Abstract
Intensive induction regimen followed by high dose chemotherapy and autologous stem cell transplantation (auto-SCT) is frequently used to improve outcome in patients with mantle-cell lymphoma (MCL). The comparative impact of conventional vs. intensive induction regimen before transplantation is unknown. Forty eight patients with MCL receiving SCT at our institution between January 2000 and December 2010 were included in this study. At the time of initial presentation, 43 (89.5%) had stage IV disease and 18(37.5%) received more than one chemotherapy regimens prior to transplantation. Forty patients underwent auto-SCT, 7 allo-SCT; one patient had an allo-SCT for relapsed disease after auto-SCT. At the time of this analysis (median follow-up 6 years from diagnosis and 4 years from transplantation), 40 patients (88%) were alive with a 5 year disease free survival of 74.8%. Age, disease stage, number of regimens pre-SCT, pre-SCT disease status, and type of SCT had no impact on long-term outcome. Importantly, there was no difference among the type of induction regimen on outcome in this cohort receiving SCT. Based on our data, we believe that future studies should focus on strategies to prevent disease relapse rather than comparing induction regimens prior to stem cell transplantation.
Keywords: mantle cell lymphoma, stem cell transplantation, induction regimen
Introduction
Mantle cell lymphoma (MCL) is a rare disease that constitutes about 6% of non-Hodgkin lymphoma (NHL). It is believed that the prognosis and outcome is driven by genetic loci that encode tumor suppressor genes [1-3]. Therapeutic modalities, specifically high dose therapy and transplant approaches have been developed to overcome poor prognosis of MCL. Such treatment has been applied to majority of patients irrespective of clinical presentation or prognostic indices. Standard treatment for this disease is lacking due to the deficits in conducting large prospective controlled trials. Practice patterns therefore vary considerably between centers.
Newer drugs and drug combinations (bortezomib based regimens), that may be helpful have not been adequately tested compared with conventional immunochemotherapy regimens in patients receiving autologous stem cell transplantation (auto-SCT) for MCL [4, 5]. Transplantation after attaining a response to chemotherapy appears to be superior to chemotherapy alone [6, 7]. Therefore there is a tendency to consider aggressive induction therapy followed by high dose chemotherapy and auto-SCT among patients who can tolerate intensive regimens [8-11]. Allogeneic stem cell transplantation is reserved for patients who experience disease relapse following autologous stem cell transplantation [12-14]. It is unknown if incorporation of newer agents can change the natural history of the disease and improve outcome [15].
We hypothesize that in the rituximab era, patients achieving a partial or a complete response to any induction therapy benefit from high dose therapy and auto-SCT as a consolidation. We report the outcome data of patients undergoing SCT following various induction regimens. This is the initial report to present outcome analysis following transplantation on patients receiving bortezomib based regimens compared with other standard or intensive therapies.
Patients and methods
Forty eight consecutive patients with MCL among 270 NHL patients older than age 18 years receiving high dose chemotherapy and SCT between January 2000 and December 2010 at Vanderbilt University Medical Center adult transplant program, were included in this study. All patients had received planned rituximab based induction chemotherapy pre-SCT. This study was approved by the Institutional Review Board of Vanderbilt University Medical Center. All patients provided informed consent in accordance with the Declaration of Helsinki.
Clinical information was reviewed, and baseline characteristics, including common pre-transplant and transplant variable information were recorded. Disease progression, overall survival (OS), disease free survival (DFS), non-relapse mortality (NRM) and relapse rate were defined by standard criteria. Survival parameters were measured from the time of diagnosis and also from time of transplantation.
Induction regimens
Intensive induction regimens were defined as those containing high-dose cytarabine (1.5-3 g/m2 every 12 hrs for six doses) and included hyper fractionated cyclophosphamide (300mg/m2 every 12 hrs for six doses), vincristine (max 2mg days 1 and 11), adriamycin(50mg/m2), dexamethasone(40mg on days 1-4 and 11-14) (Hyper-CVAD/Ara-C/MTX), whereas standard induction regimens included, CHOP; cyclophosphamide (750mg/m2), doxorubicin (50mg/m2), vincristine(max 2 mg), prednisone(100mg days 1-5) (CHOP) or BR-CVAD ; with bortezomib (1.3 mg/m2 on days 1 and 4), cyclophosphamide (300mg/m2 every 12 hrs for six doses), vincristine (1mg/m2), adriamycin (50mg/m2; CIVI) and dexamethasone (40mg days 1-4). All patients received rituximab immunotherapy (375mg/m2) incorporated in the induction regimen with each cycle.
