Abstract
The blood-testis barrier (BTB) is one of the tightest blood-tissue barriers in the mammalian body. It divides the seminiferous epithelium of the seminiferous tubule, the functional unit of the testis, where spermatogenesis takes place, into the basal and the adluminal (apical) compartments. Functionally, the BTB provides a unique microenvironment for meiosis I/II and post-meiotic spermatid development which take place exclusively in the apical compartment, away from the host immune system, and it contributes to the immune privilege status of testis. However, the BTB also poses major obstacles in developing male contraceptives (e.g., adjudin) that exert their effects on germ cells in the apical compartment, such as by disrupting spermatid adhesion to the Sertoli cell, causing germ cell exfoliation from the testis. Besides the tight junction (TJ) between adjacent Sertoli cells at the BTB that restricts the entry of contraceptives from the microvessels in the interstitium to the adluminal compartment, drug transporters, such as P-glycoprotein and multidrug resistance-associated protein 1 (MRP1), are also present that actively pump drugs out of the testis, limiting drug bioavailability. Recent advances in drug formulations, such as drug particle micronization (<50 μm) and co-grinding of drug particles with ß-cyclodextrin have improved bioavailability of contraceptives via considerable increase in solubility. Herein, we discuss development in drug formulations using adjudin as an example. We also put emphasis on the possible use of nanotechnology to deliver adjudin to the apical compartment with multidrug magnetic mesoporous silica nanoparticles. These advances in technology will significantly enhance our ability to develop effective non-hormonal male contraceptives for men.
Keywords: Testis, male contraceptive, blood-testis barrier, adjudin, nanotechnology
INTRODUCTION
The testes produce an upward of ~300 million spermatozoa per day in a man since puberty at ~12 years of age via spermatogenesis without interruption to support reproduction [1, 2]. Spermatogenesis takes place in the seminiferous tubule which is the functional unit that produces spermatozoa via cycles of spermatogonial self-renewal, germ cell differentiation and meiosis I/II, spermiogenesis and spermiation [3, 4]. For over a century, the seminiferous tubules in mammalian testes have been known to be protected by a unique blood-tissue barrier known as the blood-testis barrier (BTB) [5–8]. As noted in the cross-section of a seminiferous tubule, the BTB that locates near the basement membrane also segregates the seminiferous epithelium into the basal and the adluminal (apical) compartment (Fig. 1). Thus, all the events of meiosis I/II and post-meiotic spermatid development take place behind the BTB in the adluminal compartment (Fig. 1). In contrast to other blood-tissue barriers, which are constituted almost exclusively by the tight junction (TJ) barrier of endothelial cells of microvessels, such as the blood-brain barrier (BBB) [9] and the blood-retinal barrier [10], the BTB is constituted not just by TJ between adjacent Sertoli cells, but also by coexisting basal ectoplasmic specialization (basal ES, a testis-specific actin-rich adherens junction (AJ)) and gap junction, as well as intermediate filament-based desmosome. While the BTB is one of the tightest blood-tissue barriers, it undergoes extensive remodeling during the seminiferous epithelial cycle in particular at stage VIII when preleptotene spermatocytes are being transported across the BTB to enter the adluminal compartment to prepare for meiosis I/II. Studies have shown that these coexisting junctions are necessary to maintain the BTB function to avoid any disruption even transiently during the epithelial cycle [11, 12] and to ensure normal junction remodeling events such as during the transport of preleptotene spermatocytes across the immunological barrier at stage VIII of the epithelial cycle [13]. As such, the BTB poses a major obstacle to allow non-hormonal male contraceptives to get access to the seminiferous epithelium in particular if these drugs exert their effects behind the immunological barrier, such as the adluminal compartment wherein germ cell meiosis and post-meiotic spermatid development take place.
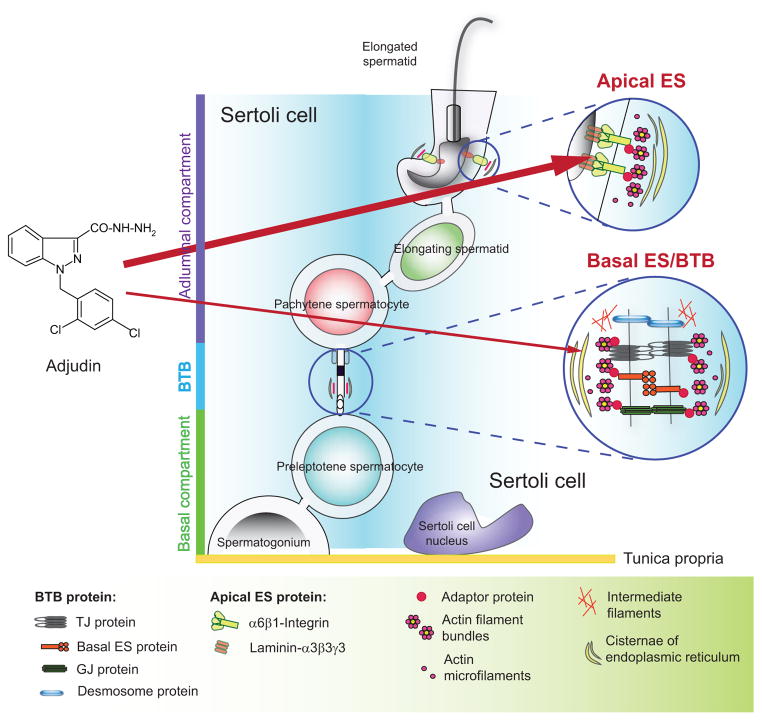
Fig. (1).
A schematic drawing that illustrates the site of action of adjudin in the mammalian testis. Studies have shown that adjudin (left panel) exerts its effects primarily at the ectoplasmic specialization (ES) (right panel) in the seminiferous epithelium of mammalian testes [36, 131, 132]. Adjudin is more effective to perturb the apical ES function versus the basal ES function at the BTB. This is likely due to the presence of only a single array of actin filament bundles at the apical ES versus two arrays of actin filament bundles at the basal ES at the BTB [36, 133]. Furthermore, the BTB is constituted by actin-based tight junction (TJ) and GJ (gap junction) besides basal ES, as well as intermediate filament-based desmosome; whereas the apical ES does not have other parallel junctions at the Sertoli-spermatid interface. As such, apical ES is rapidly disrupted, usually within 6- to 9-hr following exposure of adult rats to adjudin by oral gavage [36, 134], but the basal ES/BTB is not disrupted until 2-wk following treatment to the rats unless a high acute dose of adjudin is used [133].
