Abstract
Thirty-five consecutive patients with follicular lymphoma (FL) receiving SCT at our institution between January 2000 and December 2010 were included in this study. At the time of presentation, 30 (86%) had advanced stage disease and 25 (71%) received three or more chemotherapy regimens prior to transplantation. In all, 12 (34%) patients were in complete response pre-SCT following salvage therapy. At the time of analysis (median follow-up 6 years from diagnosis and 4 years from transplantation), 24 patients were alive with an estimated 5-year OS and PFS of 66.5 and 53%, respectively. OS and PFS in patients receiving auto-SCT (91.7%, 73.3%) were superior compared with patients receiving allo-SCT (53.9%, 43%). Our data support early use of auto-SCT in patients with FL and suggest the need to improve allo-SCT outcome. Integrating novel agents in a combined modality approach may improve long-term outcome in FL.
Keywords: follicular lymphoma, relapse, SCT, survival
Introduction
Follicular lymphoma (FL) is the second most common lymphoma diagnosed in the United States and Western Europe, with an incidence of 15 000 new cases per year in the United States. Despite advances in therapeutic regimens, FL remains an incurable disease with Immunochemotherapy alone. A continuous pattern of relapse with short disease-free interval or histological transformation is commonly encountered, making long-term disease control a challenge.1–4
Auto-SCT is feasible with low TRM in FL, but questions remain relating to the optimal timing of this procedure, type of conditioning regimen, long-term disease control and late effects. Allo-SCT used mostly in heavily pretreated patients is associated with high TRM especially after myeloablative conditioning (MST) and is applicable to only a selected group. To decrease the rate of TRM, reduced-intensity conditioning (RIC) allo-SCT is being increasingly performed.5 The long-term survival data of patients undergoing auto-SCT compared with allo-SCT are limited.1,3,6–9
The current study provides comparative long-term outcome of patients receiving SCT for recurrent FL and supports the need to individualize treatment approach in FL patients relapsing after initial chemotherapy.
Patients and Methods
Thirty-five consecutive patients >18 years of age with a confirmed diagnosis of FL (grade 1 or 2), receiving high-dose chemotherapy and SCT between January 2000 and December 2010 at Vanderbilt University Medical Center adult transplant program, were included in this retrospective analysis (Table 1). All patients received planned rituximab-based induction chemotherapy pre-SCT. Patients were required to have chemotherapy-sensitive disease documented pre-SCT after salvage chemotherapy. Patients with evidence of transformation or concomitant presentation with grade 3 FL were excluded. This study was approved by the Institutional Review Board of Vanderbilt University Medical Center. All patients provided informed consent in accordance with the Declaration of Helsinki.
Table 1. Patient characteristics.
| Patients (n = 35) | Allo-SCT (n = 23) | Auto-SCT (n =12) | P-value |
|---|---|---|---|
| Median age (year) | 52 (35 – 64) | 61 (range 50 – 62) | 0.012 |
| Gender (male) | 16 (70%) | 8 (67%) | 0.86 |
| Stage | 0.69 | ||
| I and II | 1 (4%) | 4 (33%) | |
| III and IV | 22 (96%) | 8 (67%) | |
| Median number of extranodal sites | 2 (0 – 3) | 1 (0 – 2) | 0.98 |
| Median numbers of chemotherapy regimens | 3 (1 – 6) | 3 (2 – 5) | 0.71 |
| Rituximab maintenance pre-SCT | 0.86 | ||
| Yes | 7 (30%) | 4 (33%) | |
| No | 16 (70%) | 8 (67%) | |
| Disease status pre-SCT | 0.93 | ||
| CR | 8 (35%) | 4 (33%) | |
| PR | 15 (65%) | 8 (67%) | |
| Stem cell dose CD34+ cells/kg | 7.35 × 106 (range, 3.87–16.48) | 2.8 × 106 (range 2.3–9.8) | 0.004 |
Clinical information was reviewed, and baseline characteristics, including common pre-transplant and transplant variable information, were recorded. Disease progression, OS, PFS, non-relapse mortality (NRM) and relapse rate were defined by standard criteria.
Stem cells were collected using approved institutional PBSC mobilization protocols. All patients received standard care per institutional protocol regarding antimicrobial prophylaxis, surveillance cultures, monitoring for viral infections and treatment.
