Abstract
Geographic atrophy (GA) and choroidal neovascularization (CNV), the two late forms of age-related macular degeneration (AMD), are generally considered two distinct entities. However, GA and CNV can occur simultaneously in the same eye, with GA usually occurring first. The prevalence of this combined entity is higher in histological studies than in clinical studies. No distinct systemic or genetic risk characteristics are associated with the combined GA/CNV entity, although on clinical exam and retinal imaging it can feature drusen or subretinal drusenoid deposits. GA and CNV may exist within the spectrum of a single disease, or they may be two very different diseases. Therapy with anti-vascular endothelial growth factor (anti-VEGF) is often successful for CNV, but some evidence suggests increased rates of GA development in eyes treated with anti-VEGF. In this article, we review the current literature regarding epidemiology, clinical presentation, and treatment options for patients with the combined GA/CNV entity.
Keywords: geographic atrophy, choroidal neovascularization, age-related macular degeneration
INTRODUCTION
Age-related macular degeneration (AMD), a major cause of blindness worldwide, is a degenerative disease of the macula, often leading to progressive vision loss. According to the World Health Organization global eye disease survey report, 14 million people are blind or severely visually impaired due to AMD [1]. AMD has an early and a late stage, with visual impairment occurring during the late stage of the disease. AMD is quite prevalent in the elderly population; in one large population-based study of subjects 75 years of age and older, 30% had signs of early AMD, and 7% had signs of late AMD [2]. Currently, “late AMD” is defined by the presence of 1 of 2 key features: growth of new choroidal vessels breaking through into the neuroretina, known as “choroidal neovascularization” (CNV), or progressive atrophy of the retinal pigment epithelium (RPE), choriocapillaris and photoreceptor layers, known as “geographic atrophy” (GA) [3]. GA and CNV are not necessarily mutually exclusive and can occur simultaneously in the same eye. This co-occurrence of the 2 subtypes of late AMD is generally not considered a distinct entity in clinical and research settings; therefore, less is known about eyes progressing to the combined phenotype [4]. In this article, we review the current literature regarding the epidemiology, genetics, clinical presentation, and treatment options for the combined GA/CNV phenotype of AMD.
BACKGROUND
Early vs. Late AMD
The Age-Related Eye Disease Study (AREDS) established a staging schema for AMD [5]. In the early stage, patients are typically asymptomatic, with drusen seen on fundus exam and retinal imaging. Commonly utilized imaging techniques include color fundus photography, spectral domain optical coherence tomography (SD-OCT), near-infrared reflectance (NIR-R), and fundus autofluorescence (FAF). Drusen consist of a heterogeneous mixture of esterified lipids, amyloid, vitronectin, complement elements, and several proteins, including apolipoprotein B and apolipoprotein E [6–9]. Other early AMD findings include pigmentary changes and subretinal drusenoid deposits (SDD) [10, 11]. The time of progression from early to late AMD differs from patient to patient. An analysis based on the Beaver Dam Eye Study demonstrated that, in subjects aged 43–86 years with signs of early AMD in both eyes, the cumulative 15-year incidence is 13.5% for GA and 14.8% for CNV [12]. Late AMD is diagnosed based on characteristic clinical exam and imaging findings, which will be discussed in the following two sections.
General Characteristics of GA
GA is a progressive process leading to a slow, irreversible decline in visual function. Clinicopathologic studies have defined GA as areas of cell death in the RPE, outer neurosensory retina, and choriocapillaris [3, 13–15]. Unlike CNV, GA usually spares the foveal center until late in its course. On color fundus photography, it presents as unilobular or multilobular sharply delineated atrophic areas of severe depigmentation or absence of RPE cells, with a minimum diameter of 175 micrometers, through which larger choroidal vessels can be easily visualized [16]. GA can be further visualized using NIR-R, near infrared autofluorescence (NIA), FAF, and SD-OCT imaging [17]. On NIR-R, atrophic patches are hyperreflectant, and on NIA and AF these patches are hypofluorescent, due to absence of RPE cells [13, 18, 19]. While FAF has traditionally been used to supplement color fundus photography in the evaluation of GA, work by Forte et al. suggests that NIA might detect RPE cell loss at GA borders earlier than FAF [19], and another study by Kellner et al. suggests that NIA and FAF are equally capable of GA detection [20]. An advantage of FAF in imaging GA is that it demonstrates the multilobular nature of most GA lesions clearly when this is not apparent on fundus photography or NIR-R imaging [21]. On SD-OCT, RPE atrophy is seen. Multiple pathological pathways, including immunologic [22], vascular [23], and oxidative stress-induced [14, 15], have been proposed to be responsible for GA onset and growth. More recently, SDD aka reticular macular disease (RMD) have been strongly associated with GA prevalence and growth [21, 24].