During the last two decades, significant advances are made in developing effective hormonal male contraceptives [14–16]. The approach is to use injectable androgen (e.g., testosterone undecanoate with or without a progestin such as levonorgestrel or etonogestrel), oral androgen (e.g., dimethandrolone undecanoate) or androgen implants in men to block spermatogenesis by down-regulating intratesticular testosterone level [15, 17–21], which must be maintained at a level of ~100-fold over the blood level in the systemic circulation in both humans and rodents to sustain spermatogenesis [22–24]. In short, this approach disrupts the hypothalamic-pituitary-testicular axis via an elevated serum testosterone level through the injectable androgen or androgen implants, which shuts down the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus, thereby suppressing the secretion of luteinizing hormone (LH) from the pituitary gland, leading to a reduced androgen production by Leydig cells in the interstitium to disrupt spermatogenesis [15, 25]. However, since the hypothalamic-pituitary-testicular axis is disrupted, other androgen-dependent biological functions (e.g., blood pressure) and organs (e.g., prostate) as well as muscle mass in men can be affected, leading to possible side-effects and concerns among men [26–29]. Furthermore, it takes several weeks for a male pill, including androgen-based implants or injectable androgen, to become effective, which inevitably associates with low patient compliance [30]. Additionally, it has been reported that some men are less susceptible to injectable testosterone known as non-responders, such as among Chinese men, leading to variations in androgen-mediated suppression of spermatogenesis [31]. Therefore, there is a need to develop non-hormonal contraceptives which preferably exert their effects locally in the testis without perturbing the hypothalamic-pituitary-testicular axis and serum androgen level. At present, several candidate compounds are being vigorously investigated by investigators, among them adjudin (1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydra-zide) [32, 33] and gamendazole [34, 35] appear to be two promising lead compounds. Both drugs are derivatives of lonidamine [1-(2,4-dichlorobenzyl)-1H-indazole-3-carboxylic acid], and are known to induce reversible germ cell exfoliation from the seminiferous epithelium by disrupting the testis-specific atypical adherens junction (AJ) known as apical ectoplasmic specialization (apical ES) between germ cells (step 8–19 spermatids in the rat testis) and Sertoli cells [36]. Studies have shown that adjudin possesses several added health benefits, such as anti-cancer [37], anti-neuroinflammatory and anti-neurodegenerative activity [38, 39] as well as anti-ototoxicity [40], suggesting its use as a male contraceptive has other added health benefits. While adjudin had passed the standard acute toxicity, mutagenesis, and chromosomal aberration tests conducted by licensed toxicologists, it failed the subchronic toxicity test in which rats were treated with adjudin by oral gavage at 50 mg/kg b.w. for 29 consecutive days since liver inflammation and skeletal muscle atrophy were detected in 3 out of 10 male rats even though the females did not display signs of similar liver and skeletal muscle damage [41]. These findings thus illustrate the margin between the safety and efficacy of adjudin must be considerably widened before it can be developed as a male contraceptive. This is not entirely unexpected since adjudin displays a relatively poor bioavailability largely because of its poor water solubility. According to a study using [3H]-adjudin, fewer than 1% of adjudin could reach the testis following its administration by oral gavage in male rats [32]. Furthermore, the bioavailability of adjudin is compromised by the BTB which hinders drugs including male contraceptives such as adjudin from being transported from the interstitial space to the adluminal compartment of the seminiferous epithelium. This by and large is due to the presence of robustly expressed drug transporters by Sertoli cells at the BTB, including ATP-binding cassette (ABC)-transporters such as P-glycoprotein and multidrug resistance-associated protein 1 (MRP1), which are found on the plasma membranes of Sertoli cells, germ cells, peritubular myoid cells as well as the endothelial cells that constitute the microvessels in the interstitium [5, 8, 42]. Furthermore, ABC transporter breast cancer resistance protein (BCRP) is also robustly expressed by peritubular myoid cells in the tunica propria, and by endothelial cells of the microvessels in the interstitium [43], but not by Sertoli cells at the BTB or germ cells in the seminiferous epithelium. These efflux drug pumps are working in concert to actively pump exogenous drugs out of the testis if they somehow penetrated the BTB via specific drug transporters such as influx drug pumps. Also, these efflux drug pumps prevent drugs from entering the seminiferous epithelium, thereby limiting drug bioavailability in the testis. Thus, it has become imperative to develop better formulations based on current advances (e.g., slow release formulations) to increase bioavailability of non-hormonal male contraceptives so as to widen the margin between drug efficacy and toxicity. Herein, we briefly review some of the development (including the use of nanotechnology) in drug formulations that target tissue barriers, which will be applicable to develop new formulations of adjudin for its delivery to the testis.
OVERCOMING TISSUE/CELL BARRIERS FOR DRUG DELIVERY
Before a drug can exert its effects in a target organ such as the testis, it has to overcome one of the three anatomical primary barriers: the epidermal barrier of the skin to enter the systemic circulation, the gut barrier of the gastro-intestinal tract, or the epithelial lining of the respiratory tract for its initial uptake. If a drug can penetrate one of these barriers, an internal or secondary barrier is usually present to serve as a second line of defense to protect vital organs which include the blood-brain barrier (BBB) that guards the brain, the blood-ocular/retinal barrier that protects the eye, the maternal-fetal barrier in the placenta that guards the fetus, the blood-epididymal barrier that protects the epididymis, and the BTB that sequesters the seminiferous epithelium of the seminiferous tubules, among others. In short, the presence of these internal barriers further hinders a drug’s bioavailability and its pharmacological actions, thus limiting its clinical uses. To improve drug bioavailability, two approaches are being actively pursued. The first approach attempts to penetrate tissue/cell barriers via the paracellular pathway, and the second focuses on increasing the transcellular drug uptake. Table 1 summarizes recent advances in both approaches to deliver drugs behind tissue/cell barriers.
Table 1.
Typical strategies used for delivery of selected therapeutic agents across tissue/cell barriers*.
| Strategies | Drugs | Targeted Tissue Barrier(s) | References | |
|---|---|---|---|---|
| The Paracellular Ap- proach | Attachment of surfactant-like agents to the drug (e.g., sodium caprate) | Berberine | Gut barrier | [135] |
| Delivery of specific peptides to modulate tight junction proteins (e.g., synthetic peptide corresponding to the C-terminal ex tracellular loop 1 of rat claudin-1 that transiently perturbs the TJ- permeability barrier) | DAMGO, tetrodotoxin | Perineurial barrier | [50] | |
| Delivery of specific siRNA or shRNA that targets junction proteins (e.g., claudin-5 shRNA or siRNA) | Small molecules (<1 kDa) (e.g., sunitinib malate) | Blood-brain barrier, inner-blood- retina barrier | [44, 56, 136] | |
| The Transcellular Ap- proach | Design of lipophilic drugs (e.g. ester-linked/acetylated prodrugs or drug-in-liposome) | Thiorphan | Blood-brain barrier, blood- cerebrospinal fluid barrier | [137, 138] |
| Design of prodrugs that target specific protein transporters or receptors on the cell surface (e.g. valacyclovir that targets trans porter PEPT1 & 2 or a vitamin B12 based insulin conjugate) | Acyclovir Insulin |
Renal epithelial barrier, Gut barrier | [139] [140] |
|
| Drug particle microniztion (e.g., preparation of drugs using super- critical fluid technology) | Tetracycline | Gut barrier | [70] | |
| Nanoparticulate strategies (e.g., combination of lipid-based or polymer-based nanoparticles) | Nitrendipine Rivastigmine |
Blood-brain Barrier | [141] [142] |
This Table is not intended to be exhaustive. Only selective examples are shown herein to support discussion for delivery of male contraceptives (see text for detail). Thus, the use of different delivery routes such as nasal or transdermal delivery to circumvent biological barriers are not included. DAMGO, [D-Ala2, N-MePhe4, Gly5-ol]-enkephalin; PEPT1 & 2, peptide transporter 1 and 2.
The Paracellular Approach
The paracellular barrier is an important mechanism used by internal tissue/cell barriers to limit drug bioavailability. Thus, modifications of paracellular barrier permeability by pharmaceutical formulation are widely used in the field. Agents such as siRNA, medium chained fatty acids, antibodies and peptides have shown promising regulatory effects by specifically targeting epithelial junctions [44–48]. Multiple peptides have been reported to perturb TJ-permeability function by targeting the extracellular domains of crucial TJ-integral membrane proteins. For instance, a 22-amino acid peptide corresponding to a stretch of sequence of the second extracellular loop of rat occludin is known to induce reversible disruption of the Sertoli cell TJ-barrier in vitro and/or in vivo [48, 49]. Also, a synthetic peptide designated C1C2 which derived from rat claudin-1 is capable of modulating TJ function in rat perineurium to facilitate the entry of antinociceptive drug into the peripheral nervous system [50]. Recently, a peptide likely produced endogenously during spermiation at late stage VIII of the epithelial cycle via the action of MMP-2 on laminin chains at the apical ES [51, 52] has been shown to disrupt the Sertoli cell BTB function transiently with high potency [46, 53]. In short, this peptide coordinates the events of spermiation to release mature sperm and BTB remodeling to facilitate preleptotene spermatocyte transport across the immunological barrier, both of which take place at stage VIII of the epithelial cycle but at the opposite ends of the epithelium [54]. Moreover, this biologically active peptide designated F5-peptide derived from the laminin-γ3 chain also induces germ cell exfoliation effectively when administered to the testis intratesticularly [46], illustrating its potential as a reversible male contraceptive. This latter observation also suggests that besides exerting its effects at the Sertoli cell BTB, the F5-peptide can potentiate apical ES disruption to facilitate sperm release at spermiation. Collectively, these findings suggest an attractive multidrug approach of using F5-peptide and adjudin in which F5-peptide perturbs the paracellular barrier at the BTB to facilitate the entry of adjudin into the adluminal compartment to induce germ cell loss. This thus widens the gap between the efficacy and toxicity of adjudin.
