Abstract
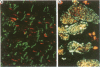
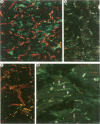
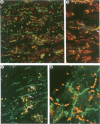
OBJECTIVE: To examine the distribution pattern of intercellular junctions (the mechanically coupling desmosomes and the electrically coupling gap junctions) in hypertrophic cardiomyopathy (HCM) hearts showing myofibre disarray. DESIGN: Samples from six necropsied hearts were studied, representing the interventricular septum and the free walls of the left and right ventricles. Immunohistochemical labelling of desmoplakin was used as a marker for desmosomes, and of connexin43 as a marker for gap junctions, in single and double stainings. The slides were examined by confocal laser scanning microscopy. RESULTS: Marked disorganisation of intercalated discs was observed in areas featuring myofibre disarray. Besides overall derangement, localised abnormalities in desmosome organisation were evident, which included: (1) the formation of abnormally enlarged megadiscs; (2) the presence of intersecting disc structures; and (3) aberrant side to side desmosomal connections. Gap junctional abnormalities included: (1) random distribution of gap junctions over the surface of myocytes, rather than localisation to intercalated discs; (2) abundant side to side gap junction connections between adjacent myocytes; and (3) formation of abnormally shaped gap junctions. Circles of myocytes continuously interconnected by gap junctions were also observed. Regions of the diseased hearts lacking myofibre disarray, and control hearts of normal patients and patients with other cardiac diseases, did not show these alterations. CONCLUSIONS: The disorganisation of the intercellular junctions associated with myofibre disarray in HCM may play an important role in the pathophysiological manifestations of the disease. The remodelling of gap junction distribution may underlie the formation of an arrhythmogenic substrate, thereby contributing to the generation and maintenance of cardiac arrhythmias associated with HCM.
Full text
PDF

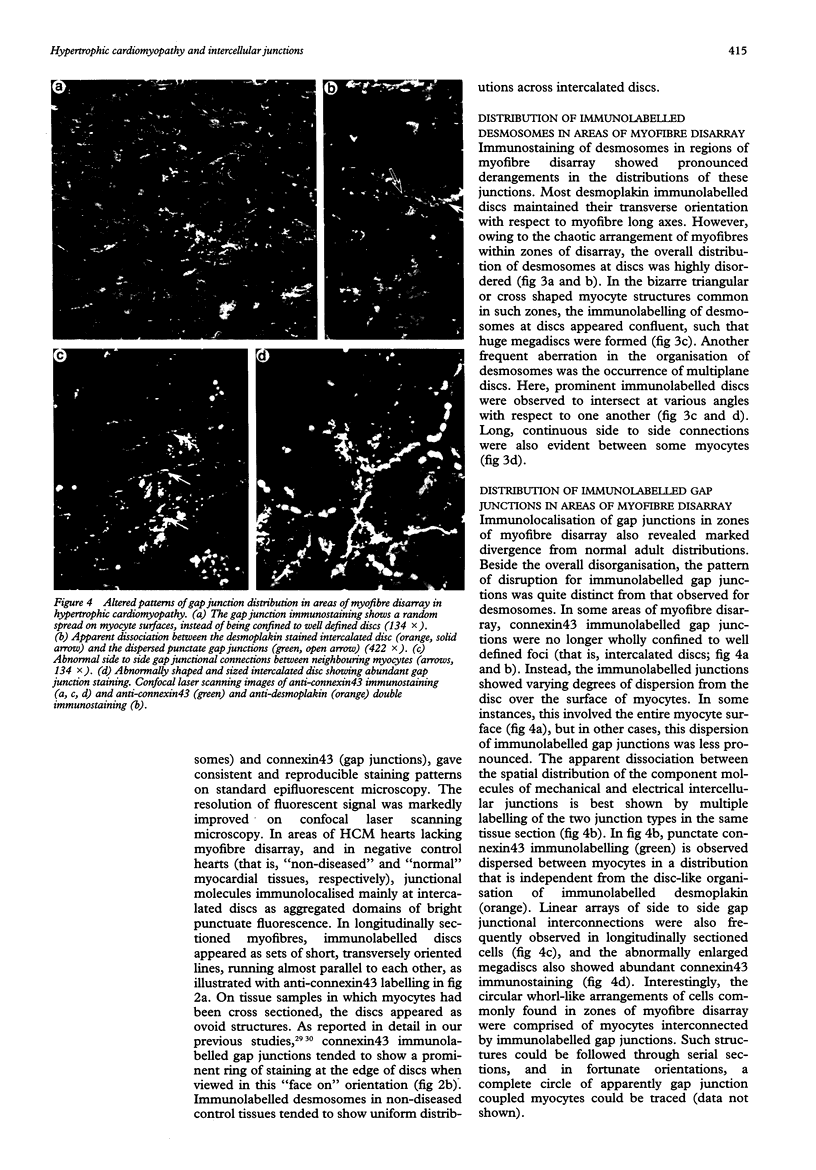


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnemann J., Sullivan K. H., Magee A. I., King I. A., Buxton R. S. Stratification-related expression of isoforms of the desmosomal cadherins in human epidermis. J Cell Sci. 1993 Mar;104(Pt 3):741–750. doi: 10.1242/jcs.104.3.741. [DOI] [PubMed] [Google Scholar]
- Bonne G., Carrier L., Bercovici J., Cruaud C., Richard P., Hainque B., Gautel M., Labeit S., James M., Beckmann J. Cardiac myosin binding protein-C gene splice acceptor site mutation is associated with familial hypertrophic cardiomyopathy. Nat Genet. 1995 Dec;11(4):438–440. doi: 10.1038/ng1295-438. [DOI] [PubMed] [Google Scholar]
- Bonow R. O. Left ventricular diastolic function in hypertrophic cardiomyopathy. Herz. 1991 Feb;16(1):13–21. [PubMed] [Google Scholar]
- Dillon S. M., Allessie M. A., Ursell P. C., Wit A. L. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988 Jul;63(1):182–206. doi: 10.1161/01.res.63.1.182. [DOI] [PubMed] [Google Scholar]
- Geisterfer-Lowrance A. A., Kass S., Tanigawa G., Vosberg H. P., McKenna W., Seidman C. E., Seidman J. G. A molecular basis for familial hypertrophic cardiomyopathy: a beta cardiac myosin heavy chain gene missense mutation. Cell. 1990 Sep 7;62(5):999–1006. doi: 10.1016/0092-8674(90)90274-i. [DOI] [PubMed] [Google Scholar]
- Gourdie R. G. A map of the heart: gap junctions, connexin diversity and retroviral studies of conduction myocyte lineage. Clin Sci (Lond) 1995 Mar;88(3):257–262. doi: 10.1042/cs0880257. [DOI] [PubMed] [Google Scholar]