General Characteristics of CNV
CNV is defined by the growth and invasion of new fragile choroidal vessels through Bruch’s membrane. It is characterized on retinal imaging by the following features: hemorrhages, exudates, detachment of the RPE or retina, and/or subsequent disciform scars [14, 15, 25]. CNV historically has been classified into 3 types based on fluorescein angiography (FA) imaging findings: classic, occult, and minimally classic. Classic CNV corresponds to early fluorescein leakage on FA and is localized above the RPE on SD-OCT, whereas occult CNV is anatomically confined to the area below the RPE and is defined by late fluorescein leakage and poorly defined margins on FA. Lesions that combine both patterns, with a predominance of occult CNV, are called minimally classic lesions [14, 25]. A more modern classification of CNV lesions is based on both the origin and extent of neovascularization: Type I vessels originate from the choroid and remain sub-RPE, Type II vessels also originate from the choroid but break through the RPE while remaining sub-retinal, and Type III vessels originate from the retinal arteries. Type III CNV has also been referred to as retinal angiomatous proliferation (RAP) [26, 27], to emphasize that it is not truly choroidal in origin. Several pathological factors are thought to be involved in CNV. Hypotheses include an immunologic response to RPE damage or degenerative changes of the choroidal vasculature (which might account for CNV development via an ischemic response) [14, 25]. The common endpoint of these pathological cascades is their ability to trigger the secretion of angiogenic factors, such as vascular endothelial growth factor (VEGF), with subsequent choroidal vessel growth.
THE COMBINED GA/CNV PHENOTYPE OF AMD
Prevalence and Incidence
Population-based studies give us insight into the prevalence of each form of late AMD. In subjects aged 75 years and older, the Beaver Dam Eye Study reported a GA prevalence of 2% and a CNV prevalence of 5.2% [2]. Similarly, in the Blue Mountains Eye Study, CNV was twice as common as GA [28]. While these studies report on each form of late AMD individually, the seemingly distinct entities, GA and CNV, can coexist in the same eye. Of note, several older histopathological studies have reported the prevalence of the combined GA/CNV entity, which may not always be evident clinically. In a study of 46 eyes with a clinical diagnosis of GA, Sarks et al. found that 15 eyes had subclinical CNV on histology [29]. In 2 other histopathological studies, Green et al. found that 22 eyes of 63 patients with clinical bilateral CNV also had areas of RPE atrophy on histology, and 86/760 eyes with a pre-mortem diagnosis of AMD demonstrated both CNV and RPE atrophy histologically [30, 31].
The incidence of the combined GA/CNV phenotype has been reported in several clinical studies, with widely varying results, perhaps not surprisingly given that they are from different populations. Sarks et al. demonstrated that 7/208 patients (3.4%) clinically diagnosed with GA in at least one eye developed CNV in the GA study eye after an average follow-up period of 6.2 months [32]. Schatz et al. reported that 10/50 eyes (20%) diagnosed with GA developed CNV after 2–6 years of follow-up [33]. Sunness et al. reported the 2- and 4-year incidences of CNV in the GA study eye as 6% and 17%, respectively, for 152 patients with a baseline diagnosis of GA in at least one eye [34]. The Macular Photocoagulation Study (MPS) Group found that the 5-year incidence of CNV in an eye with GA was 45% for 11 patients in their initial study and 49% for 20 patients in the following study [35, 36]; the higher rates are likely a consequence of small sample size in the MPS Group studies. The Beaver Dam Eye Study found that, of eyes with GA at baseline, 10.9% (6/55) progressed to CNV over 5 years; of note, development of CNV in GA eyes was more frequent if CNV was present in the fellow eye [37]. The difference in the follow-up period and in the number of participants recruited could explain the disparity in the incidence rates among the various studies. AREDS found that 0.4% of 3,212 eyes free of late AMD at baseline developed concomitant GA and CNV after 5 years of follow-up [38]. Both MPS and AREDS were conducted at retinal centers, which may not be representative of all populations. In a study of the simultaneous occurrence of GA and CNV, Grob et al. found that GA tends to occur before CNV [39].