Stem cell mobilization, conditioning regimens and transplantation procedures
Stem cells were collected using approved institutional peripheral blood stem cell mobilization protocols. Stem cells were mobilized with G-CSF alone or with high dose chemotherapy (most patients received cyclophosphamide) and G-CSF in combination. CBV (cyclophosphamide 7200mg/m2, etoposide 2000 mg/m2, and BCNU 400 mg/ m2) was the conditioning regimen in 85% of patients receiving auto-SCT, other received BEAM (BCNU, etoposide, ara-C and melphalan) conditioning regimen followed by auto-SCT. Reduced intensity conditioning (fludarabine plus busulfan, n=6; others= 1) was most commonly used regimen in patients receiving allo-SCT (MRD=3; MUD=5).
Mantle Cell International Prognostic Index (MIPI) score
A simplified MIPI score was calculated on all available patients with advanced stage disease. Based on the ECOG performance status, age, LDH ratio and WBC at presentation an individual score was generated and risk groups were assigned as previously described [16, 17].
Response criteria
Response criteria were based on guidelines from the International workshop on NHL. Complete remission (CR) was defined as complete radiological regression of all previous measurable disease or BM involvement. Partial response (PR) was defined as a reduction of 50% or greater reduction in the sum of the products of the longest and perpendicular diameter of measurable lesions within a 30-day period prior to transplant and at days +30 and +100 [18]. Based on these criteria, all data were individually verified regarding the best response status prior SCT.
Statistical Analysis
Patient characteristics were compared between patients who received intensive and standard induction regimens by t-test as appropriate. OS, DFS, NRM were reported by using Kaplan–Meier analysis. The difference between survival curves was tested for statistical significance using log rank test. Multivariate analysis for time to event end points was done using the Cox proportional hazards regression model. Univariate analysis was performed on patients, disease and transplantation related variables to see the impact on long term outcome (Table 1). Chi-Square test was used to determine the relationship between all categorical variables. For association between continuous variables and categories Mann–Whitney U rank sum tests were used. All p-values reported were two-sided and statistical significance was declared at p<0.05. Statistical analyses were performed using SPSS software (v.19) (SPSS, Chicago, IL, USA).
Table 1. Baseline characteristics.
| Patients | N= 48 pts | ||
|---|---|---|---|
| Sex (male) | 34 (71%) | ||
|
| |||
| Age (median) | 56 yrs (34-71) | ||
|
| |||
| Stage | |||
| I and II | 5 | ||
| III and IV | 43 | ||
|
| |||
| Median no of regimens prior to SCT | 1(1-5) | ||
| 1 | 30 | ||
| 2 or more | 18 | ||
|
| |||
| Regimens | |||
| R-CHOP | 30 (median age:58 <40-71>) | ||
| R-Hyper-CVAD/Ara-c/MTX | 9 (median age: 54<48-65>) | ||
| R-bortezomib-CVAD | 9 (median age: 57 <34-63>) | ||
|
| |||
| Number of patients in CR prior to SCT | 37 | ||
|
| |||
| Type of Transplantation | |||
| Auto- SCT | 40 (33 in CR /8 in PR) | ||
| Allo-SCT | 8 (4 in CR/3 in PR) | ||
| RIC/MRD | 2 | ||
| RIC/MUD | 5 | ||
| Myeloablative/MRD | 1 | ||
|
| |||
| PS | 0-1 | ||
| Median ratio of LDH: upper limit of normal | 0.73 (range: 0.54- 2.44) | ||
| Median WBC count at diagnosis (109/l) | 6.8 (1.8- 22.9) | ||
|
| |||
| MIPI score (Type of transplant) | Auto | Allo | |
| Low | 22 | 3 | |
| Intermediate | 12 | 1 | |
| High | 2 | 1 | |
| N/A (n=7) | |||
|
| |||
| MIPI score (Regimen used) | R-CHOP | R-Hyper-CVAD | R-Bortezomib-CVAD |
| Low | 11 | 7 | 7 |
| Intermediate | 9 | 1 | 1 |
| High | 5 | ||
|
| |||
| Conditioning Regimen | |||
| CBV | 40 | ||
| Flu/Bu | 5 | ||
| FCR | 1 | ||
| TBI based | 2 | ||
SCT: Stem cell transplantation; R-CHOP: Rituximab, Cyclophosphamide, Adriamycin, Vincristine, prednisone; R-Hyper-CVAD/Ara-C/MTX: Hyper fractionated cyclophosphamide, vincristine, Adriamycin, Dexamethasone, Cytarabine and methotrexate; CR: complete remission, Auto-SCT: autologous ; Allo-SCT: allogeneic stem cell transplantation, RIC: reduced intensity conditioning, MRD- Matched related donor MUD: matched unrelated donor, PS: performance status, LDH: lactate dehydrogenase, WBC: white blood cells, MIPI: mantle cell lymphoma international prognostic index; CBV: cyclophosphamide, BCNU and etoposide, Flu/Bu: Fludarabine/ Busulfan; FCR: Fludarabine, Cyclophosphamide and rituximab ; TBI: Total body Irradiation.