- Gourdie R. G., Green C. R., Severs N. J., Thompson R. P. Immunolabelling patterns of gap junction connexins in the developing and mature rat heart. Anat Embryol (Berl) 1992;185(4):363–378. doi: 10.1007/BF00188548. [DOI] [PubMed] [Google Scholar]
- Gourdie R. G., Severs N. J., Green C. R., Rothery S., Germroth P., Thompson R. P. The spatial distribution and relative abundance of gap-junctional connexin40 and connexin43 correlate to functional properties of components of the cardiac atrioventricular conduction system. J Cell Sci. 1993 Aug;105(Pt 4):985–991. doi: 10.1242/jcs.105.4.985. [DOI] [PubMed] [Google Scholar]
- Green K. J., Geiger B., Jones J. C., Talian J. C., Goldman R. D. The relationship between intermediate filaments and microfilaments before and during the formation of desmosomes and adherens-type junctions in mouse epidermal keratinocytes. J Cell Biol. 1987 May;104(5):1389–1402. doi: 10.1083/jcb.104.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiowski W., Linder L., Kleinbloesem C., van Brummelen P., Bühler F. R. Blood pressure control by the renin-angiotensin system in normotensive subjects. Assessment by angiotensin converting enzyme and renin inhibition. Circulation. 1992 Jan;85(1):1–8. doi: 10.1161/01.cir.85.1.1. [DOI] [PubMed] [Google Scholar]
- Lane N. J., Swales L. S. Dispersal of junctional particles, not internalization, during the in vivo disappearance of gap junctions. Cell. 1980 Mar;19(3):579–586. doi: 10.1016/s0092-8674(80)80034-1. [DOI] [PubMed] [Google Scholar]
- Luque E. A., Veenstra R. D., Beyer E. C., Lemanski L. F. Localization and distribution of gap junctions in normal and cardiomyopathic hamster heart. J Morphol. 1994 Nov;222(2):203–213. doi: 10.1002/jmor.1052220207. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Anan T. J., Roberts W. C. Quantitative analysis of the distribution of cardiac muscle cell disorganization in the left ventricular wall of patients with hypertrophic cardiomyopathy. Circulation. 1981 Apr;63(4):882–894. doi: 10.1161/01.cir.63.4.882. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Bonow R. O., Cannon R. O., 3rd, Leon M. B., Epstein S. E. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (1). N Engl J Med. 1987 Mar 26;316(13):780–789. doi: 10.1056/NEJM198703263161305. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Edwards J. E., Henry W. L., Clark C. E., Bingle G. J., Epstein S. E. Asymmetric septal hypertrophy (ASH) in infancy. Circulation. 1974 Oct;50(4):809–820. doi: 10.1161/01.cir.50.4.809. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Roberts W. C. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum of patients with hypertrophic cardiomyopathy. Circulation. 1979 Apr;59(4):689–706. doi: 10.1161/01.cir.59.4.689. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Sato N., Roberts W. C., Edwards J. E., Chandra R. S. Quantitative analysis of cardiac muscle cell disorganization in the ventricular septum. Comparison of fetuses and infants with and without congenital heart disease and patients with hypertrophic cardiomyopathy. Circulation. 1979 Sep;60(3):685–696. doi: 10.1161/01.cir.60.3.685. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Spirito P., Wesley Y., Arce J. Development and progression of left ventricular hypertrophy in children with hypertrophic cardiomyopathy. N Engl J Med. 1986 Sep 4;315(10):610–614. doi: 10.1056/NEJM198609043151003. [DOI] [PubMed] [Google Scholar]
- Maron B. J., Tajik A. J., Ruttenberg H. D., Graham T. P., Atwood G. F., Victorica B. E., Lie J. T., Roberts W. C. Hypertrophic cardiomyopathy in infants: clinical features and natural history. Circulation. 1982 Jan;65(1):7–17. doi: 10.1161/01.cir.65.1.7. [DOI] [PubMed] [Google Scholar]
- McKenna W. J., Camm A. J. Sudden death in hypertrophic cardiomyopathy. Assessment of patients at high risk. Circulation. 1989 Nov;80(5):1489–1492. doi: 10.1161/01.cir.80.5.1489. [DOI] [PubMed] [Google Scholar]