Clinical Characteristics
The GA component of the combined GA/CNV phenotype has a presentation similar to GA alone. It progresses slowly over time, leading to a gradual loss of visual acuity, and atrophic areas continue to enlarge independently of CNV development [34]. Likewise, the CNV component of this entity has the same clinical manifestations as CNV alone, including subretinal hemorrhage, exudates in the retinal layer, RPE or retinal detachment, and/or a sudden loss of visual acuity [14, 34, 39]. CNV is active over a shorter period of time than GA [34]. Thus, CNV may be clinically silent, and the appearance of the fundus may be limited to that of GA, leading to underestimation of the actual prevalence of the GA/CNV phenotype. Conversely, the effects of neovascularization, including hemorrhages and exudates, often obscure the central areas of atrophy, also leading to underestimation of the GA/CNV phenotype. In both types of late AMD, drusen and/or SDD can be present [15, 40] and visualized on various imaging modalities, as described earlier, although SDD seen on imaging paradoxically appears to decrease in eyes that progress to CNV [10, 41], whereas SDD are strongly associated with GA at all stages [21, 24, 42].
Systemic and Genetic Risk Factors
Risk factors for the subtypes of late AMD have been thoroughly described in the literature. Advanced age, cigarette smoking, low intake of antioxidants, elevated body mass index, family history of AMD, hypertension, large soft drusen, and SDD have been found to increase the risk for both GA and CNV [24, 43–50]. However, few studies have concentrated on the risk factors for the combined GA/CNV phenotype. Sunness et al. studied the clinical and systemic risk factors for CNV development in eyes clinically diagnosed with GA [34]. Systemic factors, such as gender, age, hypertension, and vitamin use, were not associated with the risk of CNV development in eyes with GA. The effect of smoking was not evaluated due to the limited number of smokers among participants. However, a major clinical risk factor found in the Sunness et al. study was the status of the fellow eye; having CNV in the fellow eye significantly increased the risk of developing CNV in the GA study eye [34]. Other ocular factors studied by Sunness et al., such as total atrophic area, configuration of the atrophy, RPE degeneration, phakic status, iris color, and peripapillary atrophy, were not significantly associated with the occurrence of CNV in eyes with GA [34]. The MPS Group studies showed that hypertension, presence of 5 or more drusen, focal hyperpigmentation, and 1 or more large drusen are risk factors for CNV development. However, neither of the MPS Group studies focused on the combined GA/CNV form, and few patients with GA were included [35, 36]. One recent study showed that the combined GA/CNV entity tends to occur at an older age than GA or CNV alone and is associated with late AMD in the fellow eye, suggesting that the combined phenotype may be a later stage along the same spectrum of disease [4]. There were no differences in rates of hypertension, diabetes, or dyslipidemia, or in smoking status or family history of AMD, between the combined phenotype group and the group with either GA or CNV [4].
Regarding genetic risk factors, GA and CNV have a similar profile. Recently, certain genetic variants have been shown to be strong risk factors for both forms of late AMD, especially variants in the age-related maculopathy susceptibility 2 (ARMS2) and complement factor H (CFH) genes [49, 51–53]. Grob et al. found no correlation between the following AMD genes and the combined GA/CNV phenotype: complement component 3 (C3), complement component 2 (C2), CFH, high temperature requirement factor A1 (HTRA1), and ARMS2, although there were higher rates of ARMS2 and HTRA1 risk alleles in the combined phenotype group (not statistically significant) [39]. Another study found no difference in CFH allele frequencies between subjects with the combined GA/CNV phenotype and those with GA or CNV alone, but contrary to the Grob. et al. study, there was a lower frequency (albeit not statistically significant) of the ARMS2 risk allele among patients with the combined GA/CNV form [4]. It appears that the combined phenotype does not have a unique genetic profile, although further research remains to be done.
Treatment and the Role of Anti-VEGF Intravitreal Injections
Currently, very different treatment modalities exist for the two late forms of AMD. The Age-Related Eye Disease Study (AREDS) demonstrated that nutritional supplements can slow the onset of GA [5], although there is no proven treatment for GA once it occurs. Beta-carotene, part of the original AREDS formulation, was shown to increase the risk of lung cancer in patients with smoking history [54, 55]. AREDS2, the follow-up study to AREDS, showed beta-carotene could be safely replaced by the antioxidants zeaxanthin and lutein [56, 57]. Thus, the current formulation includes vitamin E, vitamin C, zinc, copper, lutein, and zeaxanthin.
Intravitreal anti-VEGF therapies are the standard of care for symptomatic CNV, as well as for maintenance treatment, with many patients experiencing significant improvements in visual acuity [58–61]. Currently, there are 3 medications in widespread use: bevacizumab (Avastin; Genentech, Inc., South San Francisco, CA), ranubizumab (Lucentis; Genentech, Inc., South San Francisco, CA), and aflibercept (Eyelea; Regeneron, Inc., Tarrytown, NY). Anti-VEGF injections were recently studied for their efficacy in treating CNV with underlying GA. Amaro et al. studied the effect of anti-VEGF treatment on the combined GA/CNV phenotype in a case series of 11 eyes [62]. Favorable anatomical and visual function outcomes were found after treatment with either ranibizumab or bevacizumab. However, Amaro et al. mentioned that the improvement in visual acuity in their study was not as remarkable as in other anti-VEGF trials, incriminating underlying GA [62]. Querques et al. found that treatment of CNV with ranibizumab in eyes with concomitant GA resulted in significant reduction of the morphological manifestations of CNV at 24 months of treatment in 21 naïve eyes; however, visual acuity deteriorated, perhaps because the underlying GA prevented any favorable outcome in visual function [63].