R-CHOP: Cyclophosphamide 750 mg/m2 iv day 1; Adriamycin 50 mg/m2 iv day 1; Vincristine 1.4 mg/m2 iv day 1 (capped at 2 mg); Prednisone 100 mg/day p.o. × 5 days; Rituximab 375 mg /m2 iv day 1 Cycles are given every 21 days.
R-Hyper-CVAD/Ara-c/MTX: Cycles 1,3, and 5: Cyclophosphamide 300 mg/m2 iv over 2 hours, every 12 hours, for 6 doses on days 1-3; Mesna 600 mg/m2 iv daily, day 1-3, starting 1 hour before cyclophosphamide and completed 12 hours after the last cyclophosphamide dose; Vincristine 2 mg iv day 4 and 11; Doxorubicin 50 mg/m2 iv over 2 hours on day 4; Dexamethasone 40 mg/day po days 1 to 4 and 11 to 14. Cycles 2, 4, and 6: Methotrexate 1 gm/m2 iv over 24 hours on day 1 loading dose 100 mg/m2 over 2 hours, then 900 mg/m2 over 23 hours; Ara-C 3 gm/m2 iv over 2 hours. Rituximab 375 mg/m2 with each cycle. Cycles are given at 21 day intervals if possible.
Bortezomib-R-CVAD: Cyclophosphamide 300 mg/m2 iv over 3 hours, every 12 hours, for 6 doses on days 1-3. Mesna 60mg/m2/dose iv, q4h × 18 during cytoxan; Vincristine 1mg iv day 3; Doxorubicin 25 mg/m2 iv continuous infusion, over 24 hours x2 day 1 and day 2; Rituximab 375 mg/m2 iv day 1; Dexamethasone 40 mg/day po days 1 to 4 bortezomib 1.3 mg/m2 iv day 1 and 4. Cycles are given every 21 days.
Results
The baseline features of these patients are listed in Table 1.
At diagnosis of MCL, median age of patients was 56 years (range 34-71) and 34 (71%) were male. At the time of initial presentation, one patient had stage I disease, 4 with stage II, 17 stage III and 26 patients were diagnosed with stage IV disease, and 8% of patients had blastoid variant on pathology.
Nine (19%) received intensive induction regimen with R-Hyper-CVAD alternating with high dose cytarabine and methotrexate. The median age of patients receiving R-hyper-CVAD regimen was 54 yrs (range 48-65). Nine patients received therapy with BR- CVAD therapy prior to auto-SCT (median age 57 yrs, 34-63) whereas 30 patients (62%) received other standard regimens such as R-CHOP. The median age of patients receiving R-CHOP was 58 yrs (range 40-71). There was no statistical difference among these age groups.
Thirty patients (62%) received one chemotherapy regimen prior to transplantation, and 18 patients (38%) received two or more salvage therapies prior to transplantation. Thirty seven patients (77%) were in complete remission (CR) at the time of transplantation. All patients had chemotherapy-sensitive disease before transplantation.
MIPI score was available on 41 of 48 patients. All patients had an Eastern Cooperative Oncology Group performance score of 0 to 1, the median ratio of lactate dehydrogenase to its upper limit of normal was 0.73 (range, 0.54 to 2.44), and median WBC count was 6.8 (range, 1.8 to 22.1×109/L). Twenty five (61%) patients at diagnosis had a low-risk MIPI score (auto-SCT 22 [88%], allo-SCT 3 [12%]); Thirteen patients had an intermediate-risk score (auto-SCT 12 [92%], allo-SCT 1 [8%]) and 3 patients had a high-risk score. Similarly, patients receiving induction therapy with R-hyper-CVAD had MIPI scores of low among 7 patients and intermediate in one patient. Patients receiving R-CHOP therapy had a low score among 11 patients, intermediate among 9 patients and a high score in 5 patients. MIPI score of low and intermediate among 7 and one patient respectively in patients treated with BR-CVAD therapy.
Seven patients received allo-SCT and one patient had an allo-SCT for relapsed disease after auto-SCT. CBV was the conditioning regimen in 34 patients receiving auto-SCT. Seven patients underwent RIC allo-SCT and myeloablative conditioning followed by allo-SCT was performed in one patient. Median follow-up was 28 months (range 0-138 months) from the time of stem cell transplantation and 47 months (range 11-147 months) from the time of diagnosis for the entire cohort.
