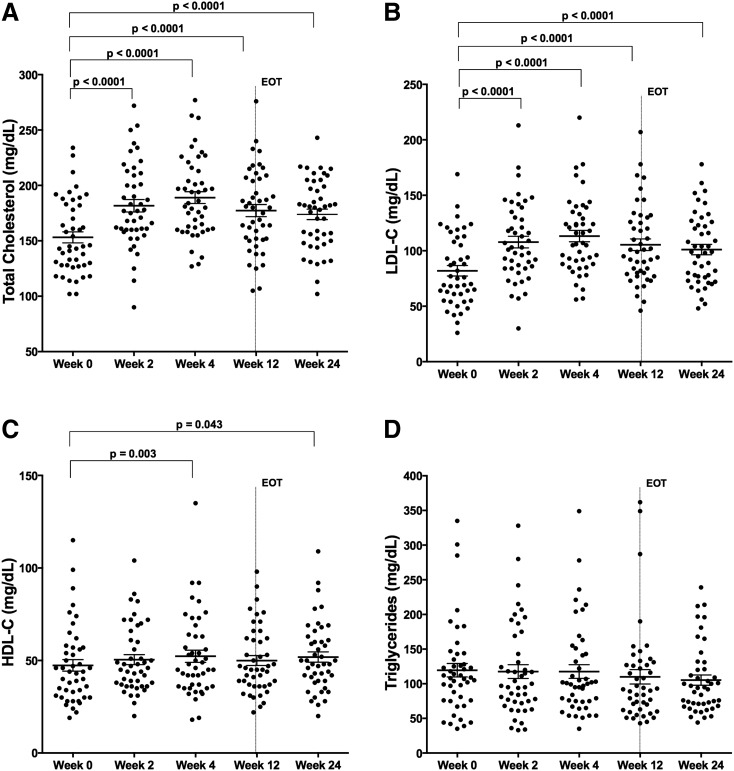
In HIV/HCV-coinfected subjects treated with SOF/LDV for 12 weeks, total serum cholesterol content increased by week 2 of treatment (153 ± 5 to 182 ± 6 mg/dl, p < 0.0001), the first on-treatment time point assessed, a change sustained during therapy at week 4 (189 ± 5 mg/dl, p < 0.0001), week 12 (end-of-treatment) (177 ± 5 mg/dl, p < 0.0001), and week 24 (174 ± 5 mg/dl, p < 0.0001) (Fig. 1A). Serum LDL-C also increased by week 2 of treatment (82 ± 5 to 108 ± 5 mg/dl, p < 0.0001), a change sustained through week 4 (113 ± 5 mg/dl, p < 0.0001), week 12 (105 ± 5 mg/dl, p < 0.0001), and week 24 (101 ± 5 mg/dl, p < 0.0001) (Fig. 1B). HDL-C increased only slightly at week 4 (47 ± 3 to 52 ± 3 mg/dl, p = 0.0013) and week 24 (52 ± 3 mg/dl, p = 0.043) (Fig. 1C), while there was no significant change in triglyceride concentration (Fig. 1D). No differences in lipid measurements at baseline or during treatment were observed between subjects treated or untreated with ARVs, and the results did not change significantly when patients on stable lipid-modulating therapy were excluded (data not shown).
FIG. 1.
Low-density lipoprotein (LDL)-C cholesterol concentration increases on therapy in HIV/hepatitis C virus (HCV)-coinfected subjects. Total cholesterol and serum LDL-C concentrations change on HCV therapy with sofosbuvir/ledipasvir (SOF/LDV) for 12 weeks in HIV/HCV-coinfected subjects (A, B), with less notable changes in high-density lipoprotein (HDL)-C and triglycerides (C, D). Shown are individual values and means with standard error (A–D) (n = 45). p-values represent values significantly different by one-way ANOVA with a multiple t-test correction test for values in relation to baseline values (week 0). EOT, end of treatment.