While Amaro et al. and Querques et al. analyzed eyes with GA already present, some eyes will develop new GA after CNV onset. Some researchers and clinicians believe that onset and progression of GA in eyes previously affected by CNV may be accelerated as a result of anti-VEGF injections; this hypothesis is based on suggestive retrospectively reviewed data from the Inhibition of VEGF in Age-related choroidal Neovascularization (IVAN), Comparison of Age-Related Macular Degeneration Treatments Trials (CATT), and the pHase III, double-masked, multicenter, randomized, Active treatment-controlled study of the efficacy and safety of 0.5 mg and 2.0 mg Ranibuzumab administered monthly or on an as-needed Basis in patients with subfoveal neOvasculaR age-related macular degeneration (HARBOR) trials [64–66]. Importantly, this theory has not been tested in a prospective study, and patients may be experiencing natural progression of their AMD rather than a treatment-induced effect [65, 67]. Animal models suggest that VEGF, secreted by the RPE, is critical to choriocapillaris and RPE maintenance; mice lacking certain isoforms of VEGF, including VEGFA, were shown to have atrophy of the RPE similar to GA, with decreased autofluorescence, accumulation of sub-RPE deposits, and loss of barrier properties [68, 69]. Clinically, the macular appearance can vary after anti-VEGF treatment and can involve RPE disturbances, including pigmentary changes and atrophy, as well as atrophy of the choriocapillaris, which could represent precursor lesions to GA or outright GA [70]. Additionally, some patients in another study were reported to experience a decline in visual acuity [71]. Young et al. showed that, in CNV eyes treated with a treat-and-extend protocol of bevacizumab or ranibizumab, progression of RPE and choroidal atrophy was associated with the number of intravitreal injections [72]. The CATT study found that the 2-year incidence of GA in treated eyes was approximately 18%. Further, it showed that the rate of GA onset was higher among patients who were treated monthly with ranibizumab compared to those treated with ranibizumab as needed and those treated with bevacizumab either monthly or as needed [64, 65] and that visual prognosis was quite poor if GA involved the fovea [65]. The study found that ranibizumab, as compared to bevacizumab, was associated with a 43% increased risk of GA development; it was proposed that the distinct molecular composition of ranibizumab vs. bevacizumab might account for the higher rate of GA onset in patients treated with ranibizumab [65]. On reanalysis of CATT study imaging, Grunwald et al. found no difference in GA onset between patients treated monthly and those treated as needed, although there was a higher incidence of GA and a greater area of yearly GA growth in those treated with ranibizumab as compared to those who received bevacizumab [73]. Subfoveal localization of CNV was associated with a slower GA growth rate, compared to CNV not localized to the fovea; similarly, GA near the fovea grew more slowly than GA further from the fovea. Classic CNV was associated with a faster rate of GA growth than minimally classic or occult types. The number of injections was not associated with GA growth rate [73]; in contrast to Lois et al., who showed, using FAF imaging, that GA onset was associated with the number of intravitreal anti-VEGF injections [74]. Of the 1185 participants receiving anti-VEGF therapy that Grunwald et al. assessed, 120 (10.1%) developed GA within the first year of the study, and an additional 36 (3.0%) developed GA within the second year. Of note, most new GA was localized within areas of CNV involvement [73]. Further, the CNV-associated GA was clinically indistinguishable from normal de novo GA seen in non-CNV eyes and grew at similar rates as demonstrated in GA AMD studies [75, 76], suggesting its similarity to normal GA [73]. McLeod et al. showed increased choriocapillaris loss in areas adjacent to CNV [77], which could explain why more GA was found in these areas within the CATT study analysis [73]. Jaffe et al. found that GA developed in 18% of patients treated with anti-VEGF therapy for CNV [78]. Cho et al. showed that, in subjects with RAP, a subtype of CNV, 16/43 eyes (37.2%) treated with ranibizumab developed GA over the 2-year follow-up period, with baseline subfoveal choroidal thinning, SDD, and GA in the fellow eye being significant risk factors for GA in the study eye [79]. Xu et al. showed that eyes with Type I CNV were less likely to progress to GA after anti-VEGF treatment than eyes with other types [50].