Outcome analysis
Conventional prognostic factors such as age (<55 vs. 55-68), disease stage (early vs. advance), number of regimens pre-SCT (1 vs. >1), pre-transplant radiation therapy, pre-SCT disease status (CR vs. PR), auto vs. allo-SCT had no impact on overall survival. There was no impact of early lymphocyte recovery on transplant outcome.
OS, DFS and NRM
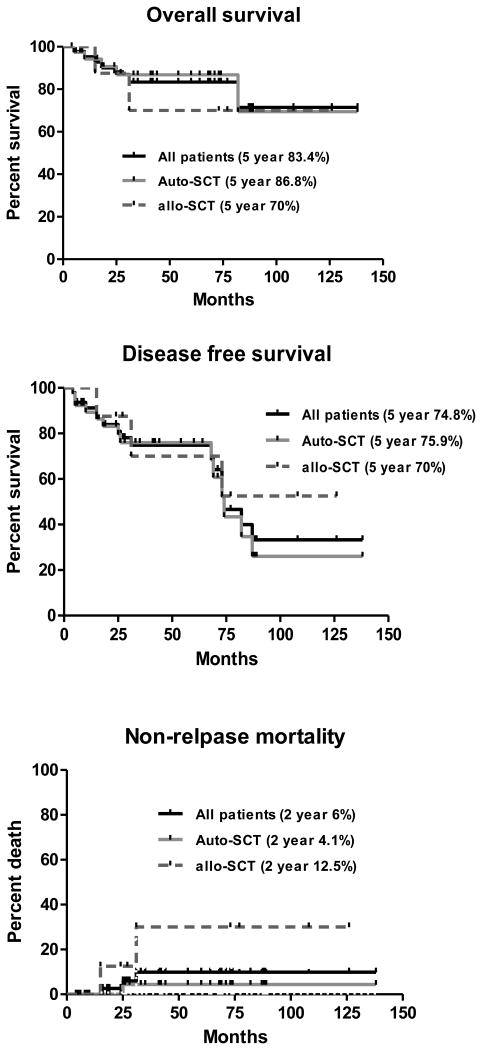
At the time of the analysis, 40 patients (88%) were alive (34 of 40 after auto-SCT and 6 of 8 after allo-SCT; with an actuarial overall survival rate of 83.4±9.9% (auto-SCT 86.8%, allo-SCT 70%), the estimated 5 year DFS was 74.8% (auto-SCT 75.9%, allo-SCT 70%), and cumulative non-relapse mortality (NRM) was 6±4.2% for all patients (auto-SCT 4.1%, allo-SCT 12.5%) (Fig 1a-d).
Figure 1.
Outcome of patients undergoing stem cell transplantation for Mantle Cell Lymphoma (all patients): a. overall survival; b. disease-free survival; c. non-relapse mortality; and d. relapse rate
Impact of MIPI score on outcome after auto-SCT
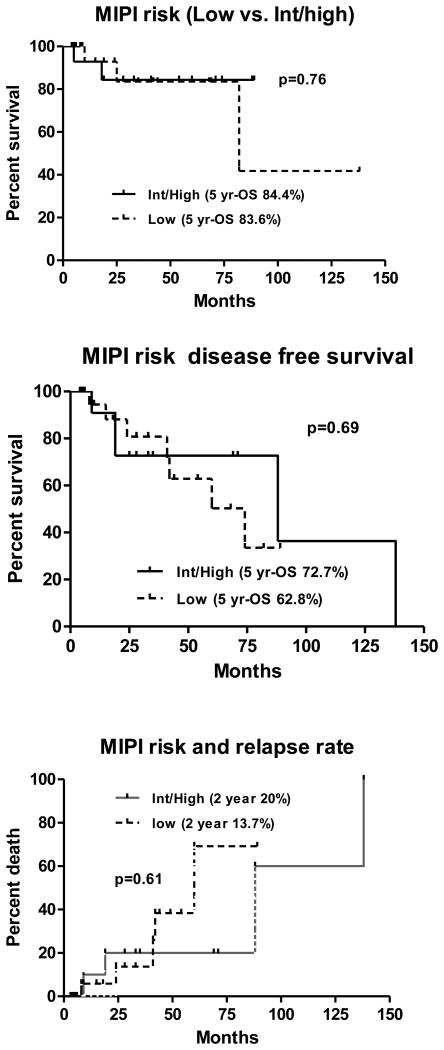
Patients with low MIPI scores (0-3) were compared with patients with intermediate to high MIPI scores (>4). The 5-yr DFS and OS of patients with low MIPI was 62.8% and 83.6% vs. 72.7% and 84.4% for patients with high MIPI score (p=0.76) (Fig 2a-b). Two year relapse rate for patients with low MIPI score was 13.7% compared with patients with high MIPI score at 20% (p=0.99) (Fig 2c). There was no difference in outcome among patients with various prognostic factors undergoing stem cell transplantation.