Transplantation procedures
Auto-SCT
Stem cells were mobilized using high-dose chemotherapy (CY) and G-CSF in combination. CBV (CY 7200 mg/m2, etoposide 2000 mg/m2 and BCNU 400 mg/m2) was the conditioning regimen in 87% of patients receiving auto-SCT, others received BEAM (BCNU, etoposide, cytarabine and melphalan) conditioning regimen followed by auto-SCT.
Allo-SCT
In all, 14 patients received RIC (fludarabine plus BU, n = 12; others = 2) and 9 received myeloablative regimen followed by a PBSC transplant (minimal residual disease = 17; matched unrelated donor = 6). All patients received GVHD prophylaxis with calcineurin inhibitor and either MTX (myeloablative conditioning) or mycophenolate mofetil (RIC). The diagnosis and grading of acute and chronic GVHD were based on standard criteria.
Response criteria
Response criteria were based on guidelines from the International workshop on non-Hodgkin's lymphoma. CR was defined as complete radiological regression of all previous measurable disease or BM involvement. Partial response was defined as a reduction of 50% or greater reduction in the sum of the products of the longest and perpendicular diameter of measurable lesions within a 30-day period prior to transplant and at days +30 and +100. Based on these criteria, all data were individually verified regarding the best response status prior to SCT.
Statistical analysis
Patient characteristics were compared between the two SCT groups by t-test as appropriate. OS, PFS and NRM were reported using Kaplan-Meier analysis. The difference between survival curves was tested for statistical significance using log-rank test. The Cox proportion hazard regression model was used to assess patient characteristics to predict outcome variables. Univariate analysis was performed on patients, disease and transplantation-related variables to see the impact on long-term outcome (Table 1). Chi-square test was used to determine the relationship between all categorical variables. For association between continuous variables and categories, Mann-Whitney U-rank sum tests were used. All the P-values reported were two-sided, and statistical significance was declared at P<0.05. Statistical analyses were performed using SPSS software (v.19) (SPSS, Chicago, IL, USA).
Results
The baseline features are listed in Table 1
The median age of patients at the time of transplantation was 54 years (range 35–64), and 24 (69%) were male. At the time of presentation, 2 patients had stage I disease, 3 with stage II, 7 with stage III and 23 patients were diagnosed with stage IV disease. The follicular international prognostic index was available in a few patients and therefore not reported.
Thirty-four (97%) patients received two or more salvage therapies prior to transplantation. Three (9%) had auto-SCT prior to allo-SCT. Twelve patients (34%) were in CR at the time of transplantation and all patients had chemotherapy-sensitive disease before transplantation. The median stem cell dose prior to auto-SCT was 2.81 × 106 CD34/kg (range: 2.3–9.8), and for patients receiving allo-SCT the median stem cell dose was 7.35 × 106 CD34/kg (range: 3.87–16.48). There was no significant difference between patient groups receiving auto-SCT and allo-SCT except that patients receiving allo-SCT were significantly younger (P = 0.012) and received higher stem cell dose (P = 0.004) (Table 1).
Outcome analysis
OS, DFS and NRM
At the time of analysis, 24 patients were alive with an actuarial OS rate of 66.5%, the estimated 5-year DFS was 53% and NRM was 28.6% for all patients. Grade II–IV acute GVHD occurred in 6 (26%) patients and chronic GVHD in 5 (22%) patients. Three patients had extensive chronic GVHD.
Univariate and multivariate analysis
Age, stage of the disease, the number of chemotherapy regimens, pre-SCT rituximab maintenance, type of conditioning regimens, disease status (CR or partial response) pre-SCT, absolute lymphocyte count at day 14 or day 28 and stem cell dose had no impact on long-term outcome.