On the other hand, it may be a viable approach by manipulating the Sertoli cell TJ-barrier permeability through a down-regulation on the expression of TJ integral membrane or adaptor proteins (e.g., ZO-1, oc-cludin and claudins) to improve drug bioavailability behind the BTB via the use of specific siRNA or shRNA. Studies in other tissue barriers have shown that an enhanced uptake of neuropeptide thyrotropin-releasing hormone behind the BBB in the mouse brain is observed within 48 hr following the administration of claudin-5 siRNA [55]. Furthermore, shRNA specific to claudin-5 can facilitate the entry of low-molecular weight drugs sunitinib malate (532 Da) and 17-AAG (585 Da) through blood-retinal barrier [56]. Nonetheless, the delivery of siRNA duplexes or shRNA is also hindered by the biological barriers, in part due to the negatively charged backbone of the nucleic acid, compounded with the intrinsic instability against endonu-cleases and rapid clearance [57, 58]. Thus, much work is needed to develop a better delivery system for successful application of siRNA or shRNA-based therapy. Recent attempts that protect siRNA with TEA-core PAMAM dendrimer [59], polyethylenimine/poly (lactide-co-glycolide) matrix [60], and chitosan derivatives [61, 62] have sparked some excitements for better siRNA delivery. Since studies have shown that the Sertoli cell TJ-permeability function can be transiently perturbed by using RNAi by targeting proteins that maintain the actin microfilament bundles at the basal ES/BTB such as palladin [63], ezrin [64], and Eps8 [65], a multidrug approach by combining adjudin and one of these siRNA duplexes should be considered in future investigations.
The Transcellular Approach
This approach aims at circumventing biological barriers by enhancing cellular uptake of drug molecules. There are several approaches to improve drug bioavailability via the transcellular pathway:
Drug Micronization
Micronization technology is the most widely used approach that substantially decreases the size of drug particles so that pharmaceutical agents can readily penetrate through cell membrane in a target organ. Adjudin per se is practically insoluble in water, and only ~1 mg/ml in ethanol but ~133 mg/ml in DMSO (a cytotoxic agent), which thus partially accounts for its poor bioavailability. Previous effort has been made to micronize adjudin by pulverization, which shrunk the particle size of adjudin to ~50 μm, thus significantly lowered the effective dose to ~16 mg/kg b.w. along with improved drug solubility [66]. However, conventional micronization methods such as ultrafine milling, spray-drying and liquid anti-solvent crystallization often result in variable distribution of drug particle size, which thus hinders their use for effective and consistent absorption. Consequently, other alternatives have been investigated during the past decade, which include supercritical fluid (SCF) techniques [67, 68]. By manipulating temperature and pressure, it is now possible to produce much smaller drug particles with significantly narrowed size distribution. In addition, with appropriate solvents/co-solvents and procedures, the entire process can take place at ambient temperature, which is particularly ideal for the production of heat-sensitive drugs [68]. For instance, using SCF-CO2 method, the production of more stable and uniform liposomes can be achieved that serve as better carriers for drugs [69]. Earlier SCF technologies such as the Rapid Expansion of Supercritical Solutions (RESS), Gas Anti Solvent (GAS) and Supercritical Anti Solvent (SAS) were used to produce particles of ~0.7–5.0 μm in size [70]. Recent SCF processes are capable of obtaining pharmaceutical particles at a nanometer range, such as <300 nm, which thus significantly increases dissolution rate and oral bioavailability of different drugs [70, 71]. Thus, work is in progress in our laboratory to encapsulate the practically water insoluble adjudin in liposomes or to prepare uniform fine particles of adjudin with the use of SCF technology to improve its bioavailability and efficacy.
Protein Transduction Domain for Delivery of Macromolecules
The transcellular entry of proteins via the plasma membrane was first reported in 1988 when the HIV TAT (HIV trans-activator of transcription) protein was shown to be capable of entering mammalian cells to activate HIV transcription [72, 73]. Conjugation of peptide fragments from HIV TAT or full length functional proteins was found to be an effective approach to deliver large molecules to mammalian cells [74]. Furthermore, only the amino acid residues spanning 48–60 of the TAT protein is necessary to induce effective cellular internalization [75]. For instance, administration of a 120 kDa TAT(47–57 amino acid residues)-β-galactosidase fusion protein to mice by i.p. was found to be effectively delivered to lung, heart muscle, and spleen in vivo [76]. Thus, following the discovery of this stretch of sequence of TAT from 47–57, a 11-amino acid peptide, known as Protein/Peptide Trans-duction Domain (PTD), multiple PTDs have since been identified and designated Cell Penetrating Peptides (CPPs) [77], such as low molecular weight protamine (LMWP) which is an effective CPP [78]. Most PTDs are basic peptides composed of multiple Arg residues between 9 and 20 amino acid residues, and can effectively transport proteins, peptides, siRNA, and siRNA nanoparticles across cell membranes [79]. It is now generally accepted that protein transduction occurs by first binding of the PTD with or without a cargo protein/molecule to the plasma membrane, to be followed by endocytic vesicle-mediated internalization, and the release of the cargo to the cell cytosol [79], analogous to the endocytic vesicle-mediated trafficking events that take place at the Sertoli cell BTB [80, 81]. In short, the use of PTD-conjugated adjudin or other potential male contraceptive is a novel and alternative approach of delivering adjudin behind the BTB for male contraception. For instance, a short PTD peptide can be conjugated to adjudin via the use of a heterobifunctional cross-linker (e.g., SFB, succinimidyl 4-formyl-benzoate) which generate a benzaldehyde at the N-terminus of the PTD peptide, which then reacts spontaneously with adjudin at its hydrazide group in physiological buffer through a stable hydrazone linkage as recently reported by conjugating adjudin to keyhole limpet hemocyanin (KLH, an adjuvant), for producing an anti-adjudin antibody [82]. Such a conjugate is likely to have better penetrability across the testis, which should be explored in future studies.
Receptor-Mediated Transcellular Drug Transport
Integral membrane receptors or peptide transporters (e.g., OATPs) are potential targets for drug delivery and also drug selectivity. In short, conjugation of a drug to a synthetic peptide or recombinant protein that bears structural resemblance to the physiologically occurring substrates or ligands can be a novel approach to deliver drugs across plasma membrane [83]. In the mammalian testis, follicle-stimulating hormone (FSH) receptors are restricted to Sertoli cells [84, 85]. Thus, FSH is a promising carrier to deliver male contraceptives such as adjudin selectively to testis. In a proof-of-concept study, a FSH mutant was prepared through site-directed mutagenesis by deleting two glycosylation sites in the α subunit and one glycosylation site in the β subunit [41]. These modifications rendered the FSH mutant a loss of hormonal activity without compromising its receptor-binding capability. The FSH mutant was then conjugated to adjudin through a hydrazone bond [41]. This adjudin-FSH mutant conjugate was highly effective in inducing reversible infertility in adult rats with a significantly lower dose [41]. However, this approach is prohibitively expensive in particular if this adjudin-FSH conjugate contraceptive drug is to be used in developing countries. Also, it requires parenteral administration, posing an acceptability obstacle, unless this mutant-adjudin conjugate can overcome the proteolysis of FSH polypeptide in the GI tract when the conjugate is orally administered, such as using the approach developed for oral delivery of insulin [86], including the use of nanoparticles containing absorption enhancers (e.g., surfactants, zonula occludens toxins) and proteolytic enzyme inhibitors (e.g., bacitracin, aprotinin, soybean trypsin inhibitor, polymer-inhibitor conjugates) [86–88]. A recent study had also used this approach to conjugate a permanent male contraceptive melphalan (also a cytotoxic and gonado-toxic nitrogen mustard alkylating agent that kills murine testicular cells) to FSH-ß peptide to be used to target melphalan to the testis for chemical sterilization in wild-life animals and also pets such as cats and dogs [89].