Figure 2.
Mantle cell lymphoma International Prognostic index score (low vs. high plus intermediate) and outcome after autologous stem cell transplantation: a. overall survival; b. disease-free survival; c. relapse rate
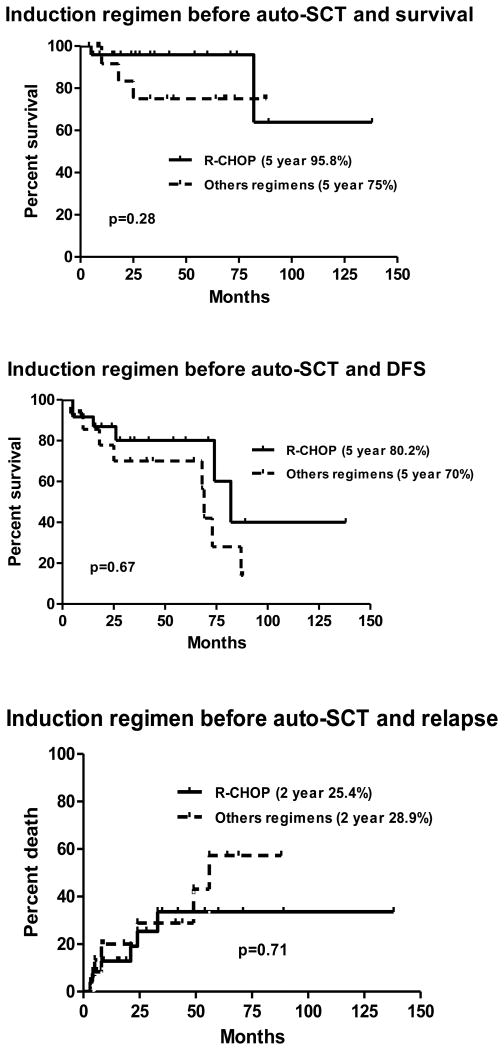
Pre-SCT disease status and induction regimen on survival
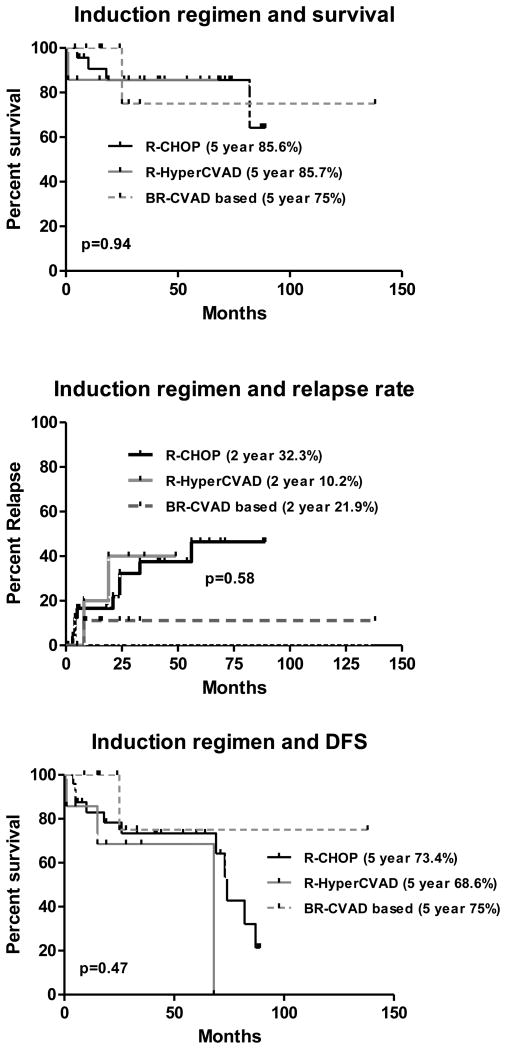
There was no difference in overall survival of patients undergoing auto-SCT in either a complete or a partial response. The estimated 5 year DFS and OS of patients receiving R-CHOP was 80.2% and 95.8% compared with patients receiving other regimens was 70% and 75% respectively (p=0.28) (fig: 3a-b). The relapse rate among patients receiving R-CHOP or other regimens was not significantly different (Fig 3c). There was no impact of various induction regimens on overall survival of patients. The estimated 5 year DFS and OS of patients receiving R-CHOP, R-Hyper-CVAD/ Ara-C/MTX and BR-CVAD were 73.4%, 85.6%; 68.6%, 85.7% and 75%, 75% respectively (fig 4a-b). Two year relapse rate of patients receiving R-CHOP was 32.3%, R-Hyper-CVAD was 10.2% and BR-CHOP was 21.9% (p=0.58) (Fig 4c).
Figure 3.