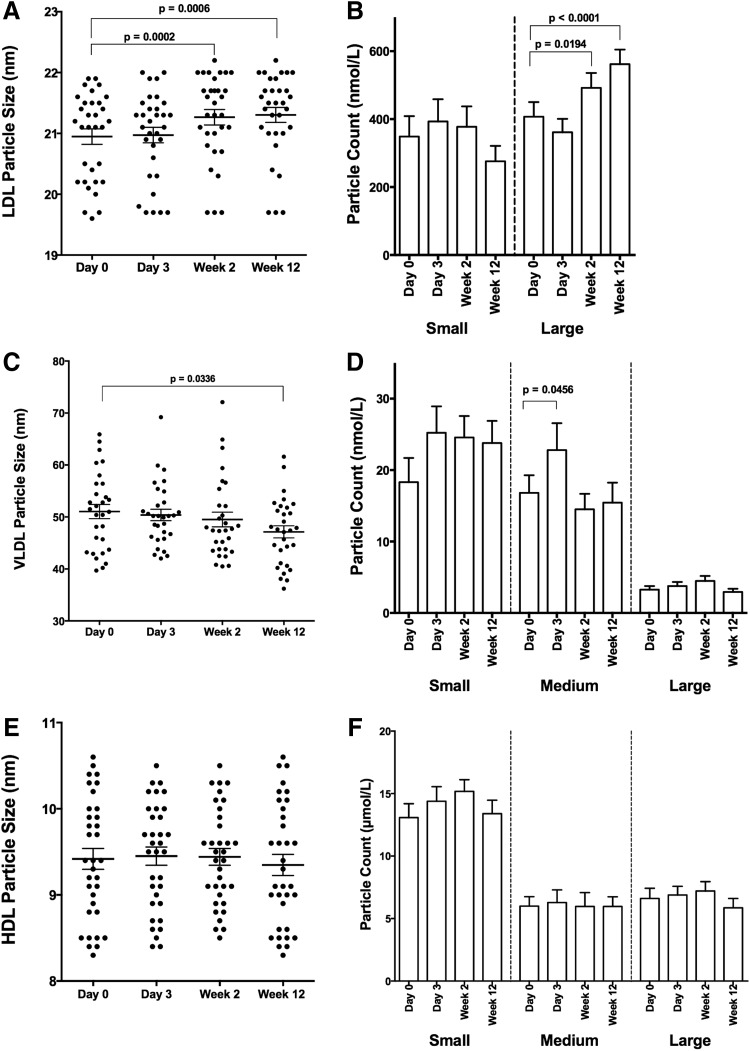
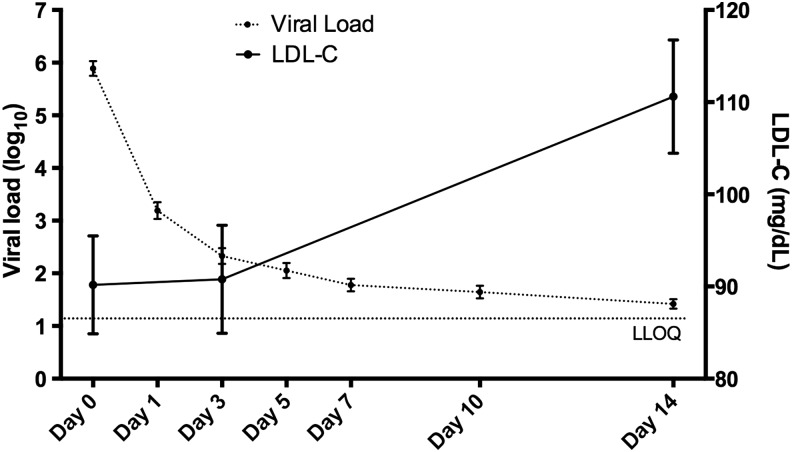
As noted in these and most DAA trials, there is a precipitous drop in HCV serum viral load with initiation of DAA therapy.32 In representative studies, baseline HCV RNA ranging from 6.2 to 6.8 log10 IU/ml dropped to <25 IU/ml by week 2 of treatment in 81–93% of subjects.34–36 HCV circulates in lipoviral particles, and clearance of HCV could directly impact cholesterol levels and account for the observed changes in Fig. 1. To assess the relationship between serum LDL changes and viral load decline, an in depth NMR LipoProfile analysis was performed on serum from 34 HIV/HCV-coinfected subjects early in therapy on pretreatment, day 3, week 2, and week 12 samples to enumerate LDL particle size and number. Interestingly, LDL particle size (LDL-Z) did not significantly change early in therapy at day 3 (21.0 ± 0.13 to 21.0 ± 0.13 nm, p = 0.96) but had changed notably by week 2 (21.3 ± 0.13 nm, p = 0.0002), a change sustained through week 12 (21.3 ± 0.12 nm, p = 0.0006) and accounted for by an increase in large LDL particle count (LDL-P) (Fig. 2A and B). In contrast, early changes in particle size of VLDL (VLDL-Z) and HDL (HDL-Z) were not observed, other than a marginal reduction in VLDL-Z by week 12 of therapy with no change in particle count (HDL-P and VLDL-P) (Fig. 2C–F). While viral load had dropped >3.5 log10 IU/ml by day 3 (5.89 ± 0.14 to 2.33 ± 0.15 log10 IU/ml, p < 0.0001), there was no significant difference from baseline in serum LDL-C (90.2 ± 5.3 to 90.8 ± 5.8 mg/dl, p = 0.99) (Fig. 3), indicating that the changes in serum LDL-C do not directly correlate with peripheral HCV clearance measured in serum.