NANOPARTICLES (NPS) FOR DELIVERY OF NON-HORMONAL MALE CONTRACEPTIVES
Nanoparticles, with particle sizes ranging between 1 and 1000 nm, have been intensively investigated for the delivery of numerous therapeutic agents mostly as carriers for anti-cancer drugs for chemotherapy. Conventional drug molecules are either conjugated onto the surface of NPs or encapsulated into the core, if NPs carrying multiple drugs are delivered to a target organ to improve the efficacy. These ‘nanodrugs’ are designed to transport drugs that have low aqueous solubility or are susceptible to enzymatic cleavage since drugs are placed inside the core of the iron-based nanoparticles until they are uptaken by cells in a target organ, such as with the aid of a magnetic field [90, 91]. Furthermore, small NPs (i.e., <100 nm) are having molecular sizes that facilitate their passage across internal biological barriers [92, 93]. NPs are usually made of nanomaterials such as lipids, metallic materials, polymers (e.g., chitosan), dentrimers, nanocrystals (e.g., semi-conductor quantum dots), carbon nanotubes, mesoporous materials and iron oxide-based magnetic nanomaterials. Among them, mesoporous materials are emerging as leading carriers for drugs including male contraceptive adjudin. For instance, mesoporous silica nanoparticles (MSN) are nanoparticles of ~40–70 nm in diameter with orderly arranged pores called mesopores (or tunable pore sizes) of ~3.8–6.1 nm, high surface areas (700–1100 m2/gm) and large pore volumes (0.44–1.54 cm3/gm) [94–96]. MSN are emerging vehicles for drug delivery [97–99], including small molecular drugs [e.g., anticancer drugs paclitaxel (water insoluble) and doxorubicin (water soluble), anti-hypertension drug telmisartan (poorly water soluble)] [100–102], proteins [97, 103–105] and siRNAs [106–109]. MSN are often loaded with multiple drugs [105, 106, 108, 110] so that drugs with different modes of action can be targeted to a specific organ/tissue to exert their effects to correct a pathological condition. More important, the use of MSN has now entered preclinical development stage for cancer treatment [111–113]. Furthermore, magnetic-based MSN (MMSN), in which MSN is constructed with a magnetic Fe3O4 core surrounded by hexagonally arranged mesopores (Fig. 2), is shown to have better cellular uptake to deliver multidrugs simultaneously to target tissues/organs via the use of strong magnet fielded established in the target organ [110, 114–116]. Studies have also shown that MMSN or MSN enter cells via either endocytic vesicle-mediated pathway [116, 117] or GTPase (e.g., Rac1, Cdc42)-mediated macropinocytosis [118]. Administration of amorphous nanosilica particles to mouse testes (via i.v.) was shown to penetrate the BTB, leading to accumulation of NPs in the Sertoli and germ (e.g., spermatocytes) cells without causing testicular injury [119]. Also, mesopores protect bioactive drugs from undesired enzymatic degradation before reaching the target cells/tissues due to the inaccessibility of the inner surface to enzymes in the systemic circulation and/or tissues (e.g., intestine) [120]. Thus, it is possible that F5-peptide and adjudin can be loaded into hexagonally arranged mesopores of MMSN for their administration orally without the use of a needle, and they can be delivered to the testis specifically via the use of a magnetic field, such as by placing neodymium (NdFeB) permanent magnets in men’s shorts to generate a strong magnetic field. Upon released into the Sertoli cell cyto-sol, F5-peptide disrupts the BTB to facilitate entry of adjudin to the adluminal compartment to induce germ cell loss from the seminiferous epithelium (Fig. 2). To further optimize the targeting of this multi-drug MMSN to the testis, an anti-FSH receptor IgG or recombinant FSH can be incorporated onto the surface of the adjudin/F5-peptide containing MMSN, so that the nanoparticles can home-in to the Sertoli cell for specific delivery besides under the influence of an external magnetic field (e.g., NdFeB magnets) that is placed near the testis. This approach thus avoids parenteral administration, improving acceptability.
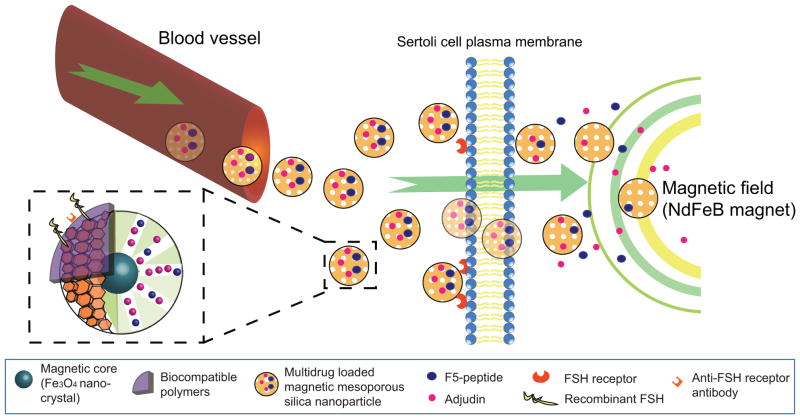
Fig. (2).
A schematic drawing illustrating the strategy that can be used to prepare multidrug MMSN (magnetic mesoporous silica nanoparticles) as non-hormonal male contraceptive. Adjudin together with F5-peptide can be loaded into the hexagonally arranged mesopores of MMSN with a magnetic Fe3O4 core. When administered orally, multidrug MMSN enter the blood vessel as demonstrated in published studies via macropinocytosis (see text for detail). The presence of a static magnetic field in the target organ, such as the testis, can be created by placing permanent neodymium (NdFeB) magnet near the testis such that MMSN enter the Sertoli cell preferentially. The targeted delivery of MMSN to the Sertoli cell in the testis can be further improved by coating either recombinant FSH or anti-FSH receptor antibody on the biocompatible polymers since FSH receptor is expressed only in Sertoli cells in the mammalian body. Thus, MMSN can reach Sertoli cell cytosol via endocytosis and/or macropinocytosis. As such, adjudin and/or F5-peptide can be released from the MMSN and exerts their effects at the ES in the testis to induce transient BTB disruption and germ cell exfoliation. In short, this multidrug MMSN can be further modified by including: (i) a fluorescence tag (e.g., FITC or Cy3) to track its cellular uptake, (ii) an anti-FSH receptor IgG or FSH recombinant protein so that it can be better targeted to the Sertoli cell (even without the magnetic core), (iii) inclusion of an efflux drug transporter inhibitor and/or specific siRNA duplexes to inactivate P-glycoprotein at the BTB if necessary, and (iv) biocompatible polymers to improve its bioavailability and/or resistant to degradation. It is likely that the BTB will eventually be disrupted even when a lower dose of adjudin is used by adopting the multi-drug MMSN nanotechnology for its delivery because of the F5-peptide intrinsic BTB-disrupting activity, and there are concerns about the production of anti-sperm antibodies in men receiving adjudin/F5-peptide MMSN. However, since germ cells would be depleted from the seminiferous epithelium before the BTB is compromised given that the apical ES is more susceptible to adjudin treatment than the basal ES/BTB [36, 131, 132], the production of anti-sperm antibodies is not likely to be an issue.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
As briefly discussed above, there are major advances in the field that will assist the optimization of delivering adjudin to the testis to serve as a non-hormonal male contraceptive. Both paracellular and transcellular approaches exhibit marked advantages in reducing side-effects and enhancing bioavailability of adjudin. It is obvious that much research is needed to gain a full understanding of the mechanisms under which adjudin perturbs germ cell adhesion (in particular spermatids) and BTB integrity following long exposure, and to better understand factors that limit the bioavailability of adjudin. It is noted that by modulating the BTB integrity, even transiently, this may expose meiotic germ cells and developing haploid spermatids to an unfavorable environment such as the systemic circulation, causing unwanted immunological responses. As discussed above, BTB restructuring near the basal compartment and apical ES reorganization during spermiation in the adluminal compartment are tightly coordinated events through an autocrine-based axis in the testis [54]. The reversible and transient disruption of BTB through paracellular approach using siRNA duplexes or biological peptides, although highly effective, may cause unwanted effects such as by perturbing meiosis I/II or escalating germ cell exfoliation that leads to a prolonged recovery phase. Therefore, much challenge remains. Perhaps the nanoparticle-based multidrug approach is one of the most promising leads for future studies. Another challenge for non-hormonal male contraceptive development is the fact that the testis is equipped with an array of drug transporters, both efflux and influx drug pumps, that actively involved in determining the level of adjudin available to the testis following its administration by oral gavage [5, 8, 42, 121]. Furthermore, besides Sertoli cells that constitute the BTB, peritubular myoid cells in the tunica propria also express drug efflux transporters, such as BCRP (breast cancer resistance protein) [43, 122–124]. Moreover, germ cells, in particular spermatogonia, spermatocytes and spermatids including elongating/elongated spermatids, also express multiple drug transporters [5, 8, 42]. These findings illustrate that the bioavailability of adjudin in the testis is determined, at least in part, by an interaction of adjudin to these drug transporters [125]. In fact, studies have shown that the knockdown of P-glycoprotein, an efflux drug transporter, enhances the influx transport of adjudin across the Sertoli cell BTB [126], and studies by utilizing molecular modeling have identified putative docking pocket of adjudin with BCRP [127, 128] and P-glycoprotein [129]. Thus, an inhibitor or a set of inhibitors against drug transporters can also be included in the MMSN-based nanoparticles to optimize the entry of adjudin to the testis specifically (Fig. 2). Recently, SLC15A1, a peptide transporter which predominantly locates at the peritubular myoid cells in rat testes has been found to play a role in mediating the transport of F5 peptide into the seminiferous epithelium following administration of F5 peptide to the testis via intratesticular injection in rodents [130]. This finding indicates that transporters are at play to mediate influx and efflux of macromolecules in the testis and SLC15A1 may be a promising target to facilitate the transport of the F5-adjudin multidrug into the seminiferous epithelium. While much research is needed in the years to come, recent advances in the field have shed new lights in developing novel delivery strategies to the testis, making drugs such as adjudin to be more effective non-hormonal male contraceptives by exerting their effects behind the BTB in the adluminal compartment.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIHCD, U54 HD029990, Project 5 to CYC and R01 HD056034 to CYC).