Auto-SCT vs Allo-SCT
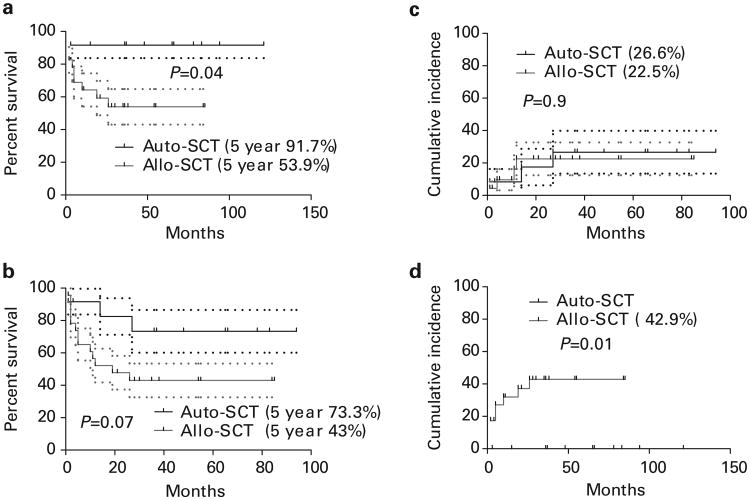
The 5-year OS of patients (Figure 1) receiving auto-SCT was significantly higher than that of patients receiving allo-SCT (91.7 vs 53.9%, P = 0.01). The 5-year PFS of auto-SCT and allo-SCT was 73.3% vs 43% (P = 0.07). There was no significant difference in the rate of relapse among auto-SCT and allo-SCT recipients (26.6% vs 22.5%). NRM in patients receiving allo-SCT was 42.9%. Patients undergoing auto-SCT did not experience NRM (Figure 1). These data were also confirmed by the Cox proportion hazard regression model, which confirmed type of SCT being the only variable affecting survival in the multivariate analysis (HR 6.2, P = 0.03 for OS and HR 4.3, P = 0.052 for PFS).
Figure 1.
(a) OS, (b) PFS, (c) relapse and (d) NRM of patients undergoing auto-SCT and allo-SCT.
Discussion
Our results indicate promising outcome after auto-SCT in selected patients, but post-SCT relapse remains a challenge. Patients receiving allo-SCT had high NRM. Based on our data, we cannot definitively conclude which arm is more efficacious because of patient selection process for either SCT procedure. We are also limited by small sample size. We support early auto-SCT and need to design safer allo-SCT approach to improve outcome after allo-SCT in FL.
The B-cell lymphoma-specific non-myeloablative (NST) regimen fludarabine, CY and rituximab (FCR) is reportedly safe and effective, and this regimen resulted in a TRM of 10%, with 85% patients alive without disease at 8 years.1 Similarly, recently published BMT-CTN results using FCR allo-SCT (n = 8) showed a disease-free survival of 86%.3 Our limited data in recent years also confirmed (unpublished data) effectiveness of FCR regimen in recurrent B-cell lymphoid malignancies.10 Currently, there is an ongoing BMT-CTN study (no. 0701) for relapsed FL patients with the goal of validating the promising results of FCR allo-SCT.
Favorable outcomes were also reported by the Cancer and Leukemia Group B 109901 trial in 16 patients with relapsed chemo-sensitive FL using the NST regimen consisting of fludarabine and CY. This study reported a 3-year EFS and OS of 75% and 81%, respectively.11 Compared with studies employing NST regimen (such as fludarabine and CY or FCR), there is no evidence that the more substantial RIC regimens (fludarabine and melphalan or BU in our study) provide any advantage in disease control in patients with chemo-sensitive disease. Importantly, RIC allo-SCT appears to be associated with more severe toxicities, GVHD and NRM.12 The GELTAMO group, for example, has recently reported the outcome in 37 FL patients who underwent a minimal residual disease using melphalan and fludarabine conditioning, majority of patients had chemo-sensitive disease and 40% were in CR at the time of transplantation. After median follow-up of 52 months, the 4-year DFS for patients with PD, partial response or CR at transplantation were 29%, 48% and 64%, respectively, whereas the 4-year cumulative incidence of NRM were 71%, 33% and 26%, respectively.12 We have made similar observation in our study using fludarabine, BU RIC allo-SCT.
It is important to obtain a good remission status prior to SCT. The only factor that emerged as truly predictive of transplant outcomes is disease status at transplantation.1,4 Furthermore, intensified therapies to improve disease control prior to SCT are indeed needed to make SCT an eligible option. Various strategies are being investigated to improve the outcome in patients with refractory disease and to prevent relapses after SCT, including incorporating novel agents into the conditioning regimen to increase effectiveness without increasing toxicity and enhancing GVL effects through tumor-specific immunization or post-transplantation immunomodulation.9,13–15 Radioimmunotherapy in the peri-transplant period needs to be evaluated in patients undergoing SCT. We believe that all these need to be tested in clinical trials and we encourage participation in clinical trials.