FIG. 2.
LDL-Z and VLDL-Z change dynamically on therapy in HIV/HCV-coinfected subjects. Serum from 34 HIV/HCV-coinfected subjects achieving sustained virologic response (SVR) was analyzed using NMR LipoProfile. Particle size and count for LDL (A, B), VLDL (C, D), and HDL (E, F) are displayed. Shown are individual measurements with means and standard error for particle size and means with standard error for particle counts. p-values represent values significantly different by one-way ANOVA with a multiple t-test correction test for values in relation to baseline values (week 0).
FIG. 3.
Increase in serum LDL-C lags behind treatment-induced HCV viral decline. Viral load and LDL-C concentration from 34 HIV/HCV-coinfected subjects treated with 12 weeks of ledipasvir and sofosbuvir HCV therapy. Shown are means and standard errors. LLOQ, lower limit of quantitation using the Abbott Real-Time HCV Assay.
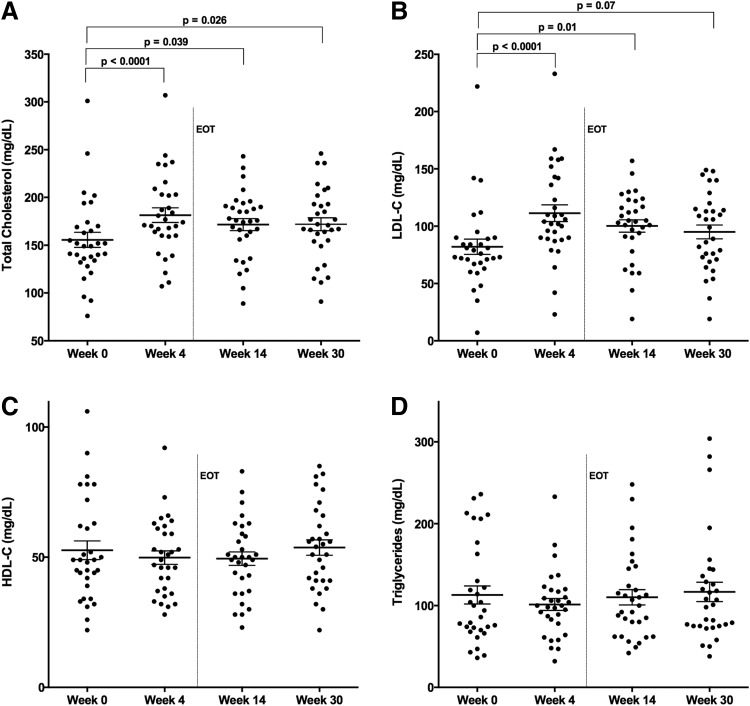
To compare changes in HCV-monoinfected subjects treated with similar IFN-free, RBV-free combination DAA therapy, we measured longitudinal cholesterol levels in subjects treated with SOF/LDV combined with GS-9451 or GS-9669 for 6 weeks.32 No differences in baseline lipid parameters were observed relative to the HIV/HCV-coinfected cohort (Table 1). Similar to HIV/HCV-coinfected subjects, in HCV-monoinfected subjects total serum cholesterol content increased by week 4 of treatment (156 ± 8 to 182 ± 8 mg/dl, p < 0.0001), the first on-treatment time point assessed in this trial (Fig. 4A). This increase was sustained posttreatment at week 14 (SVR8) (172 ± 6 mg/dl, p = 0.039) and week 30 (172 ± 7 mg/dl, p = 0.026) (Fig. 4A). Serum LDL-C also increased by week 4 of treatment (82 ± 7 to 111 ± 7 mg/dl, p < 0.0001), which was sustained posttreatment at week 14 (100 ± 5 mg/dl, p = 0.01) and week 30 (95 ± 6 mg/dl, p = 0.07) (Fig. 4B). No significant changes in HDL-C or triglyceride concentration were observed (Fig. 4C and D).