Treating the combined GA/CNV form with anti-VEGF injections is not contraindicated. However, it is important to weigh the risks and benefits of treatment and to consider a different therapeutic approach or dosing regimen in eyes that develop GA. For example, treatment with aflibercept has been shown to be as effective on a bi-monthly dosing plan as monthly injections of ranibizumab [80]. In our experience, treating the combined GA/CNV phenotype presents a challenge to the clinician. Even with treatment of the CNV, gains in visual acuity tend to be less than in eyes without GA due to the underlying RPE and photoreceptor atrophy. Patients should be counseled regarding the modest expectations of therapy.
CONCLUSION
GA and CNV are frequently considered separate subtypes of AMD. This hypothesis is supported by their distinct clinical expressions. However, GA and CNV are both associated with common variants in the CFH and ARMS2 genes, they both co-exist with drusen and SDD, and they can occur simultaneously with a frequency that is probably underestimated, suggesting a possible overlap between these apparently separate pathological cascades. To our knowledge, no distinct systemic or genetic risk profile has been associated with the combined GA/CNV entity compared to the GA and CNV subtypes. Thus, AMD may have a linear progression, with GA and CNV lying on the same disease continuum, and the combined entity may be a more advanced stage of AMD than either GA or CNV alone. Alternatively, late AMD may be two different disorders that coalesce with similar final expression. Treatment of these patients can present a challenge to the clinician, with the primary concern being limited visual acuity improvement after use of anti-VEGF agents due to underlying GA. In fact, some evidence suggests that anti-VEGF injections may accelerate the onset and/or growth of GA, although this hypothesis requires further investigation. Further studies are warranted to investigate the clinical and genetic characteristics of the combined GA/CNV entity and its relationship to the individual subtypes of late AMD.
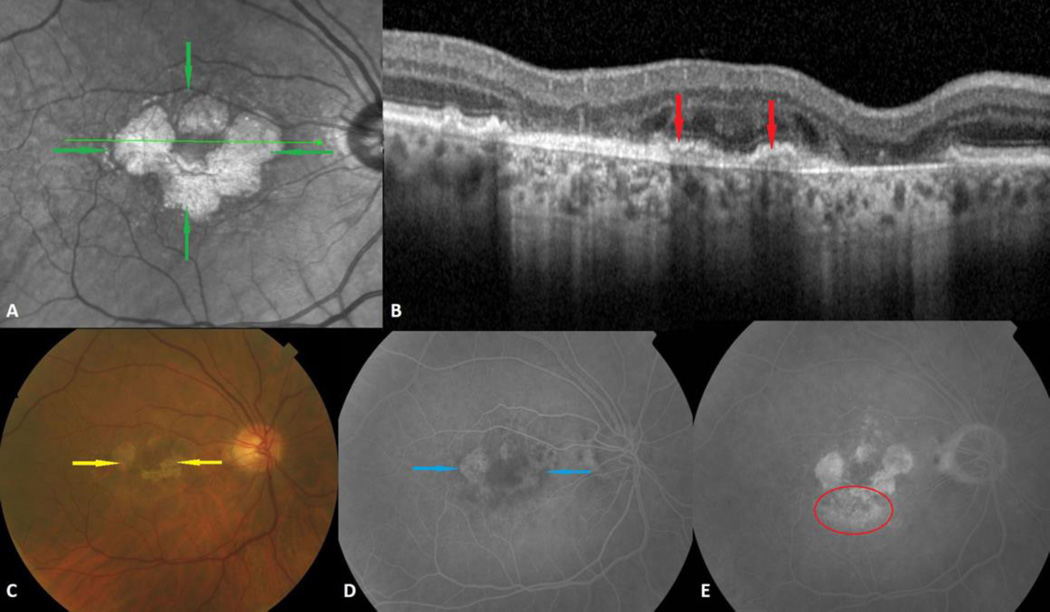
Fig. 1.
Combined geographic atrophy (GA) and choroidal neovascularization (CNV) in the right eye of a 75-year-old man. A. Multilobular GA is visible on infrared as small round or oval atrophic areas of increased reflectance (green arrows). B. Inactive CNV is shown on spectral domain optical coherence tomography as fibrovascular material below the retinal pigment epithelium (red arrows). C. The color photograph clearly shows the atrophic depigmented oval and round areas of GA (yellow arrows). D. GA is seen in the intermediate phase of the fluorescein angiogram as hyperfluorescent lobular lesions due to window defects (blue arrows). E. Occult CNV is characterized by an ill-defined area of irregular leakage and stippled hyperfluorescence in the late phase of the fluorescein angiogram (red circle).