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Amann RP, Howards SS. Daily spermatozoal production and epididymal spermatozoal reserves of the human male. J Urol. 1980;124:211–215. doi: 10.1016/s0022-5347(17)55377-x. [DOI] [PubMed] [Google Scholar]
- 2.Johnson L, Petty CS, Neaves WB. A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod. 1980;22:1233–1243. doi: 10.1093/biolreprod/22.5.1233. [DOI] [PubMed] [Google Scholar]
- 3.Ehmcke J, Schlatt S. A revised model for spermatogonial expansion in man: lessons from non-human primates. Reproduction. 2006;132:673–680. doi: 10.1530/rep.1.01081. [DOI] [PubMed] [Google Scholar]
- 4.Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist's perspective. Semin Cell Dev Biol. 2014;29:2–16. doi: 10.1016/j.semcdb.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- 8.Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 9.Easton AS. Regulation of permeability across the blood-brain barrier. Adv Exp Med Biol. 2012;763:1–19. doi: 10.1007/978-1-4614-4711-5_1. [DOI] [PubMed] [Google Scholar]
- 10.Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- 11.Yan HHN, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc Natl Acad Sci USA. 2005;102:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of blood-testis barrier via its effects on tight junction reassembly. Proc Natl Acad Sci USA. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia W, Wong CH, Lee NPY, Lee WM, Cheng CY. Disruption of Sertoli-germ cell adhesion function in the seminiferous epithelium of the rat testis can be limited to adherens junctions without affecting the blood-testis barrier integrity: An in vivo study using an androgen suppression model. J Cell Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- 14.Amory JK. Reproductive endocrinology: Male hormonal contraceptive passes efficacy test in China. Nat Rev Endocrinol. 2009;5:359–360. doi: 10.1038/nrendo.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page ST, Amory JK, Bremner WJ. Advances in male contraception. Endocr Rev. 2008;29:465–493. doi: 10.1210/er.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Swerdloff RS. Hormonal approaches to male contraception. Curr Opin Urol. 2010;20:520–524. doi: 10.1097/MOU.0b013e32833f1b4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surampudi P, Page ST, Swerdloff RS, Nya-Ngatchou JJ, Liu PY, Amory JK, Leung A, Hull L, Blithe DL, Woo J, Bremner WJ, Wang C. Single, escalating dose pharmacokinetics, safety and food effects of a new oral androgen dimethandrolone undecanoate in man: a prototype oral male hormonal contraceptive. Andrology. 2014;2:579–587. doi: 10.1111/j.2047-2927.2014.00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilani N, Roth MY, Amory JK, Swerdloff RS, Dart C, Page ST, Bremner WJ, Sitruk-Ware R, Kumar N, Blithe DL, Wang C. A new combination of testosterone and nestorone transdermal gels for male hormonal contraception. J Clin Endocrinol Metab. 2012;97:3476–3486. doi: 10.1210/jc.2012-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mommers E, Kersemaekers WM, Elliesen J, Kepers M, Apter D, Behre HM, Beynon J, Bouloux PM, Costantino A, Gerbershagen HP, Gronlund L, Heger-Mahn D, Huhtaniemi I, Koldewijn EL, Lange C, Lindenberg S, Meriggiola MC, Meuleman E, Mulders PF, Nieschlag E, Perheentupa A, Solomon A, Vaisala L, Wu FC, Zitzmann M. Male hormonal contraception: a double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2008;93:2572–2580. doi: 10.1210/jc.2008-0265. [DOI] [PubMed] [Google Scholar]
- 20.Liu PY, Swerdloff RS, Anawalt BD, Anderson RA, Bremner WJ, Elliesen J, Gu YQ, Kersemaekers WM, McLachlan RI, Meriggiola MC, Nieschlag E, Sitruk-Ware R, Vogelsong K, Wang XH, Wu FC, Zitzmann M, Handelsman DJ, Wang C. Determinants of the rate and extent of spermatogenic suppression during hormonal male contraception: an integrated analysis. J Clin Endocrinol Metab. 2008;93:1774–1783. doi: 10.1210/jc.2007-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth MY, Shih G, Ilani N, Wang C, Page ST, Bremner WJ, Swerdloff RS, Sitruk-Ware R, Blithe DL, Amory JK. Acceptability of a transdermal gel-based male hormonal contraceptive in a randomized controlled trial. Contraception. 2014;90:407–412. doi: 10.1016/j.contraception.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zirkin BR. Spermatogenesis: its regulation by testosterone and FSH. Semin Cell Dev Biol. 1998;9:417–421. doi: 10.1006/scdb.1998.0253. [DOI] [PubMed] [Google Scholar]
- 23.Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann NY Acad Sci. 2005;1061:208–220. doi: 10.1196/annals.1336.023. [DOI] [PubMed] [Google Scholar]
- 24.Turner TT, Jones CC, Howards SS, Ewing LL, Zegeye B, Gunsalus GL. On the androgen micro-environment of maturing spermatozoa. Endocrinology. 1984;115:1925–1932. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- 25.McLachlan RI, O'Donnell L, Meachem SJ, Stanton PG, De Kretser DM, Pratis K, Robertson DM. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179. doi: 10.1210/rp.57.1.149. [DOI] [PubMed] [Google Scholar]
- 26.Mok KW, Mruk DD, Lie PP, Lui WY, Cheng CY. Adjudin, a potential male contraceptive, exerts its effects locally in the seminiferous epithelium of mammalian testes. Reproduction. 2011;141:571–580. doi: 10.1530/REP-10-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moulana M, Lima R, Reckelhoff JF. Metabolic syndrome, androgens, and hypertension. Curr Hypertens Rep. 2011;13:158–162. doi: 10.1007/s11906-011-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilani N, Swerdloff RS, Wang C. Male hormonal contraception: potential risks and benefits. Rev Endocr Metab Disord. 2011;12:107–117. doi: 10.1007/s11154-011-9183-3. [DOI] [PubMed] [Google Scholar]
- 29.Mostaghel EA, Lin DW, Amory JK, Wright JL, Marck BT, Nelson PS, Matsumoto AM, Bremner WJ, Page ST. Impact of male hormonal contraception on prostate androgens and androgen action in healthy men: a randomized, controlled trial. J Clin Endocrinol Metab. 2012;97:2809–2817. doi: 10.1210/jc.2012-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mruk DD, Cheng CY. Delivering non-hormonal contraceptives to men: advances and obstacles. Trends Biotechnol. 2008;26:90–99. doi: 10.1016/j.tibtech.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu ST, Gui YL, Lin CH, He CH. Hormonal contraception in Chinese men: variations in suppression of spermatogenesis with injectable testosterone undecanoate and levonorgestrel implants. Asian J Androl. 2004;6:41–46. [PubMed] [Google Scholar]
- 32.Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, Lee NP, Lui WY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohyd-razide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohyd-razide. Biol Reprod. 2001;64:1500–1508. doi: 10.1095/biolreprod64.5.1500. [DOI] [PubMed] [Google Scholar]
- 34.Tash JS, Chakrasali R, Jakkaraj SR, Hughes J, Smith SK, Hornbaker K, Heckert LL, Ozturk SB, Hadden MK, Kinzy TG, Blagg BSJ, Georg GI. Gamendazole, an orally active indazole carboxylic acid male contraceptive agent, targets HSP90AB1 (HSP90BHETA) and EEF1A1 (eEF1A), and stimulates Il1a transcription in rat Sertoli cells. Biol Reprod. 2008;78:1139–1152. doi: 10.1095/biolreprod.107.062679. [DOI] [PubMed] [Google Scholar]
- 35.Tash JS, Attardi B, Hild SA, Chakrasali R, Jakkaraj SR, Georg GI. A novel potent indazole carboxylic acid derivative blocks spermatogenesis and is contraceptive in rats after a single oral dose. Biol Reprod. 2008;78:1127–1138. doi: 10.1095/biolreprod.106.057810. [DOI] [PubMed] [Google Scholar]