FIG. 4.
LDL-C cholesterol concentration increases on therapy in HCV-monoinfected subjects. Total cholesterol and serum LDL-C concentrations change during HCV therapy with SOF/LDV combined with GS-9541 or GS-9669 for 6 weeks in HCV-monoinfected subjects (A, B) while HDL-C and triglyceride values are unchanged (C, D). Shown are individual values and means with standard error (A–D) (n = 31). p-values represent values significantly different by one-way ANOVA with a multiple t-test correction test for values in relation to baseline values (week 0). EOT, end of treatment (week 6).
Discussion
In this study, we observed a rapid and sustained increase in serum LDL-C in HCV-monoinfected and HIV/HCV-coinfected patients during and following successful treatment of HCV infection with IFN-free DAA therapy. The increase in serum LDL-C during HCV therapy was independent of the type of HCV therapy, and is likely reflective of the host's response to HCV suppression. These findings are consistent with increases in cholesterol observed during IFN-free treatment of HCV-monoinfected individuals,12 and suggest that HIV coinfection does not substantially modulate the HCV-induced perturbation in serum cholesterol and the host response to HCV clearance. As HCV is eradicated with increasing frequency in HIV/HCV-coinfected subjects, these findings are significant in a climate where serum LDL is commonly used to prognosticate and pharmaceutically modulate cardiovascular risk.
The consistent observation that chronic HCV infection is associated with reduced serum LDL concentrations but increased cardiovascular risk reflects a distinction from traditional use of serum LDL for cardiovascular risk assessment.15,16,37 The relative impact of the observed increase in LDL concentration associated with HCV clearance, shown here and in other studies, with the decrease observed in other biomarkers of cardiovascular disease observed with HCV clearance on subsequent risk for cardiovascular events requires prospective evaluation.38
We also demonstrate that an LDL increase occurs with slower kinetics than the decline in HCV viral load in serum during treatment (Fig. 3), suggesting cholesterol changes are not directly due to clearance of lipoviral particles containing HCV. Interestingly, a recent study examining viral kinetics in serum and liver during treatment with telaprevir/IFN/RBV revealed a slower HCV RNA decline in the liver compared to plasma.39 Given the estimated 7-day half-life of infected hepatocytes,40 a persistent burden of intrahepatic HCV is likely still present at day 3 of treatment in this trial, but has likely declined significantly by week 2. Thus, changes in serum LDL-C may be a reflection of intrahepatic HCV burden and direct intracellular viral modulation of cholesterol metabolism. This difference could also reflect a delay in intrahepatic lipid biosynthesis and/or exocytosis relative to suppression of HCV replication.
One limitation of this study is that fasting was not requested prior to serum blood draws, which could result in variability in lipid measurements, in particular triglyceride and VLDL-C concentrations, known to be more affected by nonfasting.41 In addition, the durability of the observed changes in lipids should be assessed prospectively posttreatment, as cholesterol values could change over time as the host reestablishes metabolic homeostasis in the absence of HCV.
In conclusion, there is a sustained increase in LDL-C levels in patients who achieve SVR with DAA therapy irrespective of HIV coinfection. Whether HCV clearance and changes in LDL will impact the higher rates of cardiovascular events associated with chronic HCV infection, and observed in some studies of HIV/HCV-coinfected relative to HIV-monoinfected subjects, will require prospective study in large patient cohorts.16,37 In HIV-infected subjects receiving IFN-free treatment for HCV, lipids should be monitored posttreatment for cardiovascular risk assessment and determining indications for lipid-modulating therapy.