Standard induction regimen (CHOP) vs. other regimens and outcome after autologous stem cell transplantation: a. overall survival; b. disease-free survival; c. relapse rate
Figure 4.
Type of induction regimen and outcome after autologous stem cell transplantation: a. overall survival; b. disease-free survival; c. relapse rate
Discussion
Mantle cell lymphoma represents a spectrum of clinical symptoms from an indolent to that of a highly aggressive nature [19, 20]. The observation that intense induction regimens may be superior to standard chemotherapeutic regimens has led to the development of several phase II studies with improved long-term outcome [21-25]. Patients uniformly treated with high-dose induction regimens may experience improved long-term outcomes after auto-SCT in first remission when compared with historical controls. However, methods to compare outcomes between studies using varied induction regimens remain problematic due to inherent treatment bias as younger patients with high-risk disease features and good performance status are offered intensive regimens, whereas older and possibly patients with low risk disease may have been offered less aggressive therapies. Thus, the relative benefit of these initial intensive strategies remains uncertain. Despite selection bias in choosing induction regimen, our data showed no difference in long term outcome between intensive vs. standard induction regimen prior to SCT as long as patients had chemosensitive disease (CR or PR).
The MCL-2 Nordic trial reported results on patients treated with R-maxi-CHOP alternating with high-dose cytarabine and consolidation with BEAM supported by in vivo rituximab-purged autologous stem cells. In this study, with a median observation of 4 years, the 6-year OS and DFS were 70% and 66% respectively [26]. Our results appear similar to those reported in the Nordic study. Recently published study by Chang et al report their results following induction therapy with BR-CVAD (without SCT). The overall response rate in this study was 90% with a CR rate of 77%. The long term follow up data on these patients is pending at this time. The CALGB published results of intensive Immunochemotherapy induction regimen followed by auto-SCT showing a 3 year DFS and OS of 63% and 83% respectively[27].
The MIPI score has been recently generated as a prognostic tool specific for patients with MCL. Its scoring is based on a complex model using four clinical variables (age, performance status, LDH with upper limit of normal [ULN], and white blood cell count) [28-30]. Budde et al., reported that the MIPI score at diagnosis is independently associated with OS and PFS of patients with MCL who received auto-SCT regardless of the type of induction regimen received and the timing of transplantation [31]. The Nordic Lymphoma Group showed a positive correlation of the MIPI and sMIPI with the overall survival (OS) of patients uniformly treated on the Nordic Lymphoma Group MCL2 protocol followed by auto-SCT. In contrast, van't Veer et al found no prognostic value of MIPI in patients with MCL who were responsive to standard or intensive induction regimen followed by auto-SCT[25]. Similar to this study, we did not see difference in long term outcome between intensive vs. standard induction responders prior to auto-SCT even when adjusted for MIPI score.
Our data showed that, in the absence of available clinical trials standard induction regimen is an acceptable alternative compared to intensive induction regimen in patients receiving auto-SCT consolidation in selected induction responders. On the other hand, about 5% of blastoid variants MCL shows a much more aggressive clinical course in most studies and might preclude this decision. Greater than 80% of MCLs present with intermediate characteristics and more than half patients will respond to standard induction regimen. Those who do not get initial response can be considered for intensive re-induction followed by auto-SCT and our data supports benefits of SCT in this group as well, as long as chemosensitivity is documented before auto-SCT even after re-induction regimens. Our data showed no difference in outcome in patients achieving CR after one or more than one regimen and PR before auto-SCT. This, however, needs to be confirmed in larger prospective studies.
The emerging role of reduced intensity conditioning (RIC) regimens has created a paradigm shift by reducing transplant related mortality (TRM) therefore offering more individuals the option of allo-SCT in mantle cell lymphoma. We report a 5 year survival rate of 70% with and NRM of 12.5% in albeit a highly selected group receiving allogeneic stem cell transplantation. In the era of improved supportive care, decreasing transplant related mortality setting, RIC allo-SCT should be offered for otherwise fit patients who have relapsed following high dose chemotherapy (ie, auto-SCT). MD Anderson published the most encouraging long term results of non-myeloablative stem cell transplantation in 35 patients with relapsed MCL[32]. Six year OS was 53%, DFS was 46%. No patients had grade 3 to 4 acute GVHD. Chronic GVHD was noted in 60%. This study is encouraging and potentially could be offered upfront to a selected group of younger patients.
The major limitation of this study is the small sample size, its retrospective nature and inherent treatment selection bias at the time of diagnosis. This study does not compare various induction regimens in patients who have not received consolidation with transplantation. Therefore we are limited in our conclusions and apply only to patients who have undergone stem cell transplantation. Despite these limitations, the current study highlights the long term survival of patients receiving auto-SCT after a variety of induction regimens.