Acknowledgments
This work was supported by an individual investigator research award from the Foundation Fighting Blindness (RTS), National Institutes of Health/National Eye Institute grant R01 EY015520 (RTS), and unrestricted funds from Research to Prevent Blindness (RTS). The funding organizations had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit for publication. The authors wish to thank Jennifer Dalberth for her help in editing the manuscript.
Footnotes
DISCLOSURE STATEMENT
The authors have no conflicts of interest to disclose.
METHOD OF LITERATURE SEARCH
We conducted an electronic search via PubMed for articles published up to October 2015. The search terms used were as follows: “geographic atrophy,” “choroidal neovascularization,” “simultaneous,” and “age-related macular degeneration.” We selected relevant publications to be included in this review. We also searched the reference lists of those articles, and relevant articles were included in the review.
REFERENCES
- 1.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 4.Saade C, Ganti B, Marmor M, Freund KB, Smith RT. Risk characteristics of the combined geographic atrophy and choroidal neovascularisation phenotype in age-related macular degeneration. Br J Ophthalmol. 2014;98(12):1729–1732. doi: 10.1136/bjophthalmol-2014-305005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research G: A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudolf M, Clark ME, Chimento MF, Li CM, Medeiros NE, Curcio CA. Prevalence and morphology of druse types in the macula and periphery of eyes with age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49(3):1200–1209. doi: 10.1167/iovs.07-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li CM, Clark ME, Rudolf M, Curcio CA. Distribution and composition of esterified and unesterified cholesterol in extra-macular drusen. Exp Eye Res. 2007;85(2):192–201. doi: 10.1016/j.exer.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14(7):835–846. [PubMed] [Google Scholar]
- 9.Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99(23):14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148(5):733–743. e732. doi: 10.1016/j.ajo.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33(2):265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114(2):253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 14.van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014;232(2):151–164. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 15.Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann B, Gotzinger E, Pircher M, Sattmann H, Schuutze C, Schlanitz F, Ahlers C, Schmidt-Erfurth U, Hitzenberger CK. Segmentation and quantification of retinal lesions in age-related macular degeneration using polarization-sensitive optical coherence tomography. J Biomed Opt. 2010;15(6):061704. doi: 10.1117/1.3499420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choudhry N, Giani A, Miller JW. Fundus autofluorescence in geographic atrophy: a review. Semin Ophthalmol. 2010;25(5–6):206–213. doi: 10.3109/08820538.2010.518121. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28(3):385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 19.Forte R, Querques G, Querques L, Massamba N, Le Tien V, Souied EH. Multimodal imaging of dry age-related macular degeneration. Acta Ophthalmol. 2012;90(4):e281–e287. doi: 10.1111/j.1755-3768.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 20.Kellner U, Kellner S, Weinitz S. Fundus autofluorescence (488 NM) and near-infrared autofluorescence (787 NM) visualize different retinal pigment epithelium alterations in patients with age-related macular degeneration. Retina. 2010;30(1):6–15. doi: 10.1097/iae.0b013e3181b8348b. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Blonska AM, Pumariega NM, Bearelly S, Sohrab MA, Hageman GS, Smith RT. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33(9):1850–1862. doi: 10.1097/IAE.0b013e31828991b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O'Neill LA, Hollyfield JG, Humphries P. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med. 2012;18(5):791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spraul CW, Lang GE, Grossniklaus HE. Morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(13):2724–2735. [PubMed] [Google Scholar]
- 24.Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE, Jr, Freund KB, Yannuzzi LA, Smith RT. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7362–7369. doi: 10.1167/iovs.12-11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veritti D, Sarao V, Lanzetta P. Neovascular age-related macular degeneration. Ophthalmologica. 2012;227(Suppl 1):11–20. doi: 10.1159/000337154. [DOI] [PubMed] [Google Scholar]
- 26.Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. 2010;30(9):1333–1349. doi: 10.1097/IAE.0b013e3181e7976b. [DOI] [PubMed] [Google Scholar]
- 27.Gass JD. Biomicroscopic and histopathologic considerations regarding the feasibility of surgical excision of subfoveal neovascular membranes. Am J Ophthalmol. 1994;118(3):285–298. [PubMed] [Google Scholar]