- 36.Cheng CY. Toxicants target cell junctions in the testis - insights from the indazole-carboxylic acid model. Spermatogenesis. 2014;4:e981485. doi: 10.4161/21565562.2014.981485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie QR, Liu Y, Shao J, Yang J, Liu T, Zhang T, Wang B, Mruk DD, Silvestrini B, Cheng CY, Xia W. Male contraceptive Adjudin is a potential anti-cancer drug. Biochem Pharmacol. 2013;85:345–355. doi: 10.1016/j.bcp.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu T, Zhang T, Yu H, Shen H, Xia W. Adjudin protects against cerebral ischemia reperfusion injury by inhibition of neuroinflammation and blood-brain barrier disruption. J Neuroinflammation. 2014;11:107. doi: 10.1186/1742-2094-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao J, Liu T, Xie QR, Zhang T, Yu H, Wang B, Ying W, Mruk DD, Silvestrini B, Cheng CY, Xia W. Adjudin attenuates lipopolysaccharide (LPS)- and ischemia-induced microglial activation. J Neuroimmunol. 2013;254:83–90. doi: 10.1016/j.jneuroim.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quan Y, Xia L, Shao J, Yin S, Cheng CY, Xia W, Gao WQ. Adjudin protects rodent cochlear hair cells against gentamicin ototoxicity via the SIRT3-ROS pathway. Sci Rep. 2015;5:8181. doi: 10.1038/srep08181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mruk DD, Wong CH, Silvestrini B, Cheng CY. A male contraceptive targeting germ cell adhesion. Nat Med. 2006;12:1323–1328. doi: 10.1038/nm1420. [DOI] [PubMed] [Google Scholar]
- 42.Mruk DD, Su L, Cheng CY. Emerging role for drug transporters at the blood-testis barrier. Trends Pharmacol Sci. 2011;32:99–106. doi: 10.1016/j.tips.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian X, Cheng YH, Mruk DD, Cheng CY. Breast cancer resistance protein (Bcrp) and the testis - an unexpected turn of events. Asian J Androl. 2013;15:455–460. doi: 10.1038/aja.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell M, Hanrahan F, Gobbo OL, Kelly ME, Kiang AS, Humphries MM, Nguyen AT, Ozaki E, Keaney J, Blau CW, Kerskens CM, Cahalan SD, Callanan JJ, Wallace E, Grant GA, Doherty CP, Humphries P. Targeted suppression of claudin-5 decreases cerebral oedema and improves cognitive outcome following traumatic brain injury. Nat Commun. 2012;3:849. doi: 10.1038/ncomms1852. [DOI] [PubMed] [Google Scholar]
- 45.Kato-Nakano M, Suzuki M, Kawamoto S, Furuya A, Ohta S, Nakamura K, Ando H. Characterization and evaluation of the antitumour activity of a dual-targeting monoclonal antibody against claudin-3 and claudin-4. Anticancer Res. 2010;30:4555–4562. [PubMed] [Google Scholar]
- 46.Su L, Mruk DD, Lie PP, Silvestrini B, Cheng CY. A peptide derived from laminin-gamma3 reversibly impairs spermatogenesis in rats. Nat Commun. 2012;3:1185. doi: 10.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindmark T, Kimura Y, Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J Pharmacol Exp Ther. 1998;284:362–369. [PubMed] [Google Scholar]
- 48.Chung NP, Mruk D, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversiblyin vivo. Biol Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- 49.Wong V, Gumbiner B. A synthetic peptide corresponding to the extracellular domain of occludin perturbs the tight junction permeability barrier. J Cell Biol. 1997;136:399–409. doi: 10.1083/jcb.136.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwanziger D, Hackel D, Staat C, Bocker A, Brack A, Beyermann M, Rittner H, Blasig IE. A peptidomimetic tight junction modulator to improve regional analgesia. Mol Pharm. 2012;9:1785–1794. doi: 10.1021/mp3000937. [DOI] [PubMed] [Google Scholar]
- 51.Siu MK, Cheng CY. Interactions of proteases, protease inhibitors, and the beta1 integrin/laminin gamma3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- 52.Yan HH, Cheng CY. Laminin alpha 3 forms a complex with beta3 and gamma3 chains that serves as the ligand for alpha 6beta1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- 53.Yan HH, Mruk DD, Wong EW, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell M, Kiang AS, Kenna PF, Kerskens C, Blau C, O'Dwyer L, Tivnan A, Kelly JA, Brankin B, Farrar GJ, Humphries P. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med. 2008;10:930–947. doi: 10.1002/jgm.1211. [DOI] [PubMed] [Google Scholar]
- 56.Campbell M, Humphries MM, Kiang AS, Nguyen AT, Gobbo OL, Tam LC, Suzuki M, Hanrahan F, Ozaki E, Farrar GJ, Kenna PF, Humphries P. Systemic low-molecular weight drug delivery to preselected neuronal regions. EMBO Mol Med. 2011;3:235–245. doi: 10.1002/emmm.201100126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fukuda AM, Badaut J. siRNA treatment: "a sword-in-the-stone" for acute brain injuries. Genes Cancer. 2013;4:435–456. doi: 10.3390/genes4030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bora RS, Gupta D, Mukkur TK, Saini KS. RNA interference therapeutics for cancer: challenges and opportunities. Mol Med Rep. 2012;6:9–15. doi: 10.3892/mmr.2012.871. [DOI] [PubMed] [Google Scholar]
- 59.Kala S, Mak AS, Liu X, Posocco P, Pricl S, Peng L, Wong AS. Combination of dendrimer-nanovector-mediated small interfering RNA delivery to target Akt with the clinical anticancer drug paclitaxel for effective and potent anticancer activity in treating ovarian cancer. J Med Chem. 2014;57:2634–2642. doi: 10.1021/jm401907z. [DOI] [PubMed] [Google Scholar]
- 60.Das J, Das S, Paul A, Samadder A, Bhattacharyya SS, Khuda-Bukhsh AR. Assessment of drug delivery and anticancer potentials of nanoparticles-loaded siRNA targeting STAT3 in lung cancer, in vitro and in vivo. Toxicol Lett. 2014;225:454–466. doi: 10.1016/j.toxlet.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Mao S, Sun W, Kissel T. Chitosan–based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Sadio A, Gustafsson JK, Pereira B, Gomes CP, Hansson GC, David L, Pego AP, Almeida R. Modified-chitosan/siRNA nanoparticles downregulate cellular CDX2 expression and cross the gastric mucus barrier. PLoS One. 2014;9:e99449. doi: 10.1371/journal.pone.0099449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013;154:1907–1920. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gungor-Ordueri NE, Tang EI, Celik-Ozenci C, Cheng CY. Ezrin is an actin binding protein that regulates Sertoli cell and spermatid adhesion during spermatogenesis. Endocrinology. 2014;155:3981–3995. doi: 10.1210/en.2014-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng CY, Mruk DD. New frontiers in nonhormonal male contraception. Contraception. 2010;82:476–482. doi: 10.1016/j.contraception.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deshpande PB, Kumar GA, Kumar AR, Shavi GV, Karthik A, Reddy MS, Udupa N. Supercritical fluid technology: concepts and pharmaceutical applications. PDA J Pharm Sci Technol. 2011;65:333–344. doi: 10.5731/pdajpst.2011.00717. [DOI] [PubMed] [Google Scholar]
- 68.Martin A, Cocero MJ. Micronization processes with supercritical fluids: fundamentals and mechanisms. Adv Drug Deliv Rev. 2008;60:339–350. doi: 10.1016/j.addr.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 69.Karn PR, Cho W, Hwang SJ. Liposomal drug products and recent advances in the synthesis of supercritical fluid-mediated liposomes. Nanomedicine (Lond) 2013;8:1529–1548. doi: 10.2217/nnm.13.131. [DOI] [PubMed] [Google Scholar]
- 70.Chattopadhyay P, Gupta RB. Production of antibiotic nanoparticles using supercritical CO2 as antisolvent with enhanced mass transfer. Ind Eng Chem Res. 2001;40:3530–3539. doi: 10.1016/s0378-5173(01)00803-1. [DOI] [PubMed] [Google Scholar]
- 71.Imperiale JC, Bevilacqua G, Rosa PD, Sosnik A. Production of pure indinavir free base nanoparticles by a supercritical anti-solvent (SAS) method. Drug Dev Ind Pharm. 2013 doi: 10.3109/03639045.2013.838581. [DOI] [PubMed] [Google Scholar]