Current treatment strategies have evolved not based on the individual risk score but more on the feasibility, physician preference and patients' general condition. We speculate, more individualized approaches are foreseen as a way to improve outcome of MCL incorporating risk factors present at diagnosis, biomarkers representative of the molecular alterations, as well as quality of the response assessed by minimal residual disease[33, 34]. New drugs targeting survival pathways, including molecular alterations of the disease, are being progressively incorporated into the therapeutic armamentarium of the disease and will certainly contribute to further improve prognosis [35-38]. We believe as this study highlights that future prospective studies be designed to prevent relapse incorporating these novel agents as consolidation or maintenance strategies rather than comparing induction therapies.
Acknowledgments
Funding: This work was supported by National Center for Research Resources, National Institute of Health (grant # 5K-12 CA090625-09, N.R.)
Footnotes
Declaration of commercial interest: None
References
- 1.Rosenwald A, Wright G, Wiestner A, et al. The proliferation gene expression signature is a quantitative integrator of oncogenic events that predicts survival in mantle cell lymphoma. Cancer Cell. 2003;3:185–197. doi: 10.1016/s1535-6108(03)00028-x. [DOI] [PubMed] [Google Scholar]
- 2.Navarro A, Royo C, Hernandez L, et al. Molecular pathogenesis of mantle cell lymphoma: new perspectives and challenges with clinical implications. Semin Hematol. 2011;48:155–165. doi: 10.1053/j.seminhematol.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Czuczman MS, Leonard JP, Williams ME. Clinical roundtable monograph: Recent advances in the treatment of mantle cell lymphoma: a post-ASH 2009 discussion. Clin Adv Hematol Oncol. 2010;8:1–14. quiz 12p following page 14. [PubMed] [Google Scholar]
- 4.Chang JE, Peterson C, Choi S, et al. VcR-CVAD induction chemotherapy followed by maintenance rituximab in mantle cell lymphoma: a Wisconsin Oncology Network study. Br J Haematol. 2011 doi: 10.1111/j.1365-2141.2011.08820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kenkre VP, Long WL, Eickhoff JC, et al. Maintenance rituximab following induction chemo-immunotherapy for mantle cell lymphoma: long-term follow-up of a pilot study from the Wisconsin Oncology Network. Leuk Lymphoma. 2011;52:1675–1680. doi: 10.3109/10428194.2011.580404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabhan C, Ragam A, Bitran JD, Mehta J. Hematopoietic SCT for mantle cell lymphoma: is it the standard of care? Bone Marrow Transplant. 2010;45:1379–1387. doi: 10.1038/bmt.2010.77. [DOI] [PubMed] [Google Scholar]
- 7.Dreger P, Martin S, Kuse R, et al. The impact of autologous stem cell transplantation on the prognosis of mantle cell lymphoma: a joint analysis of two prospective studies with 46 patients. Hematol J. 2000;1:87–94. doi: 10.1038/sj.thj.6200007. [DOI] [PubMed] [Google Scholar]
- 8.Jantunen E, Canals C, Attal M, et al. Autologous stem-cell transplantation in patients with mantle cell lymphoma beyond 65 years of age: a study from the European Group for Blood and Marrow Transplantation (EBMT) Ann Oncol. 2011 doi: 10.1093/annonc/mdr035. [DOI] [PubMed] [Google Scholar]
- 9.Ritchie DS, Seymour JF, Grigg AP, et al. The hyper-CVAD-rituximab chemotherapy programme followed by high-dose busulfan, melphalan and autologous stem cell transplantation produces excellent event-free survival in patients with previously untreated mantle cell lymphoma. Ann Hematol. 2007;86:101–105. doi: 10.1007/s00277-006-0193-2. [DOI] [PubMed] [Google Scholar]
- 10.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: a study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 11.Tam CS, Khouri IF. Autologous and allogeneic stem cell transplantation: rising therapeutic promise for mantle cell lymphoma. Leuk Lymphoma. 2009;50:1239–1248. doi: 10.1080/10428190903026518. [DOI] [PubMed] [Google Scholar]
- 12.Le Gouill S, Mohty M, Guillaume T, et al. Allogeneic stem cell transplantation in mantle cell lymphoma: where are we now and which way should we go? Semin Hematol. 2011;48:227–239. doi: 10.1053/j.seminhematol.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Shea T, Johnson J, Westervelt P, et al. Reduced-Intensity Allogeneic Transplantation Provides High Event-Free and Overall Survival in Patients with Advanced Indolent B Cell Malignancies: CALGB 109901. Biol Blood Marrow Transplant. 2011;17:1395–1403. doi: 10.1016/j.bbmt.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook G, Smith GM, Kirkland K, et al. Outcome following Reduced-Intensity Allogeneic Stem Cell Transplantation (RIC AlloSCT) for relapsed and refractory mantle cell lymphoma (MCL): a study of the British Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2010;16:1419–1427. doi: 10.1016/j.bbmt.