Acknowledgments
This study was funded by the Intramural Research Programs of the National Institute of Allergy and Infectious Diseases, the Critical Care Medicine Department, and the Clinical Research Center of the National Institutes of Health.
Data were presented in part at the HIV and Liver Disease Meeting, Jackson Hole, Wyoming, September 18–20, 2014.
Author Disclosure Statement
Anu Osinusi is an employee of Gilead Sciences. For all other authors no competing financial interests exist.
References
- 1.Grinspoon S. and Carr A: Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N Engl J Med 2005;352(1):48–62 [DOI] [PubMed] [Google Scholar]
- 2.Giannarelli C, Klein RS, and Badimon JJ: Cardiovascular implications of HIV-induced dyslipidemia. Atherosclerosis 2011;219(2):384–389 [DOI] [PubMed] [Google Scholar]
- 3.Fedele F, Bruno N, and Mancone M: Cardiovascular risk factors and HIV disease. AIDS Rev 2011;13(2):119–129 [PubMed] [Google Scholar]
- 4.Riddler SA, Li X, Chu H, et al. : Longitudinal changes in serum lipids among HIV-infected men on highly active antiretroviral therapy. HIV Med 2007;8(5):280–287 [DOI] [PubMed] [Google Scholar]
- 5.Wheeler AL, Scherzer R, Lee D, et al. : HIV/hepatitis C virus coinfection ameliorates the atherogenic lipoprotein abnormalities of HIV infection. AIDS 2014;28(1):49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forrester JE, McGovern BH, Rhee MS, and Sterling RK: The individual and combined influence of HIV and hepatitis C virus on dyslipidaemia in a high-risk Hispanic population. HIV Med 2009;10(9):555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polgreen PM, Fultz SL, Justice AC, et al. : Association of hypocholesterolaemia with hepatitis C virus infection in HIV-infected people. HIV Med 2004;5(3):144–150 [DOI] [PubMed] [Google Scholar]
- 8.Bedimo R, Ghurani R, Nsuami M, et al. : Lipid abnormalities in HIV/hepatitis C virus-coinfected patients. HIV Med 2006;7(8):530–536 [DOI] [PubMed] [Google Scholar]
- 9.Cooper CL, Mills E, and Angel JB: Mitigation of antiretroviral-induced hyperlipidemia by hepatitis C virus co-infection. AIDS 2007;21(1):71–76 [DOI] [PubMed] [Google Scholar]
- 10.Duong M, Petit JM, Piroth L, et al. : Association between insulin resistance and hepatitis C virus chronic infection in HIV-hepatitis C virus-coinfected patients undergoing antiretroviral therapy. J Acquir Immune Defic Syndr 2001;27(3):245–250 [DOI] [PubMed] [Google Scholar]
- 11.Kohli A, Shaffer A, Sherman A, and Kottilil S: Treatment of hepatitis C: A systematic review. JAMA 2014;312(6):631–640 [DOI] [PubMed] [Google Scholar]
- 12.Meissner EG, Lee YJ, Osinusi A, et al. : Effect of sofosbuvir and ribavirin treatment on peripheral and hepatic lipid metabolism in chronic HCV, genotype-1 infected patients. Hepatology 2015;61:790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razavi H, Elkhoury AC, Elbasha E, et al. : Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 2013;57(6):2164–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felmlee DJ, Hafirassou ML, Lefevre M, et al. : Hepatitis C virus, cholesterol and lipoproteins—impact for the viral life cycle and pathogenesis of liver disease. Viruses 2013;5(5):1292–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Younossi ZM, Stepanova M, Nader F, et al. : Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther 2013;37(6):647–652 [DOI] [PubMed] [Google Scholar]
- 16.Chew KW, Bhattacharya D, McGinnis KA, et al. : Short Communication: Coronary heart disease risk by Framingham Risk Score in hepatitis C and HIV/hepatitis C-coinfected persons. AIDS Res Hum Retroviruses 2015;31(7):718–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartenschlager R, Lohmann V, and Penin F: The molecular and structural basis of advanced antiviral therapy for hepatitis C virus infection. Nat Rev Microbiol 2013;11(7):482–496 [DOI] [PubMed] [Google Scholar]