- Peters N. S., Severs N. J., Rothery S. M., Lincoln C., Yacoub M. H., Green C. R. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994 Aug;90(2):713–725. doi: 10.1161/01.cir.90.2.713. [DOI] [PubMed] [Google Scholar]
- Saffitz J. E., Corr P. B., Sobel B. E. Arrhythmogenesis and ventricular dysfunction after myocardial infarction. Is anomalous cellular coupling the elusive link? Circulation. 1993 May;87(5):1742–1745. doi: 10.1161/01.cir.87.5.1742. [DOI] [PubMed] [Google Scholar]
- Severs N. J., Gourdie R. G., Harfst E., Peters N. S., Green C. R. Intercellular junctions and the application of microscopical techniques: the cardiac gap junction as a case model. J Microsc. 1993 Mar;169(Pt 3):299–328. doi: 10.1111/j.1365-2818.1993.tb03308.x. [DOI] [PubMed] [Google Scholar]
- Severs N. J. Pathophysiology of gap junctions in heart disease. J Cardiovasc Electrophysiol. 1994 May;5(5):462–475. doi: 10.1111/j.1540-8167.1994.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Severs N. J., Shovel K. S., Slade A. M., Powell T., Twist V. W., Green C. R. Fate of gap junctions in isolated adult mammalian cardiomyocytes. Circ Res. 1989 Jul;65(1):22–42. doi: 10.1161/01.res.65.1.22. [DOI] [PubMed] [Google Scholar]
- Severs N. J. The cardiac gap junction and intercalated disc. Int J Cardiol. 1990 Feb;26(2):137–173. doi: 10.1016/0167-5273(90)90030-9. [DOI] [PubMed] [Google Scholar]
- Smith J. H., Green C. R., Peters N. S., Rothery S., Severs N. J. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol. 1991 Oct;139(4):801–821. [PMC free article] [PubMed] [Google Scholar]
- Spirito P., Bellone P. Natural history of hypertrophic cardiomyopathy. Br Heart J. 1994 Dec;72(6 Suppl):S10–S12. doi: 10.1136/hrt.72.6_suppl.s10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spirito P., Chiarella F., Carratino L., Berisso M. Z., Bellotti P., Vecchio C. Clinical course and prognosis of hypertrophic cardiomyopathy in an outpatient population. N Engl J Med. 1989 Mar 23;320(12):749–755. doi: 10.1056/NEJM198903233201201. [DOI] [PubMed] [Google Scholar]
- St John Sutton M. G., Lie J. T., Anderson K. R., O'Brien P. C., Frye R. L. Histopathological specificity of hypertrophic obstructive cardiomyopathy. Myocardial fibre disarray and myocardial fibrosis. Br Heart J. 1980 Oct;44(4):433–443. doi: 10.1136/hrt.44.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa G., Jarcho J. A., Kass S., Solomon S. D., Vosberg H. P., Seidman J. G., Seidman C. E. A molecular basis for familial hypertrophic cardiomyopathy: an alpha/beta cardiac myosin heavy chain hybrid gene. Cell. 1990 Sep 7;62(5):991–998. doi: 10.1016/0092-8674(90)90273-h. [DOI] [PubMed] [Google Scholar]
- Thierfelder L., Watkins H., MacRae C., Lamas R., McKenna W., Vosberg H. P., Seidman J. G., Seidman C. E. Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell. 1994 Jun 3;77(5):701–712. doi: 10.1016/0092-8674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Ursell P. C., Gardner P. I., Albala A., Fenoglio J. J., Jr, Wit A. L. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985 Mar;56(3):436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- Watkins H., Conner D., Thierfelder L., Jarcho J. A., MacRae C., McKenna W. J., Maron B. J., Seidman J. G., Seidman C. E. Mutations in the cardiac myosin binding protein-C gene on chromosome 11 cause familial hypertrophic cardiomyopathy. Nat Genet. 1995 Dec;11(4):434–437. doi: 10.1038/ng1295-434. [DOI] [PubMed] [Google Scholar]
- van der Bel-Kahn J. Muscle fiber disarray in common heart diseases. Am J Cardiol. 1977 Sep;40(3):355–364. doi: 10.1016/0002-9149(77)90157-6. [DOI] [PubMed] [Google Scholar]