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Weniger MA, Wiestner A. Molecular targeted approaches in mantle cell lymphoma. Semin Hematol. 2011;48:214–226. doi: 10.1053/j.seminhematol.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 17.Smith SD, Hsi E, Bolwell B, et al. Validation of the Mantle Cell Lymphoma International Prognostic Index: A single-center retrospective analysis. Am J Hematol. 2010;85:454–456. doi: 10.1002/ajh.21705. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Sato K, Imamura Y, et al. Small cell variant of mantle cell lymphoma is an indolent lymphoma characterized by bone marrow involvement, splenomegaly, and a low Ki-67 index. Cancer Sci. 2011;102 doi: 10.1111/j.1349-7006.2011.01988.x. Sepcover. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furtado M, Rule S. Indolent mantle cell lymphoma. Haematologica. 2011;96:1086–1088. doi: 10.3324/haematol.2011.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romaguera JE, Fayad LE, Feng L, et al. Ten-year follow-up after intense chemoimmunotherapy with Rituximab-HyperCVAD alternating with Rituximab-high dose methotrexate/cytarabine (R-MA) and without stem cell transplantation in patients with untreated aggressive mantle cell lymphoma. Br J Haematol. 2010;150:200–208. doi: 10.1111/j.1365-2141.2010.08228.x. [DOI] [PubMed] [Google Scholar]
- 22.Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG) J Clin Oncol. 2005;23:1984–1992. doi: 10.1200/JCO.2005.08.133. [DOI] [PubMed] [Google Scholar]
- 23.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 24.Mangel J, Leitch HA, Connors JM, et al. Intensive chemotherapy and autologous stem-cell transplantation plus rituximab is superior to conventional chemotherapy for newly diagnosed advanced stage mantle-cell lymphoma: a matched pair analysis. Ann Oncol. 2004;15:283–290. doi: 10.1093/annonc/mdh069. [DOI] [PubMed] [Google Scholar]
- 25.van't Veer MB, de Jong D, MacKenzie M, et al. High-dose Ara-C and beam with autograft rescue in R-CHOP responsive mantle cell lymphoma patients. Br J Haematol. 2009;144:524–530. doi: 10.1111/j.1365-2141.2008.07498.x. [DOI] [PubMed] [Google Scholar]
- 26.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoster E. Prognostic relevance of clinical risk factors in mantle cell lymphoma. Semin Hematol. 2011;48:185–188. doi: 10.1053/j.seminhematol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT) Blood. 2010;115:1530–1533. doi: 10.1182/blood-2009-08-236570. [DOI] [PubMed] [Google Scholar]
- 30.Schaffel R, Hedvat CV, Teruya-Feldstein J, et al. Prognostic impact of proliferative index determined by quantitative image analysis and the International Prognostic Index in patients with mantle cell lymphoma. Ann Oncol. 2010;21:133–139. doi: 10.1093/annonc/mdp495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Budde LE, Guthrie KA, Till BG, et al. Mantle cell lymphoma international prognostic index but not pretransplantation induction regimen predicts survival for patients with mantle-cell lymphoma receiving high-dose therapy and autologous stem-cell transplantation. J Clin Oncol. 2011;29:3023–3029. doi: 10.1200/JCO.2010.33.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam CS, Bassett R, Ledesma C, et al. Mature results of the M. D. Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma Blood. 2009;113:4144–4152. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartmann EM, Campo E, Wright G, et al. Pathway discovery in mantle cell lymphoma by integrated analysis of high-resolution gene expression and copy number profiling. Blood. 2010;116:953–961. doi: 10.1182/blood-2010-01-263806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pott C, Hoster E, Delfau-Larue MH, et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115:3215–3223. doi: 10.1182/blood-2009-06-230250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess G, Herbrecht R, Romaguera J, et al. Phase III study to evaluate temsirolimus compared with investigator's choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2009;27:3822–3829. doi: 10.1200/JCO.2008.20.7977. [DOI] [PubMed] [Google Scholar]
- 36.Goy A, Bernstein SH, Kahl BS, et al. Bortezomib in patients with relapsed or refractory mantle cell lymphoma: updated time-to-event analyses of the multicenter phase 2 PINNACLE study. Ann Oncol. 2009;20:520–525. doi: 10.1093/annonc/mdn656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Habermann TM, Lossos IS, Justice G, et al. Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol. 2009;145:344–349. doi: 10.1111/j.1365-2141.2009.07626.x. [DOI] [PubMed] [Google Scholar]
- 38.Wiernik PH, Lossos IS, Tuscano JM, et al. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]