Acknowledgments
This work was supported by National Center for Research Resources, National Institute of Health (Grant no. 5K-12 CA090625-09, NR)
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
References
- 1.Khouri IF. Allogeneic stem cell transplantation in follicular lymphoma. Best Pract Res Clin Haematol. 2011;24:271–277. doi: 10.1016/j.beha.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hari P, Carreras J, Zhang MJ, Gale RP, Bolwell BJ, Bredeson CN, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomblyn MR, Ewell M, Bredeson C, Kahl BS, Goodman SA, Horowitz MM, et al. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-hodgkin lymphoma beyond first complete response or first partial response. Biol Blood Marrow Transplant. 2011;17:1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman A. Follicular lymphoma: 2011 update on diagnosis and management. Am J Hematol. 2011;86:768–775. doi: 10.1002/ajh.22099. [DOI] [PubMed] [Google Scholar]
- 5.Barrett AJ, Savani BN. Stem cell transplantation with reduced-intensity conditioning regimens: a review of ten years experience with new transplant concepts and new therapeutic agents. Leukemia. 2006;20:1661–1672. doi: 10.1038/sj.leu.2404334. [DOI] [PubMed] [Google Scholar]
- 6.de Lavallade H, Mohty M, El-Cheikh J, Cassier PA, Faucher C, Fürst S, et al. Reduced-intensity conditioning allogeneic stem cell transplantation for patients with chemoresistant or relapsed follicular lymphoma. Br J Haematol. 2006;135:408–410. doi: 10.1111/j.1365-2141.2006.06308.x. [DOI] [PubMed] [Google Scholar]
- 7.Khouri IF, McLaughlin P, Saliba RM, Hosing C, Korbling M, Lee MS, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafey M, Lupichuk SM, Do T, Owen C, Stewart DA. Preferences of patients and physicians concerning treatment options for relapsed follicular lymphoma: a discrete choice experiment. Bone Marrow Transplant. 2011;46:962–969. doi: 10.1038/bmt.2010.225. [DOI] [PubMed] [Google Scholar]
- 9.Freytes CO, Lazarus HM. Second hematopoietic SCT for lymphoma patients who relapse after autotransplantation: another autograft or switch to allograft? Bone Marrow Transplant. 2009;44:559–569. doi: 10.1038/bmt.2009.214. [DOI] [PubMed] [Google Scholar]
- 10.Reddy N, Savani BN. Treatment options for transformed lymphoma: incorporating allogeneic stem cell transplantation in a multimodality approach. Biol Blood Marrow Transplant. 2011;17:1265–1272. doi: 10.1016/j.bbmt.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shea T, Johnson J, Westervelt P, Farag S, McCarty J, Bashey A, et al. Reduced-intensity allogeneic transplantation provides high event-free and overall survival in patients with advanced indolent B cell malignancies: CALGB 109901. Biol Blood Marrow Transplant. 2011;17:1395–1403. doi: 10.1016/j.bbmt.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinana JL, Martino R, Gayoso J, Sureda A, de la Serna J, Díez-Martín JL, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abou-Nassar KE, Stevenson KE, Antin JH, McDermott K, Ho VT, Cutler CS, et al. (90)Y-ibritumomab tiuxetan followed by reduced-intensity conditioning and allo-SCT in patients with advanced follicular lymphoma. Bone Marrow Transplant. 2011;46:1503–1509. doi: 10.1038/bmt.2010.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhmann R, Simoes B, Stanglmaier M, Yang T, Faltin M, Bund D, et al. Immunotherapy of recurrent B-cell malignancies after allo-SCT with Bi20 (FBTA05), a trifunctional anti-CD3 x anti-CD20 antibody and donor lymphocyte infusion. Bone Marrow Transplant. 2009;43:383–397. doi: 10.1038/bmt.2008.323. [DOI] [PubMed] [Google Scholar]
- 15.Hicks LK, Woods A, Buckstein R, Mangel J, Pennell N, Zhang L, et al. Rituximab purging and maintenance combined with auto-SCT: long-term molecular remissions and prolonged hypogammaglobulinemia in relapsed follicular lymphoma. Bone Marrow Transplant. 2009;43:701–708. doi: 10.1038/bmt.2008.382. [DOI] [PubMed] [Google Scholar]