- 18.Miyanari Y, Atsuzawa K, Usuda N, et al. : The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol 2007;9(9):1089–1097 [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Sun F, Owen DM, et al. : Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA 2007;104(14):5848–5853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen SU, Bassendine MF, Burt AD, et al. : Association between hepatitis C virus and very-low-density lipoprotein (VLDL)/LDL analyzed in iodixanol density gradients. J Virol 2006;80(5):2418–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merz A, Long G, Hiet MS, et al. : Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem 2011;286(4):3018–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andre P, Perlemuter G, Budkowska A, et al. : Hepatitis C virus particles and lipoprotein metabolism. Semin Liver Dis 2005;25(1):93–104 [DOI] [PubMed] [Google Scholar]
- 23.Sheridan DA, Neely RD, and Bassendine MF: Hepatitis C virus and lipids in the era of direct acting antivirals (DAAs). Clin Res Hepatol Gastroenterol 2013;37(1):10–16 [DOI] [PubMed] [Google Scholar]
- 24.Kwong GP, Ghani AC, Rode RA, et al. : Comparison of the risks of atherosclerotic events versus death from other causes associated with antiretroviral use. AIDS 2006;20(15):1941–1950 [DOI] [PubMed] [Google Scholar]
- 25.Iloeje UH, Yuan Y, L'Italien G, et al. : Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med 2005;6(1):37–44 [DOI] [PubMed] [Google Scholar]
- 26.Friis-Moller N, Sabin CA, Weber R, et al. : Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349(21):1993–2003 [DOI] [PubMed] [Google Scholar]
- 27.Aberg JA, Gallant JE, Ghanem KG, et al. : Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2014;58(1):1–10 [DOI] [PubMed] [Google Scholar]
- 28.Denue BA, Alkali MB, Abjah AU, et al. : Changes in lipid profiles and other biochemical parameters in HIV-1 infected patients newly commenced on HAART regimen. Infect Dis (Auckl) 2013;6:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osinusi A, Townsend K, Kohli A, et al. : Virologic response following combined ledipasvir and sofosbuvir administration in patients with HCV genotype 1 and HIV co-infection. JAMA 2015;313:1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sulkowski MS, Eron JJ, Wyles D, et al. : Ombitasvir, paritaprevir co-dosed with ritonavir, dasabuvir, and ribavirin for hepatitis C in patients co-infected with HIV-1: A randomized trial. JAMA 2015;313:1223–1231 [DOI] [PubMed] [Google Scholar]
- 31.Osinusi A, Meissner EG, Lee YJ, et al. : Sofosbuvir and ribavirin for hepatitis C genotype 1 in patients with unfavorable treatment characteristics: A randomized clinical trial. JAMA 2013;310(8):804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohli A, Osinusi A, Sims Z, et al. : Virological response after 6 week triple-drug regimens for hepatitis C: A proof-of-concept phase 2A cohort study. Lancet 2015;385:1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeyarajah EJ, Cromwell WC, and Otvos JD: Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 2006;26(4):847–870 [DOI] [PubMed] [Google Scholar]
- 34.Kowdley KV, Gordon SC, Reddy KR, et al. : Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370(20):1879–1888 [DOI] [PubMed] [Google Scholar]
- 35.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. : Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014;370(3):211–221 [DOI] [PubMed] [Google Scholar]
- 36.Afdhal N, Zeuzem S, Kwo P, et al. : Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370(20):1889–1898 [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Montero JV, Barreiro P, de Mendoza C, et al. : Hepatitis C virus coinfection independently increases the risk of cardiovascular disease in HIV-positive patients. J Viral Hepat 2015. [Epub ahead of print]; DOI: 10.1111/jvh.12447 [DOI] [PubMed] [Google Scholar]
- 38.Chew KW, Hua L, Bhattacharya D, et al. : The effect of hepatitis C virologic clearance on cardiovascular disease biomarkers in human immunodeficiency virus/hepatitis C virus coinfection. Open Forum Infect Dis 2014;1(3):ofu104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talal AH, Dimova RB, Zhang EZ, et al. : Telaprevir-based treatment effects on hepatitis C virus in liver and blood. Hepatology 2014;60(6):1826–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro RM, Li H, Wang S, et al. : Quantifying the diversification of hepatitis C virus (HCV) during primary infection: Estimates of the in vivo mutation rate. PLoS Pathog 2012;8(8):e1002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mora S, Rifai N, Buring JE, and Ridker PM: Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008;118(10):993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]