- 28.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102(10):1450–1460. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 29.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60(5):324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green WR, Key SN., 3rd Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 31.Green WR, Enger C. Age-related macular degeneration histopathologic studies. The 1992 Lorenz E. Zimmerman Lecture. Ophthalmology. 1993;100(10):1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- 32.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2(Pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 33.Schatz H, McDonald HR. Atrophic macular degeneration. Rate of spread of geographic atrophy and visual loss. Ophthalmology. 1989;96(10):1541–1551. doi: 10.1016/s0161-6420(89)32694-7. [DOI] [PubMed] [Google Scholar]
- 34.Sunness JS, Gonzalez-Baron J, Bressler NM, Hawkins B, Applegate CA. The development of choroidal neovascularization in eyes with the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106(5):910–919. doi: 10.1016/S0161-6420(99)00509-6. [DOI] [PubMed] [Google Scholar]
- 35.Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Macular Photocoagulation Study Group. Arch Ophthalmol. 1993;111(9):1189–1199. doi: 10.1001/archopht.1993.01090090041018. [DOI] [PubMed] [Google Scholar]
- 36.Risk factors for choroidal neovascularization in the second eye of patients with juxtafoveal or subfoveal choroidal neovascularization secondary to age-related macular degeneration. Macular Photocoagulation Study Group. Arch Ophthalmol. 1997;115(6):741–747. doi: 10.1001/archopht.1997.01100150743009. [DOI] [PubMed] [Google Scholar]
- 37.Klein R, Meuer SM, Knudtson MD, Klein BE. The epidemiology of progression of pure geographic atrophy: the Beaver Dam Eye Study. Am J Ophthalmol. 2008;146(5):692–699. doi: 10.1016/j.ajo.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis MD, Gangnon RE, Lee LY, Hubbard LD, Klein BE, Klein R, Ferris FL, Bressler SB, Milton RC Age-Related Eye Disease Study G. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grob S, Luo J, Hughes G, Lee C, Zhou X, Lee J, Du H, Ferreyra H, Freeman WR, Kozak I, Zhang K. Genetic analysis of simultaneous geographic atrophy and choroidal neovascularization. Eye (Lond) 2012;26(8):1106–1113. doi: 10.1038/eye.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damico FM, Gasparin F, Scolari MR, Pedral LS, Takahashi BS. New approaches and potential treatments for dry age-related macular degeneration. Arq Bras Oftalmol. 2012;75(1):71–76. doi: 10.1590/s0004-27492012000100016. [DOI] [PubMed] [Google Scholar]
- 41.Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011;95(7):979–985. doi: 10.1136/bjo.2010.194977. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz-Valckenberg S, Alten F, Steinberg JS, Jaffe GJ, Fleckenstein M, Mukesh BN, Hohman TC, Holz FG Geographic Atrophy Progression Study G. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(9):5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
- 43.Risk factors for neovascular age-related macular degeneration. The Eye Disease Case-Control Study Group. Arch Ophthalmol. 1992;110(12):1701–1708. doi: 10.1001/archopht.1992.01080240041025. [DOI] [PubMed] [Google Scholar]
- 44.Tomany SC, Wang JJ, Van Leeuwen R, Klein R, Mitchell P, Vingerling JR, Klein BE, Smith W, De Jong PT. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Pumariega NM, Smith RT, Sohrab MA, Letien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011;118(8):1619–1625. doi: 10.1016/j.ophtha.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Complications of Age-related Macular Degeneration Prevention Trial Research G. Risk factors for choroidal neovascularization and geographic atrophy in the complications of age-related macular degeneration prevention trial. Ophthalmology. 2008;115(9):1474–1479. 1479 e1471–1479 e1476. doi: 10.1016/j.ophtha.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL, 3rd Age-Related Eye Disease Study Research G. Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112(4):533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137(3):486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 49.Francis PJ, George S, Schultz DW, Rosner B, Hamon S, Ott J, Weleber RG, Klein ML, Seddon JM. The LOC387715 gene, smoking, body mass index, environmental associations with advanced age-related macular degeneration. Hum Hered. 2007;63(3–4):212–218. doi: 10.1159/000100046. [DOI] [PubMed] [Google Scholar]
- 50.Xu L, Mrejen S, Jung JJ, Gallego-Pinazo R, Thompson D, Marsiglia M, Freund KB. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina. 2015;35(2):176–186. doi: 10.1097/IAE.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 51.Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz SG, Agarwal A, Kovach JL, Gallins PJ, Cade W, Postel EA, Wang G, Ayala-Haedo J, Spencer KM, Haines JL, Pericak-Vance MA, Scott WK. The ARMS2 A69S variant and bilateral advanced age-related macular degeneration. Retina. 2012;32(8):1486–1491. doi: 10.1097/IAE.0b013e318240a540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Magnusson KP, Duan S, Sigurdsson H, Petursson H, Yang Z, Zhao Y, Bernstein PS, Ge J, Jonasson F, Stefansson E, Helgadottir G, Zabriskie NA, Jonsson T, Bjornsson A, Thorlacius T, Jonsson PV, Thorleifsson G, Kong A, Stefansson H, Zhang K, Stefansson K, Gulcher JR. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. PLoS Med. 2006;3(1):e5. doi: 10.1371/journal.pmed.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albanes D, Heinonen OP, Huttunen JK, Taylor PR, Virtamo J, Edwards BK, Haapakoski J, Rautalahti M, Hartman AM, Palmgren J, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62(6 Suppl):1427S–1430S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 55.