- 72.Green M, Loewenstein PM. Autonomous functional domains of chemicaclly synthesized human immuno-deficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 73.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 74.Fawell S, Seery J, Daikh Y, Moore C, Chen L, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 76.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 77.Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides A reevaluation of the mechanism of cellular uptake. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 78.He H, Ye J, Liu E, Liang Q, Liu Q, Yang VC. Low molecular weight protamine (LMWP): A nontoxic protamine substitute and an effective cell-penetrating peptide. J Control Release. 2014;193:63–73. doi: 10.1016/j.jconrel.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 79.van den Berg A, Dowdy SF. Protein transduction domain delivery of therapeutic macromolecules. Curr Opin Biotechnol. 2011;22:888–893. doi: 10.1016/j.copbio.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 80.Xiao X, Wong EWP, Lie PPY, Mruk DD, Wong CKC, Cheng CY. Cytokines, polarity proteins and endosomal protein trafficking and signaling - the Sertoli cell blood-testis barrier in vitro as a study model. Methods Enzymol. 2014;534:181–194. doi: 10.1016/B978-0-12-397926-1.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao X, Mruk DD, Wong EW, Lee WM, Han D, Wong CK, Cheng CY. Differential effects of c-Src and c-Yes on the endocytic vesicle-mediated trafficking events at the Sertoli cell blood-testis barrier: an in vitro study. Am J Physiol Endocrinol Metab. 2014;307:E553–562. doi: 10.1152/ajpendo.00176.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mruk DD, Cheng CY. Testin and actin are key molecular targets of adjudin, an anti-spermatogenic agent, in the testis. Spermatogenesis. 2011;1:137–146. doi: 10.4161/spmg.1.2.16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brandsch M, Knutter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60:543–585. doi: 10.1211/jpp.60.5.0002. [DOI] [PubMed] [Google Scholar]
- 84.Griswold MD, Heckert L, Linder C. The molecular biology of the FSH receptor. J Steroid Biochem Mol Biol. 1995;53:215–218. doi: 10.1016/0960-0760(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 85.Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- 86.Park K, Kwon IC, Park K. Oral protein delivery: Current status and future prospect. React Funct Polym. 2011;71:280–287. [Google Scholar]
- 87.Hwang SR, Byun Y. Advances in oral macromolecular drug delivery. Expert Opin Drug Deliv. 2014;31:1–13. doi: 10.1517/17425247.2014.945420. [DOI] [PubMed] [Google Scholar]
- 88.Smart AL, Gaisford S, Basit AW. Oral peptide and protein delivery: intestinal obstacles and commercial prospects. Expert Opin Drug Deliv. 2014;11:1323–1335. doi: 10.1517/17425247.2014.917077. [DOI] [PubMed] [Google Scholar]
- 89.Amory JK, Hong SW, Yu X, Muller CH, Faustman EM, Goldstein A. Melphalan, alone or conjugated to an FSH-β, peptide, kills murine testicular cells in vitro and transiently suppresses murine spermatogenesis in vivo. Theriogenology. 2014;82:152–159. doi: 10.1016/j.theriogenology.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sensenig R, Sapir Y, MacDonald C, Cohen S, Polyak B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine. 2012;7:1425–1442. doi: 10.2217/nnm.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seeney C, Ojwang JO, Weiss RD, Klostergaard J. Megnetically vectored platforms for the targeted delivery of therapeutics to tumors: history and current status. Nanomedicine. 2012;7:289–299. doi: 10.2217/nnm.11.183. [DOI] [PubMed] [Google Scholar]
- 92.Kievit FM, Zhang M. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv Mater. 2011;23:H217–247. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pietroiusti A, Campagnolo L, Fadeel B. Interactions of engineered nanoparticles with organs protected by internal biological barriers. Small. 2013;9:1557–1572. doi: 10.1002/smll.201201463. [DOI] [PubMed] [Google Scholar]
- 94.Slowing II, Vivero-Escoto JL, Wu CW, Lin VS. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 95.Roggers R, Kanvinde S, Boonsith S, Oupicky D. The practicality of mesoporous silica nanoparticles as drug delivery devices and progress toward this goal. AAPS Pharm Sci Tech. 2014;15:1163–1171. doi: 10.1208/s12249-014-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen Y, Chen H, Shi J. Drug delivery/imaging multi-functionality of mesoporous silica-based composite nanostructures. Expert Opin Drug Deliv. 2014;11:917–930. doi: 10.1517/17425247.2014.908181. [DOI] [PubMed] [Google Scholar]
- 97.Slowing II, Trewyn BG, Lin VS. Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins. J Am Chem Soc. 2007;129:8845–8849. doi: 10.1021/ja0719780. [DOI] [PubMed] [Google Scholar]
- 98.Vallet-Regi M, Balas F, Arcos D. Mesoporous materials for drug delivery. Angew Chem Int Ed Engl. 2007;46:7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 99.Zhao Y, Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VS. Capped mesoporous silica nanoparticles as stimuli-presplnsive controlled release systems for intracellular drug/gene delivery. Expert Opin Drug Deliv. 2010;7:1013–1029. doi: 10.1517/17425247.2010.498816. [DOI] [PubMed] [Google Scholar]
- 100.Liu Q, Xia W. Mesoporous Silica Nanoparticles for Cancer Therapy. In: Lee NP, Cheng CY, Luk JM, editors. New Advances on Disease Biomarkers and Molecular Targets in Biomedicine. Springer, Science+Business Media; New York: 2013. pp. 231–242. [Google Scholar]
- 101.Thomas MJ, Slipper I, Walunj A, Jain A, Favretto ME, PK, Douroumis D. Inclusion of poorly soluble drugs in highly ordered mesoporous silica nanoparticles. Int J Pharm. 2010;387:272–277. doi: 10.1016/j.ijpharm.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Zhi Z, Jiang T, Zhang J, Wang Z, Wang S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J Control Release. 2010;145:257–263. doi: 10.1016/j.jconrel.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 103.Chen YP, Chen CT, Hung Y, Chou CM, Liu TP, Liang MR, Chen CT, Mou CY. A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: superoxide dismutase. J Am Chem Soc. 2013;135:1516–1523. doi: 10.1021/ja3105208. [DOI] [PubMed] [Google Scholar]
- 104.Liu T, Liu H, Fu C, Li L, Chen D, Zhang Y, Tang F. Silica nanorattle with enhanced protein loading: a potential vaccine adjuvant. J Colloid Interface Sci. 2013;400:168–174. doi: 10.1016/j.jcis.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Zhao Y, Trewyn BG, Slowing II, Lin VS. Meso-porous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc. 2009;131:8398–8400. doi: 10.1021/ja901831u. [DOI] [PubMed] [Google Scholar]
- 106.Meng H, Mai WX, Zhang H, Xue M, Xia T, Lin S, Wang X, Zhao Y, Ji Z, Zink JI, Nel AE. Codelivery of an optimal drugs/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7:994–1005. doi: 10.1021/nn3044066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, Tamanoi F. Mesoporouis silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells. Small. 2010;6:1185–1190. doi: 10.1002/smll.200901966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meng H, Liong M, Xia T, Li Z, Ji Z, Zink JI, Nel AE. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano. 2010;4:4539–4550. doi: 10.1021/nn100690m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li X, Xie QR, Zhang J, Xia W, Gu H. The packaging of siRNA within the mesoporous structure of silica nanoparticles. Biomaterials. 2011;32:9546–9556. doi: 10.1016/j.biomaterials.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 110.Liu Q, Zhang J, Sun W, Xie QR, Xia W, Gu H. Delivering hydrophilic and hydrophobic chemotherapeutics simultaneously by magnetic mesoporous silica nanol-particles to inhibit cancer cells. Int J Nanomed. 2102;7:999–1013. doi: 10.2147/IJN.S28088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosenholm JM, Sahlgren C, Linden M. Multifunctional mesoporous silica nanoparticles for combined therapeutic, diagnostic and targeted action in cancer treatment. Curr Drug Targets. 2011;12:1166–1186. doi: 10.2174/138945011795906624. [DOI] [PubMed] [Google Scholar]