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr, Valanis B, Williams JH, Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 56.Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 57.Age-Related Eye Disease Study 2 Research G. Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL, 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agron E, Toth CA, Bernstein PS, Sperduto RD. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–149. doi: 10.1001/jamaophthalmol.2013.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 59.Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S Group AS. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 60.Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (avastin) for neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2005;36(4):331–335. [PubMed] [Google Scholar]
- 61.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. e365. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 62.Amaro MH, Roller AB. Intravitreal ranibizumab and bevacizumab therapy for choroidal neovascularization in age-related macular degeneration with extensive pre-existing geographic atrophy. Arq Bras Oftalmol. 2012;75(4):273–276. doi: 10.1590/s0004-27492012000400011. [DOI] [PubMed] [Google Scholar]
- 63.Querques G, Massamba N, Coscas F, Forte R, Souied EH. Choroidal neovascularisation complicating geographic atrophy in age-related macular degeneration. Br J Ophthalmol. 2012;96(12):1479–1483. doi: 10.1136/bjophthalmol-2012-302191. [DOI] [PubMed] [Google Scholar]
- 64.Comparison of Age-related Macular Degeneration Treatments Trials Research G. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. [Google Scholar]
- 65.Grunwald JE, Daniel E, Huang J, Ying GS, Maguire MG, Toth CA, Jaffe GJ, Fine SL, Blodi B, Klein ML, Martin AA, Hagstrom SA, Martin DF Group CR. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. doi: 10.1016/j.ophtha.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC investigators Is. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 67.Sophie R, Wang J, Campochiaro PA. Re: Grunwald et al.: risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials (ophthalmology 2014;121:150–61) Ophthalmology. 2014;121(7):e34. doi: 10.1016/j.ophtha.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 68.Saint-Geniez M, Kurihara T, D'Amore PA. Role of cell and matrix-bound VEGF isoforms in lens development. Invest Ophthalmol Vis Sci. 2009;50(1):311–321. doi: 10.1167/iovs.08-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122(11):4213–4217. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B, Marina Groups AS. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–530. doi: 10.1016/j.ophtha.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 71.Brader HS, Ying GS, Martin ER, Maguire MG Complications of Age-Related Macular Degeneration Prevention Trial Research G. Characteristics of incident geographic atrophy in the complications of age-related macular degeneration prevention trial. Ophthalmology. 2013;120(9):1871–1879. doi: 10.1016/j.ophtha.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young M, Chui L, Fallah N, Or C, Merkur AB, Kirker AW, Albiani DA, Forooghian F. Exacerbation of choroidal and retinal pigment epithelial atrophy after anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Retina. 2014;34(7):1308–1315. doi: 10.1097/IAE.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 73.Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF Comparison of Age-related Macular Degeneration Treatments Trials Research G. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015;122(4):809–816. doi: 10.1016/j.ophtha.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lois N, McBain V, Abdelkader E, Scott NW, Kumari R. Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina. 2013;33(1):13–22. doi: 10.1097/IAE.0b013e3182657fff. [DOI] [PubMed] [Google Scholar]
- 75.Joachim N, Mitchell P, Kifley A, Rochtchina E, Hong T, Wang JJ. Incidence and progression of geographic atrophy: observations from a population-based cohort. Ophthalmology. 2013;120(10):2042–2050. doi: 10.1016/j.ophtha.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 76.Lindblad AS, Lloyd PC, Clemons TE, Gensler GR, Ferris FL, 3rd, Klein ML, Armstrong JR Age-Related Eye Disease Study Research G. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127(9):1168–1174. doi: 10.1001/archophthalmol.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLeod DS, Grebe R, Bhutto I, Merges C, Baba T, Lutty GA. Relationship between RPE and choriocapillaris in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4982–4991. doi: 10.1167/iovs.09-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jaffe GJ, Martin DF, Toth CA, Daniel E, Maguire MG, Ying GS, Grunwald JE, Huang J Comparison of Age-related Macular Degeneration Treatments Trials Research G. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2013;120(9):1860–1870. doi: 10.1016/j.ophtha.2013.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho HJ, Yoo SG, Kim HS, Kim JH, Kim CG, Lee TG, Kim JW. Risk factors for geographic atrophy after intravitreal ranibizumab injections for retinal angiomatous proliferation. Am J Ophthalmol. 2015;159(2):285–292. e281. doi: 10.1016/j.ajo.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 80.Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U View, Groups VS. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]