- 112.Rosenholm JM, Mamaeva V, Sahlgren C, Linden M. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine. 2012;7:111–120. doi: 10.2217/nnm.11.166. [DOI] [PubMed] [Google Scholar]
- 113.He Q, Shi J. MSN anti-cancer nanomedicines: chemotherapy enhancement, overcoming of drug resistance, and metastasis inhibition. Adv Mater. 2014;26:391–411. doi: 10.1002/adma.201303123. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J, Li X, Rosenholm JM, Gu H. Synthesis and characterization of pore size-tunable magnetic mesoporous silica nanoparticles. J Colloid Interface Sci. 2011;361:16–24. doi: 10.1016/j.jcis.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 115.Liu Q, Zhang J, Xia W, Gu H. Towards magnetic-enhanced cellular uptake, MRI and chemotherapeutics delivery by magnetic mesoporous silica nanoparticles. J Nanosci Nanotechnol. 2012;12:7709–7715. doi: 10.1166/jnn.2012.6618. [DOI] [PubMed] [Google Scholar]
- 116.Liu Q, Zhang J, Xia W, Gu H. Magnetic field enhanced cell uptake efficiency of magnetic silica mesoporous nanoparticles. Nanoscale. 2012;4:3415–3421. doi: 10.1039/c2nr30352c. [DOI] [PubMed] [Google Scholar]
- 117.Ekkapongpisit M, Giovia A, Follo C, Caputo G, Isidoro C. Bioompatibility, endocytosis, and intracellular trafficking of mesoporous silica and polystyrene nano-particles in ovarian cancer cells: effects of size and surface charge groups. Int J Nanomed. 2012;7:4147–4158. doi: 10.2147/IJN.S33803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meng H, Yang S, Li Z, Xia T, Chen J, Ji Z, Zhang H, Wang X, Lin S, Huang C, Zhou ZH, Zink JI, Nel AE. Aspect ratio determines the quantity of mesoporous silica nanoparticle uptake by a small GTPase-dependent macropinocytosis mechanism. ACS Nano. 2011;5:4434–4447. doi: 10.1021/nn103344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morishita Y, Yoshioka Y, Satoh H, Nojiri N, Nagano K, Abe Y, Kamada H, Tsunoda SI, Nebeshi H, Yoshikawa T, Tsutsumi Y. Distribution and histologic effects of intravenously administered amorphous nanosilica particles in the testes of mice. Biochem Biophys Res Commun. 2012;420:297–301. doi: 10.1016/j.bbrc.2012.02.153. [DOI] [PubMed] [Google Scholar]
- 120.Botella P, Gao F, Corma A, Blesa J, Dong L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J Phys Chem B. 2009;113:1796–1804. doi: 10.1021/jp807956r. [DOI] [PubMed] [Google Scholar]
- 121.Su L, Mruk DD, Cheng CY. Drug transporters, the blood-testis barrier and spermatogenesis. J Endocrinol. 2011;208:207–223. doi: 10.1677/JOE-10-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qian X, Mruk DD, Wong EWP, Cheng CY. Breast cancer resistance protein regulates apical ectoplasmic specialization dynamics stage specifically in the rat testis. Am J Physiol Endocrinol Metab. 2013;304:E757–E769. doi: 10.1152/ajpendo.00645.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bart J, Hollema H, Groen HJ, de Vries EG, Hendrikse NH, Sleijfer DT, Wegman TD, Vaalburg W, van der Graaf WT. The distribution of drug-efflux pumps, P-gp, BCRP, MRP1 and MRP2, in the normal blood-testis barrier and in primary testicular tumours. Eur J Cancer. 2004;40:2064–2070. doi: 10.1016/j.ejca.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 124.Dankers ACA, Sweep FCGJ, Pertijs JCLM, Verweij V, Van den Heuvel JJMW, Koenderink JB, Russel FGM, Masereeuw R. Localization of breast cancer resistance protein (Bcrp) in endocrine organs and inhibition of its transport activity by steroid hormones. Cell Tissue Res. 2012;349:551–563. doi: 10.1007/s00441-012-1417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Su L, Mruk DD, Lee WM, Cheng CY. Drug transporters and blood--testis barrier function. J Endocrinol. 2011;209:337–351. doi: 10.1530/JOE-10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su L, Mruk DD, Lui WY, Lee WM, Cheng CY. P-glycoprotein regulates blood-testis barrier dynamics via its effects on the occludin/zonula occludens 1 (ZO-1) protein complex mediated by focal adhesion kinase (FAK) Proc Natl Acad Sci USA. 2011;108:19623–19628. doi: 10.1073/pnas.1111414108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qian X, Cheng YH, Jenardhanan P, Mruk DD, Mathur PP, Xia W, Silvestrini B, Cheng CY. Adjudin disrupts spermatogenesis by targeting drug transporters. Lesson from the breast cancer resistance protein (BCRP) Spermatogenesis. 2013;3:e24993. doi: 10.4161/spmg.24993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng YH, Jenardhanan P, Mathur PP, Qian X, Xia W, Silvestrini B, Cheng CY. Interaction of oligomeric breast cancer resistant protein (BCRP) with adjudin: a male contraceptive with anti-cancer activity. Curr Mol Pharmacol. 2014;7:147–153. doi: 10.2174/1874467208666150126154049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Su L, Jenardhanan P, Mruk DD, Mathur PP, Cheng YH, Mok KW, Bonanomi M, Silvestrini B, Cheng CY. Role of P-glycoprotein at the blood-testis barrier on adjudin distribution in the testis. A revisit of recent data. Adv Exp Med Biol. 2012;763:318–333. doi: 10.1007/978-1-4614-4711-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Su L, Zhang Y, Cheng YC, Lee WM, Ye K, Hu D. Slc15a1 is involved in the transport of synthetic F5-peptide into the seminiferous epithelium in adult rat testes. Sci Rep. 2015;5:16271. doi: 10.1038/srep16271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to reinitiate spermatogenesis in the rat testis following adjudin-induced infertility. Int J Androl. 2012;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cheng CY, Mruk DD, Silvestrini B, Bonanomi M, Wong CH, Siu MKY, Lee NPY, Mo MY. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohyd-razide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 135.Lv XY, Li J, Zhang M, Wang CM, Fan Z, Wang CY, Chen L. Enhancement of sodium caprate on intestine absorption and antidiabetic action of berberine. AAPS Pharm Sci Tech. 2010;11:372–382. doi: 10.1208/s12249-010-9386-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lecomte JM, Costentin J, Vlaiculescu A, Chaillet P, Marcais-Collado H, Llorens-Cortes C, Leboyer M, Schwartz JC. Pharmacological properties of acetorphan, a parenterally active "enkephalinase" inhibitor. J Pharmacol Exp Ther. 1986;237:937–944. [PubMed] [Google Scholar]
- 138.Lambert DM, Mergen F, Poupaert JH, Dumont P. Analgesic potency of S-acetylthiorphan after intravenous administration to mice. Eur J Pharmacol. 1993;243:129–134. doi: 10.1016/0014-2999(93)90371-n. [DOI] [PubMed] [Google Scholar]
- 139.Ganapathy ME, Huang W, Wang H, Ganapathy V, Leibach FH. Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998;246:470–475. doi: 10.1006/bbrc.1998.8628. [DOI] [PubMed] [Google Scholar]
- 140.Petrus AK, Allis DG, Smith RP, Fairchild TJ, Doyle RP. Exploring the implications of vitamin B12 conjugation to insulin on insulin receptor binding. Chem Med Chem. 2009;4:421–426. doi: 10.1002/cmdc.200800346. [DOI] [PubMed] [Google Scholar]
- 141.Manjunath K, Venkateswarlu V. Pharmacokinetics, tissue distribution and bioavailability of nitrendipine solid lipid nanoparticles after intravenous and intraduodenal administration. J Drug Target. 2006;14:632–645. doi: 10.1080/10611860600888850. [DOI] [PubMed] [Google Scholar]
- 142.Wilson B, Samanta MK, Santhi K, Kumar KP, Paramakrishnan N, Suresh B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res. 2008;1200:159–168. doi: 10.1016/j.brainres.2008.01.039. [DOI] [PubMed] [Google Scholar]